INTRODUCTION
The removal of free-living parasite larvae by aquatic predators has recently been recognized as an important factor limiting parasite transmission and hence their infection success (Thieltges et al. Reference Thieltges, Jensen and Poulin2008a ; Orlofske et al. Reference Orlofske, Jadin and Johnson2015). It has been shown that various aquatic organisms (e.g. carnivorous plants, cnidarians, bryozoans, oligochaetes, turbellarians, insect larvae, mites, molluscs, fish, newts) consume larval stages of parasites, decreasing their numbers in water (reviewed in Thieltges et al. Reference Thieltges, Jensen and Poulin2008a ; Johnson et al. Reference Johnson, Dobson, Lafferty, Marcogliese, Memmott, Orlofske, Poulin and Thieltges2010). Such predation can lead to the significant reduction (up to 99%) of the infection intensity in downstream hosts (Schotthoefer et al. Reference Schotthoefer, Labak and Beasley2007; Thieltges et al. Reference Thieltges, Jensen and Poulin2008a ; Orlofske et al. Reference Orlofske, Jadin, Preston and Johnson2012).
Bivalve molluscs are powerful biofilterers (McMahon, Reference McMahon, Thorp and Covich1991), which can effectively cleanse water of a broad spectrum of pathogens, including bacteria (Miller et al. Reference Miller, Miller, Gardner, Atwill, Byrne, Jang, Harris, Ames, Jessup, Paradies, Worcester, Melli and Conrad2006; Ismail et al. Reference Ismail, Dodd, Sassoubre, Horne, Boehm and Luthy2015), parasitic protists (Robertson, Reference Robertson2007; Lucy et al. Reference Lucy, Graczyk, Tamang, Miraflor and Minchin2008; Willis et al. Reference Willis, McClure, McClure, Spears, Davidson and Greenwood2014; Słodkowicz-Kowalska et al. Reference Słodkowicz-Kowalska, Majewska, Rzymski, Skrzypczak and Werner2015), larval stages of parasitic copepods (Molloy et al. Reference Molloy, Pietrak, Bouchard and Bricknell2011; Bartsch et al. Reference Bartsch, Robinson, Liutkus, Ang, Webb and Pearce2013) and even viruses (Faust et al. Reference Faust, Stallknecht, Swayne and Brown2009; Mezzanotte et al. Reference Mezzanotte, Marazzi, Bissa, Pacchioni, Binelli, Parolini, Magni, Ruggeri, Morghen, Zanotto and Radaelli2016, however see Stumpf et al. Reference Stumpf, Failing, Papp, Nazir, Böhm and Marschang2010). Thus, the potential of bivalves to reduce various aquatic diseases caused by microparasites (viruses, bacteria, protists) has been demonstrated, while the effect of bivalves on macroparasite transmission is still understudied (Burge et al. Reference Burge, Closek, Friedman, Groner, Jenkins, Shore-Maggio and Welsh2016). We propose that freshwater bivalves can also remove free-living larvae of fish trematodes (i.e. cercariae), which are within the size range (up to 0·25 mm) of particles filtered by bivalves (Gosling, Reference Gosling2003). Trematodes cause various diseases in humans, fish and other animals and have a great epidemiological and economic impact (King and Scholz, Reference King and Scholz2001; Ross et al. Reference Ross, Bartley, Sleigh, Olds, Li, Williams and McManus2002; Fenwick, Reference Fenwick2012; Shinn et al. Reference Shinn, Pratoomyot, Bron, Paladini, Brooker and Brooker2015). Taking into account the high filtration capacities and abundance of bivalves in different ecosystems (Burge et al. Reference Burge, Closek, Friedman, Groner, Jenkins, Shore-Maggio and Welsh2016), they hypothetically could be used to impede trematode transmission in aquaculture and for prevention of human trematodoses (e.g. schistosomiasis, opisthorchiasis).
Data about the influence of bivalves on trematode transmission are still sparse and controversial. Moreover, these data are limited to marine trematodes infecting mussels, while there is no information about freshwater parasites (e.g. fish trematodes). For example, some marine mussels (Crassostrea gigas, Mya arenaria) were reported to reduce parasitic load in host mussel species (Mytilus edulis, Cerastoderma edule) by filtering trematode cercariae (Thieltges et al. Reference Thieltges, Jensen and Poulin2008a ; Goedknegt et al. Reference Goedknegt, Welsh, Drent and Thieltges2015) and by acting as decoys for the trematodes, thus causing a dilution effect (Thieltges et al. Reference Thieltges, Reise, Prinz and Jensen2009). However, experiments with other marine (mix of C. edule, M. edulis, Ensis americanus) and freshwater (Sphaerium sp.) bivalves indicated that these molluscs did not remove cercariae (Orlofske et al. Reference Orlofske, Jadin, Preston and Johnson2012; Welsh et al. Reference Welsh, van der Meer, Brussaard and Thieltges2014). Therefore, clarification is needed of the role of bivalves in removal of the trematode larval stages and potential ecosystem effects.
The present study is the first attempt to assess the influence of freshwater mussels on the infection success of common fish trematodes. We experimentally tested (1) the ability of mussels (Anodonta anatina) to remove the cercariae of the eye fluke (Diplostomum pseudospathaceum) and (2) the influence of mussels on the transmission of this trematode to the host fish (rainbow trout, Oncorhynchus mykiss). Our hypothesis was that mussels can effectively decrease cercariae number in water, thus reducing the intensity of trematode infection in fish.
This host–parasite system was chosen because diplostomatids, including D. pseudospathaceum, are very common parasites, infecting almost all species of freshwater fishes wherever they occur (Valtonen and Gibson, Reference Valtonen and Gibson1997). They can infect rainbow trout in natural environments (Sokolov, Reference Sokolov2010) and are frequently encountered in fish farms (Karvonen et al. Reference Karvonen, Savolainen, Seppälä and Valtonen2006). Diplostomum pseudospathaceum can impair fish physiology and behaviour, including reduced vision and decreased attack distance when catching a prey (Owen et al. Reference Owen, Barber and Hart1993; Karvonen et al. Reference Karvonen, Seppälä and Valtonen2004a , Reference Karvonen, Kirsi, Hudson and Valtonen b ). Moreover, the eye fluke can manipulate host behaviour (Seppälä et al. Reference Seppälä, Karvonen and Valtonen2004; Mikheev et al. Reference Mikheev, Afonina and Pavlov2010a , Reference Mikheev, Pasternak, Taskinen and Valtonen b ; Gopko et al. Reference Gopko, Mikheev and Taskinen2015, Reference Gopko, Mikheev and Taskinen2017) often causing a deterioration of anti-predatory traits in their hosts and predisposing them to predation by avian definitive hosts (Crowden and Broom, Reference Crowden and Broom1980; Seppälä et al. Reference Seppälä, Karvonen and Valtonen2004, Reference Seppälä, Karvonen and Valtonen2005, Reference Seppälä, Karvonen and Valtonen2008; Gopko et al. Reference Gopko, Mikheev and Taskinen2017). Although praziquantel decreases the life span of Diplostomum cercariae (Voutilainen et al. Reference Voutilainen, Saarinen, Suonpää and Taskinen2009), effective treatment against diplostomosis in fish is not available. Thus, knowledge of the factors controlling the transmission of the eye fluke is important and potentially can be used in sustainable parasite control in the fish farming industry.
MATERIALS AND METHODS
The experiments were carried out in June to September 2016 at the Konnevesi research station (University of Jyväskylä, Finland). To minimize observer bias, blind methods were used in all experiments.
Study organisms
The eye fluke D. pseudospathaceum has a three-host life cycle. Infected freshwater snails (e.g. pond snail Lymnea stagnalis, the first intermediate host) produce huge numbers (up to 58 000 d−1) of free-living larval cercariae (Lyholt and Buchmann, Reference Lyholt and Buchmann1996), which infect freshwater fishes (second intermediate host). In fish, the parasites establish in the eye lenses and develop into metacercariae. Eye flukes complete their life cycle in the intestine of fish-eating birds (definitive host), where they reproduce sexually (Valtonen and Gibson, Reference Valtonen and Gibson1997).
We used L. stagnalis snails collected from Lake Konnevesi as a source of the cercariae. Infected snails were allowed to release cercariae for 3 h at 18 °C; therefore, cercariae used for experimental infections were no older than 3 h at the beginning of the experiment. Anodonta anatina mussels were collected from the shallow nearshore habitats of Lake Konnevesi. Anodonta anatina (Unionidae) is a widespread species (Lopes-Lima et al. Reference Lopes-Lima, Sousa, Geist, Aldridge, Araujo, Bergengren, Bespalaya, Bódis, Burlakova, Van Damme, Douda, Froufe, Georgiev, Gumpinger, Karatayev, Kebapçi, Killeen, Lajtner, Larsen, Lauceri, Legakis, Lois, Lundberg, Moorkens, Motte, Nagel, Ondina, Outeiro, Paunovic and Prié2016), which often shares the same microhabitats, shallow littoral areas of lakes (Taskinen and Valtonen, Reference Taskinen and Valtonen1995) with L. stagnalis (mussels are largely infaunal, partly submerged in the sand, while snails are epibenthic and feed on the aquatic vegetation). Therefore, potential predators of trematode cercariae, A. anatina and D. pseudospathaceum are sympatric throughout the littoral zone.
Rainbow trout were obtained from a commercial fish farm. Fish were reared in well water untreated with chemicals and therefore were free from macroparasites. Fish and mussels were acclimated to the conditions in the indoor 200 L flow-through tanks for at least a week at 13·1–13·8 °C before the experiments.
Testing cercariae removal by mussels
To study the removal of cercariae by filtering mussels, we measured changes in the cercariae numbers after their incubation in containers with mussels. Similar containers without mussels served as a control. Ten 2 L plastic containers were filled with filtered (100-μm mesh) lake water. An individual mussel was placed in each of the five containers 5 h prior to experiments, to acclimate the mussels to laboratory conditions (water temperature 15‒16 °C). Each mussel was observed to filter actively (siphons protruded) before the start of the experiment. The mussel length was from 78 to 93 mm (n = 5).
Cercariae produced by eight infected L. stagnalis snails were mixed and an equal volume of cercariae suspension (100 mL) was added to the containers to give a final concentration of about 6000 cercariae L−1. The average concentration of cercariae in the initial suspension was counted from three 2-mL samples. The experiment lasted for 2 h and the concentration of cercariae in each container was estimated at the beginning and at the end of the incubation from three 5-mL samples taken after gentle mixing. Cercariae were counted under a dissecting microscope (28× magnification) in a Bogorov zooplankton counting chamber (Hydro-Bios GmbH, Kiel-Altenholz, Germany) within 2 h of sampling. The average cercariae numbers were calculated for each container and used in the subsequent analysis. The initial number of cercariae in the experiment was 6520 ± 369 cercariae L−1 (mean ± s.e.). The effect of mussels on the cercariae numbers was tested using repeated-measures analysis of variance (ANOVA) because it is an appropriate analysis for monitoring temporal changes in mean values. Treatment (mussel present/absent) was a categorical predictor, while cercariae numbers at the beginning and at the end of the experiment were considered to be repeated measurements. Data were checked for normality using the Shapiro–Wilk's test (W > 0·8, P > 0·16 in all cases). The clearance rates were calculated according to Frost (Reference Frost1972). To calculate the rates of cercariae removal, we used the equations proposed for ingestion rates (Conover, Reference Conover and Kinne1978); however, it was not clear from the results of our study, whether cercariae were ingested or only damaged by mussels. After the experiment, the length and mass of each mussel were measured to allow calculation of the correlation between mussel size and reduction of cercariae numbers.
Infection experiment
To determine whether predation on parasite larvae can reduce the infection rate in the next host, we experimentally infected rainbow trout with eye fluke cercariae in the presence (predation treatment) or absence (control) of the mussel A. anatina. The three similarly designed experiments were conducted with high, medium and low exposure doses of cercariae (400, 300, 230 cercariae per fish, respectively). Exposure doses were similar to those used in previous studies (Seppälä et al. Reference Seppälä, Karvonen and Valtonen2004; Karvonen et al. Reference Karvonen, Seppälä and Valtonen2004a , Reference Karvonen, Kirsi, Hudson and Valtonen b ). Metacercariae numbers acquired by the individual rainbow trout in our experiment never exceeded 178 metacercariae/fish and median values were much lower (see Results), and were comparable to the infection intensities in naturally infected fish (Mikheev et al. Reference Mikheev, Pasternak and Valtonen2014 and references therein).
Rainbow trout were exposed to cercariae individually in 22 semi-transparent plastic containers (30 × 40 × 25 cm) filled with 12 L of lake water. Individual A. anatina mussels were placed in 11 randomly chosen containers (Anodonta treatment) and 11 containers with empty mussel shells served as controls. However, in the treatment with medium cercariae doses, the number of experimental and control containers was not equal (10 and 12 containers, respectively).
A closed empty A. anatina shell was placed in each of the control containers to minimize potential effect of the habitat structure and bottom colouration on the behaviour of rainbow trout. Juvenile rainbow trout may consider dark objects on the bottom (such as a mussel shell) as a shelter (Mikheev et al. Reference Mikheev, Afonina and Pavlov2010a , Reference Mikheev, Pasternak, Taskinen and Valtonen b ) and may ventilate less actively in the presence of a shelter due to decreased stress, which in turn, results in decreased acquisition of the cercariae (Mikheev et al. Reference Mikheev, Pasternak and Valtonen2014). Besides visual stimuli, chemical signals are also important for fish behaviour. Such signals can carry important information, like a predation threat, that markedly increases ventilation rate (Hawkins et al. Reference Hawkins, Armstrong and Magurran2004, Reference Hawkins, Magurran and Armstrong2007). However, there is no evidence that chemical cues produced by mussels could influence ventilation in fish. On the other hand, the presence of fish can induce an increase of the filtration activity of Anodonta mussels (Jokela and Palokangas, Reference Jokela and Palokangas1993). We estimated filtration rates of mussels in containers without fish. In experiments with fish and mussels, fish could stimulate filtration of cercariae, thus indirectly decreasing parasite transmission.
Fish and mussels were acclimated in experimental containers (at 15‒16 °C) for 2 h before the beginning of experiments. Mussels for each experiment were chosen randomly from the subpopulation of 25 A. anatina maintained in the laboratory in a large flow-through tank with water from Lake Konnevesi. The length of mussels ranged from 71 to 119 mm and did not differ from the length of mussel shells in control treatments (one-way ANOVA, P < 0·14 in all three experiments). Shells were washed and kept for a week in the same holding tank with living mussels prior to the experiments. Fish were exposed to parasites by adding the mixture of freshly produced D. pseudospathaceum cercariae (obtained from five infected L. stagnalis snails) to each container to give 400, 300 and 230 cercariae per fish (high, medium and low dose, respectively). Experiments were conducted in an 11-day period and started at the same time of the day (at noon) to exclude potential circadian effects. This time period was chosen for experiments because Diplostomum cercariae are mostly released in the day time (Karvonen et al. Reference Karvonen, Seppälä and Valtonen2004a , Reference Karvonen, Kirsi, Hudson and Valtonen b ), when their chances of encountering a fish host are highest.
After 3 h of exposure, fish were caught with a dip net and placed individually in 8 L flow-through tanks for 48 h, which is enough for the parasites to reach the eye lenses of fish (Owen et al. Reference Owen, Barber and Hart1993; Karvonen et al. Reference Karvonen, Seppälä and Valtonen2004a , Reference Karvonen, Kirsi, Hudson and Valtonen b ). Fish were then killed with an overdose of MS 222 (Sigma Chemical Co., St Louis, Missouri, USA), weighed and dissected.
The number of D. pseudospathaceum in the eye lenses of the fish was counted using a dissection microscope (28× magnification). After the experiment, the length and mass of the mussels were also measured to enable correlation between mussel size and the possible reduction of infection intensity in fish. The experiments were conducted with permission from the Centre for Economic Development, Transport and Environment of South Finland (licence number ESAVI/10184/04·10·07/2014). We used the minimum number of fish to produce statistically reproducible results and performed experiments in accordance with the ethical and regulatory guidelines (standards of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes and its appendix).
We used negative binomial generalized linear models (GLMs) with log link function to analyse infection intensities, since the negative binomial distribution often provides a good fit to parasite data (Wilson et al. Reference Wilson, Bjornstad, Dobson, Merler, Po-Glayen, Randolph, Read, Skorping, Hudson, Rizolli, Grenfell, Heester-Beek and Dobson2002; Alexander, Reference Alexander2012). The STATISTICA 10 (StatSoft Inc., 2011) and R Core team (2015) software were used for the statistical analysis. The three exposure doses (high, medium and low) were regarded as separate experiments, and were analysed separately. The ‘ggplot’ package (Wickham, Reference Wickham2009) was used for graphical presentation of the data.
RESULTS
Removal of cercariae by mussels
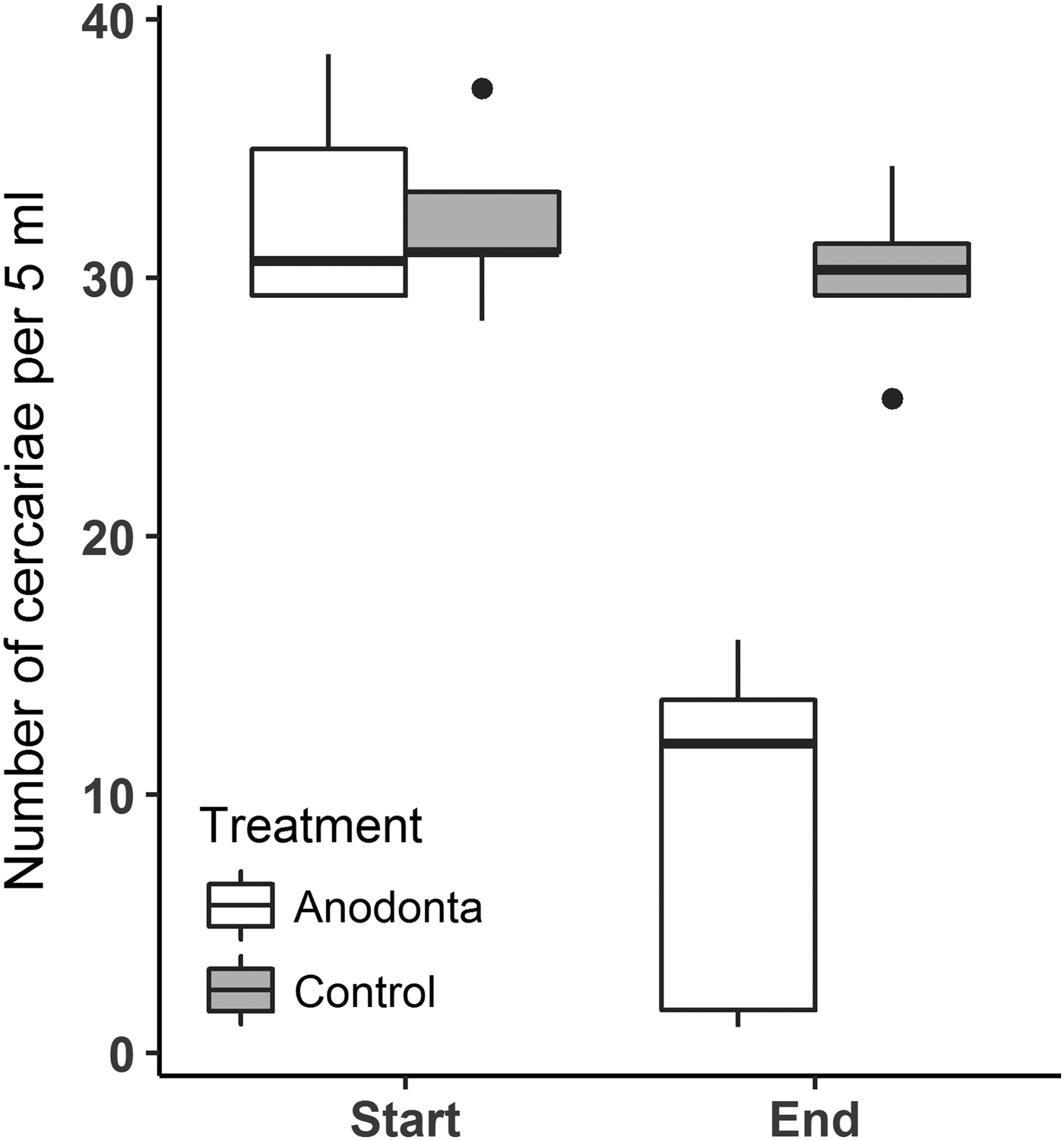
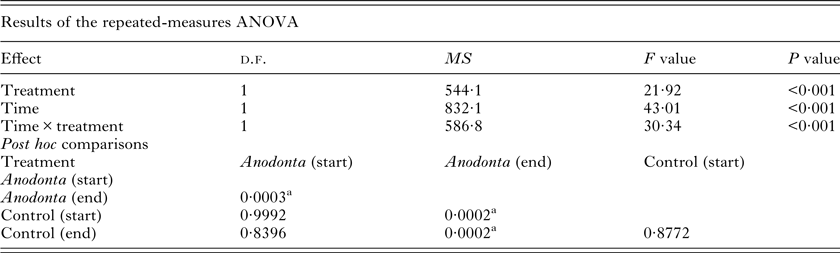
The repeated-measures ANOVA revealed a significant interaction between the treatment (Anodonta presence/absence) and the time (start/end of the experiment) on the cercariae count (Table 1, Fig. 1). Post hoc comparisons showed that A. anatina presence in the environment significantly decreased (4-fold on average) the concentration of cercariae after 2 h incubation. In contrast, cercariae numbers in the control treatment were similar at the beginning and at the end of the experiment. The initial numbers of cercariae were similar in the experimental and control treatments (Table 1, Fig. 1).
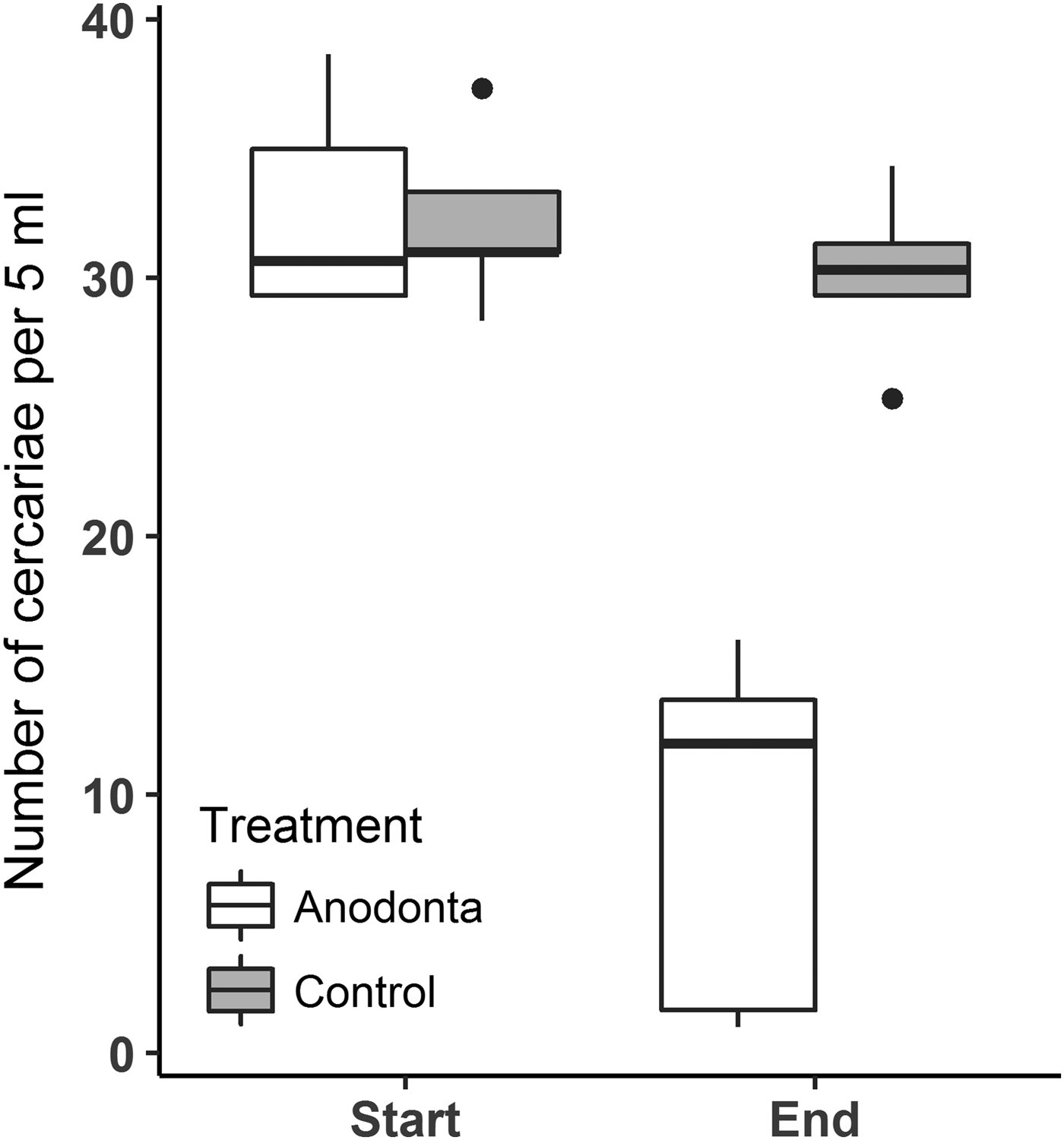
Fig. 1. Numbers of Diplostomum pseudospathaceum cercariae significantly and strongly (almost 4-fold) decreased in the presence of Anodonta anatina, while in the control treatment no decrease was found. ‘Boxes’ on the plot represent a median with the interquartile range (IQR). Whiskers extend from the highest to lower values within 1·5*IQR.
Table 1. Repeated-measures ANOVA summary for the cercariae removal experiment. The number of Diplostomum pseudospathaceum cercariae was considered as a response variable, Anodonta anatina presence/absence (treatment) as a fixed factor and time (start/end of the experiment) as repeated measurements
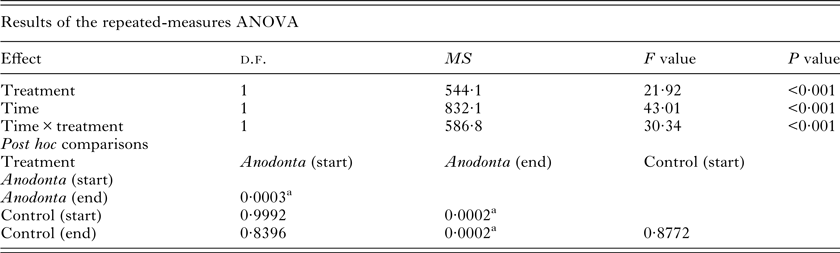
a Significant Tukey HSD post hoc comparisons.
The individual clearance rates of mussels calculated from changes in cercariae numbers in Anodonta treatment were 0·6‒3·7 L per hour (mean ± s.e. = 1·9 ± 0·6 L), meaning that all tested mussels filtered the water volume in the 2 L containers more than once during the 2 h incubation. On average, mussels reduced the number of cercariae from 6520 ± 369 to 1773 ± 628 cercariae L−1 in 2 h (Supplementary Table S1), while two individuals decreased cercariae numbers by 18- and 35-fold. Maximum individual removal rates varied from 256 to 22 563 (mean ± s.e. = 7406 ± 4357) cercariae h−1. Numbers of cercariae removed did not correlate with the mussel length (Pearson correlation, r = 0·39, P = 0·51).
Infection experiments
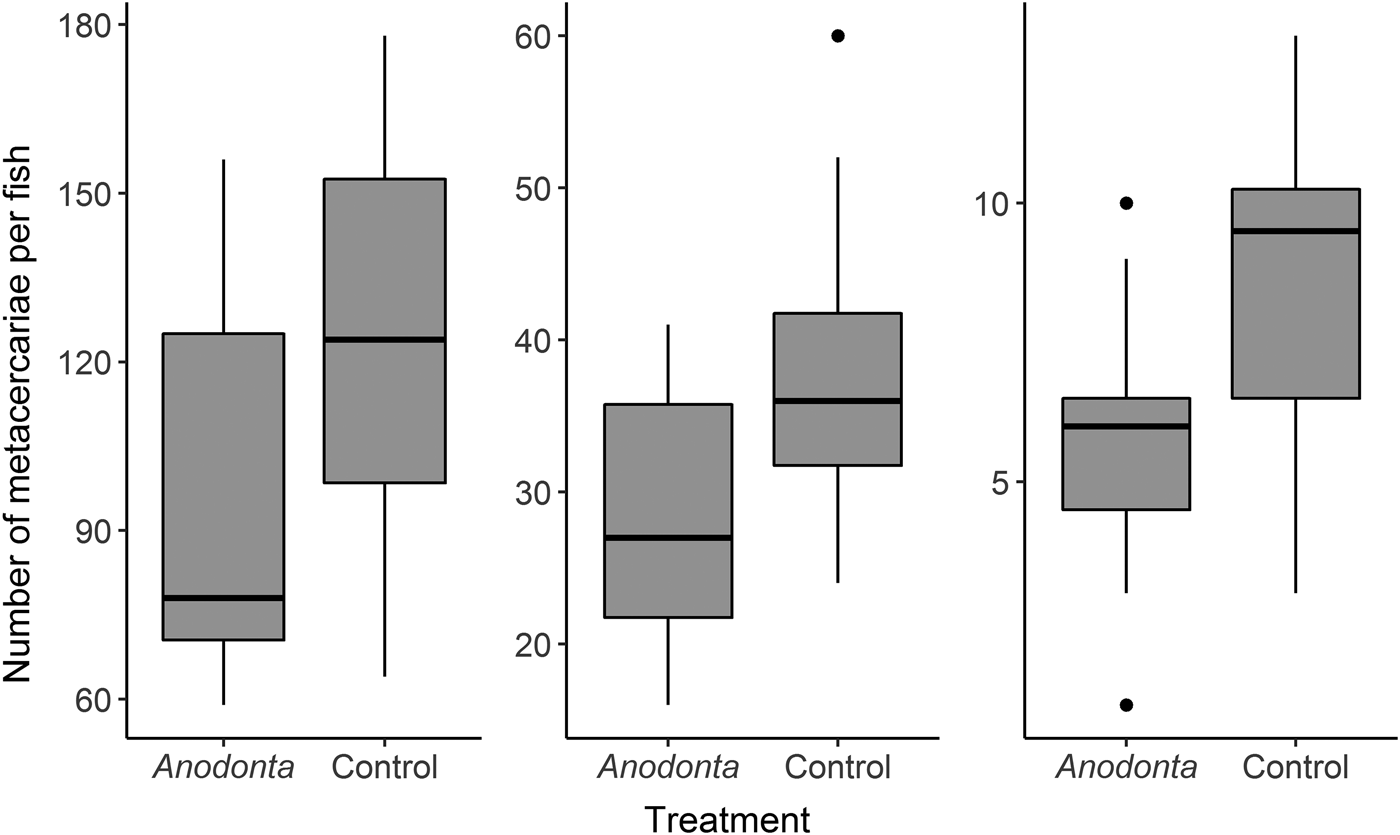
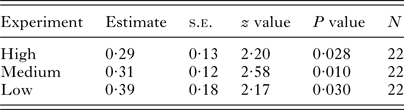
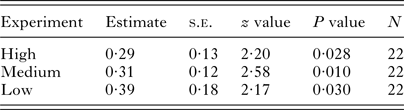
Negative binomial GLMs demonstrated a significant influence of the treatment on the infection intensities in rainbow trout in all three experiments (Table 2). Generally, mean infection intensities were 30‒40% lower in Anodonta treatment (Table 2, Fig. 2, Supplementary Table S2). When outliers were excluded, the results were still significant (P < 0·05). Mussel size did not significantly correlate with the infection intensity in fish (Pearson correlation, r < 0·12, P > 0·75 in all cases). There was no significant difference in fish mass between the treatments in any of the three experiments (t-test, P > 0·40 in all cases).
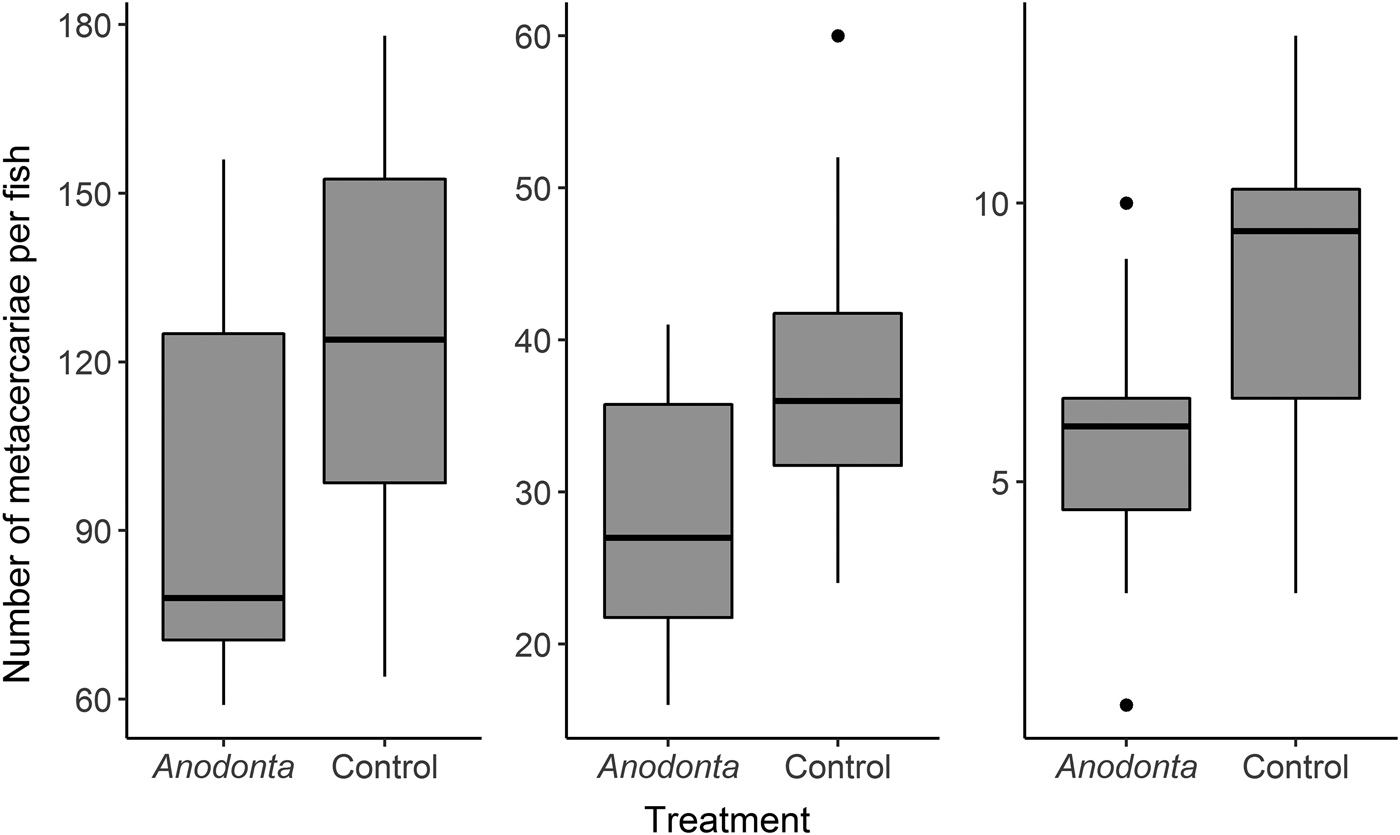
Fig. 2. Infection intensities in rainbow trout, Oncorhynchus mykiss, infected in the presence of Anodonta mussels were lower when high, medium and low amounts of Diplostomum pseudospathaceum cercariae were added to. ‘Boxes’ on plots represent a median with the interquartile range (IQR). Whiskers extend from the highest to lower values within 1·5*IQR. Outliers are plotted as black dots.
Table 2. Results of negative binomial GLM in high-, medium- and low-infection experiments. The effect of the treatment (absence/presence of Anodonta anatina in the environment) on the intensity of the Diplostomum pseudospathaceum infection in rainbow trout, Oncorhynchus mykiss, was significant in all three experiments
DISCUSSION
The removal of free-living parasite larvae by various aquatic predators has recently been recognized as an important factor limiting transmission of parasites and hence their infection success (Thieltges et al. Reference Thieltges, Jensen and Poulin2008a ; Johnson et al. Reference Johnson, Dobson, Lafferty, Marcogliese, Memmott, Orlofske, Poulin and Thieltges2010; Orlofske et al. Reference Orlofske, Jadin and Johnson2015). Our experiments showed that the common freshwater mussel A. anatina can also reduce parasite abundance in water by filtering cercariae of D. pseudospathaceum. In addition, these bivalves significantly reduced (by 30‒40%) the success of cercariae transmission to the rainbow trout, which is vulnerable to eye fluke infection both in natural conditions and at fish farms. These findings were confirmed in three independent experiments with different exposure doses. Since the mean density of Anodonta mussels in the lake littoral zone can be 15 individuals m−2 (Hanson et al. Reference Hanson, Mackay and Prepas1988), and the maximum density of unionoid mussels in a river can be over 1000 individuals m−2 (Oulasvirta, Reference Oulasvirta2011), the effect of freshwater mussels on diplostomatid cercariae concentration could be considerable. However, further laboratory and field studies are needed to test how removal of cercariae by mussels varies under different environmental conditions (e.g. temperature, light, turbidity, biotic factors) and between different mussel species.
The reduction of trematode infection in mussel hosts has earlier been reported for marine bivalves (Thieltges et al. Reference Thieltges, Jensen and Poulin2008a ; Thieltges et al. Reference Thieltges, Reise, Prinz and Jensen2009; Goedknegt et al. Reference Goedknegt, Welsh, Drent and Thieltges2015), whereas the evidence for reduction of trematode infections in fish hosts has been lacking. Furthermore, this is the first evidence for the limitation of trematode transmission by (non-host) bivalves in freshwater environments, as the only previous study focusing on this topic did not reveal an effect of freshwater fingernail clams Sphaerium on cercariae abundance (Orlofske et al. Reference Orlofske, Jadin, Preston and Johnson2012). However, the duck mussels tested in our study were considerably larger than fingernail clams, with higher filtration capacities, which could explain the observed differences in their removal of cercariae.
Our study indicated the ability of bivalves to reduce diplostomosis, which commonly causes problems at fish farms (Karvonen et al. Reference Karvonen, Seppälä and Valtonen2004a , Reference Karvonen, Kirsi, Hudson and Valtonen b ; Karvonen et al. Reference Karvonen, Savolainen, Seppälä and Valtonen2006). Therefore, bivalves might be used to control macroparasitic infections like fish and human trematodoses. Their potential effectiveness for prevention of several viral, bacterial and protozoan infections has been suggested earlier (reviewed in Burge et al. Reference Burge, Closek, Friedman, Groner, Jenkins, Shore-Maggio and Welsh2016). A wide ecological tolerance and widespread distribution of certain bivalve species make them good candidates for this role. For example, dreissenids and oysters remove pathogens effectively in different aquatic ecosystems (Graczyk et al. Reference Graczyk, Conn, Lucy, Minchin, Tamang, Moura and DaSilva2004; Lucy et al. Reference Lucy, Graczyk, Tamang, Miraflor and Minchin2008; Thieltges et al. Reference Thieltges, Reise, Prinz and Jensen2009; Conn et al. Reference Conn, Lucy, Graczyk, Nalepa and Schloesser2013; McLaughlan and Aldridge, Reference McLaughlan and Aldridge2013). Thus, unionid mussels collected from natural habitats or derived from captive breeding (Barnhart, Reference Barnhart2006; Scriven et al. Reference Scriven, Jones, Taylor, Aldridge, McIvor and Frank2011) could be used to control trematodoses, such as diplostomosis in fish farms in an ecologically sustainable way. One possible way to bridge between our small-scale laboratory research and its potential application in aquaculture would be mesocosm experiments including fish, unionid mussels and parasites. Managing parasitic infections in fish is intensively studied in semi-natural conditions (Karvonen et al. Reference Karvonen, Aalto-Araneda, Virtala, Kortet, Koski and Hyvärinen2016).
The ability of A. anatina to reduce the abundance of cercariae in the environment (on average an almost 4-fold decrease by an individual mussel in 2 h) indicates that even a small number of these biofilterers can diminish the transmission of fish parasites. The potential use of Anodonta mussels for water quality improvement and parasite control (bacterial and protist infections) has already been suggested (Hänninen et al. Reference Hänninen, Hörman, Rimhanen-Finne, Vahtera, Malmberg, Herve and Lahti2005; Lucy et al. Reference Lucy, Graczyk, Tamang, Miraflor and Minchin2008; Ismail et al. Reference Ismail, Dodd, Sassoubre, Horne, Boehm and Luthy2015; Słodkowicz-Kowalska et al. Reference Słodkowicz-Kowalska, Majewska, Rzymski, Skrzypczak and Werner2015), because their filtration capacities are among the highest in freshwater bivalves (Pusch et al. Reference Pusch, Siefert, Walz, Bauer and Wachtler2001; Stybel et al. Reference Stybel, Fenske and Schernewski2009). The filtration rates obtained in our experiment were comparable with the values calculated for this species by Kryger and Riisgård (Reference Kryger and Riisgård1988), but higher than values obtained in the experiments by McIvor (Reference McIvor2004). However, individual filtration activity of bivalves is very variable even under constant experimental conditions, following some endogenous rhythms (Kryger and Riisgård, Reference Kryger and Riisgård1988; Englund and Heino, Reference Englund and Heino1996; McIvor, Reference McIvor2004; Huyvaert et al. Reference Huyvaert, Carlson, Bentler, Cobble, Nolte and Franklin2012).
Clearance rates of bivalves are affected not only by endogenous rhythms, but also by various environmental factors, including water temperature, seston composition and concentration, light intensities, and age and size of the mollusc (Vanderploeg et al. Reference Vanderploeg, Liebig and Nalepa1995; Englund and Heino, Reference Englund and Heino1996; Eversole et al. Reference Eversole, Stuart and Brune2008; Kim et al. Reference Kim, Lee and Hwang2011). Biotic factors are also important; for example, the presence of fish can induce an increase in the filtration activity of Anodonta mussels (Jokela and Palokangas, Reference Jokela and Palokangas1993). Recently, the influence of temperature on parasite–predator interactions was discussed in the context of climate changes, and it was predicted that the temperature-driven filtration rates of bivalves will increase, leading to enhanced removal of free-living parasite stages and reduced parasite infectivity (Goedknegt et al. Reference Goedknegt, Welsh, Drent and Thieltges2015; Burge et al. Reference Burge, Closek, Friedman, Groner, Jenkins, Shore-Maggio and Welsh2016).
In addition to the negative effect on parasite transmission, filtration of cercariae by bivalves could cause other important ecosystem effects. Parasites contribute significantly to aquatic food webs (Kuris et al. Reference Kuris, Hechinger, Shaw, Whitney, Aguirre-Macedo, Boch, Dobson, Dunham, Fredensborg, Huspeni, Lorda, Mababa, Mancini, Mora, Pickering, Talhouk, Torchin and Lafferty2008; Lafferty et al. Reference Lafferty, Allesina, Arim, Briggs, De Leo, Dobson, Dunne, Johnson, Kuris, Marcogliese, Martinez, Memmott, Marquet, McLaughlin, Mordecai, Pascual, Poulin and Thieltges2008), including the suggested important role of trematode cercariae in energy transfer (Morley, Reference Morley2012). Thus, if cercariae are consumed by bivalves, trematodes could be involved in trophic pathways from bivalves to top consumers like fish and birds. However, it is not yet clear whether cercariae are damaged by filtration or/and ingested by mussels, because the gut contents of mussels was not analysed in our study or in previous studies (Thieltges et al. Reference Thieltges, Jensen and Poulin2008a ; Goedknegt et al. Reference Goedknegt, Welsh, Drent and Thieltges2015). These data are necessary to clarify the role of trematode cercariae in benthic food webs, which has earlier been assumed to be very important (Thieltges et al. Reference Thieltges, de Montaudouin, Fredensborg, Jensen, Koprivnikar and Poulin2008b ). Moreover, fish could even be attracted by bivalves in the presence of cercariae, if they seek shelter against parasites. However, further studies are needed to test if bivalves could influence fish behaviour in such a way.
It should be noted that freshwater mussels do not only act as a sink for trematode cercariae (as shown in the present study) but also as a source of cercariae. The duck mussel, A. anatina, itself serves as the first intermediate host for bucephalid trematodes Rhipdocotyle fennica and Rhipdocotyle campanula, cercariae of which infect cyprinid fish, such as roach (Rutilus rutilus) (Taskinen et al. Reference Taskinen, Valtonen and Gibson1991; Gibson et al. Reference Gibson, Taskinen and Valtonen1992). Prevalences of Rhipidocotyle infection in A. anatina can be up to 30% and numbers of tissue-dwelling Rhipidocotyle metacercariae in roach up to 900 individuals fish−1 (Taskinen et al. Reference Taskinen, Valtonen and Gibson1991). Thus, presence of A. anatina can greatly influence trematode parasitism in the fish community, for example, by decreasing Diplostomatidae eye flukes in salmonids and increasing Bucephalidae parasitism in cyprinids. Some bivalve taxa (e.g. Dreissena and Corbicula) serve as intermediate hosts for echinostomatid trematodes (Fried et al. Reference Fried, Emili and Ettinger1987; Conn and Conn, Reference Conn and Conn1995), which are important as both animal and human disease agents. Thus, these bivalves may affect the population dynamics of echinostomatids, either enhancing transmission to molluscivore definitive hosts, or reducing transmission to definitive hosts that feed on fish or amphibians.
The effect of mussels on the abundance of trematode larvae is species-specific and may affect different freshwater organisms. Based on our findings, future research could aim: (i) to clarify whether cercariae are ingested (or simply damaged) by mussels and what impact such interactions have on aquatic food webs; (ii) to study factors (biotic, abiotic) limiting the filtering of cercariae by mussels; (iii) to conduct mesocosm experiments to test the use of mussels for preventing trematodoses in semi-natural systems; and (iv) to study indirect effects of mussels on fitness and behaviour of aquatic organisms through diverse effects on their parasites (elimination and/or production of cercariae). Knowledge of interactions between parasites, their hosts and predators may help to develop methods for the control of trematode transmission and management of public health and aquaculture.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017001421.
ACKNOWLEDGEMENTS
We thank the technical staff of the Konnevesi research station (University of Jyväskylä, Finland) for their assistance with fish maintenance. We are also extremely grateful to Professor Roger I. Jones for the language check and comments. Two anonymous reviewers are acknowledged for critical comments that significantly improved the manuscript.
FINANCIAL SUPPORT
This research was supported by the Academy of Finland (J. T., mobility grant number 298911); the Ella and Georg Ehrnrooth Foundation (E. M., mobility grant); the Russian Foundation for Basic Reasearch (V. M., grant number 17-04-00247) and the Russian Science Foundation (grant number 14-14-01171).