Introduction
Air conditioning systems can harbour bacteria, fungi, viruses and protozoa, such as Acanthamoeba spp. (Ross et al., Reference Ross, Menezes, Svidzinski, Albino and Andrade2004; Ooi et al., Reference Ooi, Mak, Chen and Ambu2017). These microorganisms may remain in these locations for a long time and be dispersed in the environment through air currents (Silva et al., Reference Silva, Nazaré, Muniz and Câmara2013). The exchange of air in indoor environments does not always occur in a satisfactory way which can favour the development of microorganisms, which can eventually affect humans causing infections (Graudenz and Dantas, Reference Graudenz and Dantas2007). Indoor air quality control plays an important role in preventing infections at these sites, particularly important in hospital settings, since immunocompromised individuals are more susceptible to infections (Alves et al., Reference Alves, Moraes, Nitz, Oliveira, Hecht, Gurguel-Gonçalves and Cuba2012; Santana and Fortuna, Reference Santana and Fortuna2012).
The critical care areas of hospitals are those that show a greater probability of transmitting hospital infection, either through invasive procedures or the presence of immunocompromised patients, such as in surgical centres (SCs), intensive care unit (ICU) haemodialysis rooms, chemotherapy, transplantation, among others. The transmission occurs through direct contact with the hospital staff, from one patient to another through fomites (objects such as gloves, tools and utensils) and the hospital ventilation system (Afonso et al., Reference Afonso, Tripple, Souza, Prado and Anders2004; Leung and Chan, Reference Leung and Chan2006; Silva et al., Reference Silva, Nazaré, Muniz and Câmara2013).
Acanthamoeba spp. are among the most common protozoa in nature and identified as agents of granulomatous amoebic encephalitis (GAE), cutaneous lesions, pulmonary and kidney infections, primarily in immunocompromised patient and Acanthamoeba keratitis in immunocompetent individuals (Trabelsi et al., Reference Trabelsi, Dendana, Sellami, Sellami, Cheikhrouhou, Neji, Makni and Ayadi2012). Furthermore, Acanthamoeba spp. have been described as vehicles of pathogenic microorganisms including Legionella pneumophila, Mycobacterium spp. and Pseudomonas spp. (Marciano-Cabral et al., Reference Marciano-Cabral, Jamerson and Kaneshiro2010; Maschio et al., Reference Maschio, Corção and Rott2015; Balczun and Scheid, Reference Balczun and Scheid2017).
Species of Acanthamoeba have two stages: trophozoite, metabolically active form and cyst, stage of dormancy. Identification of Acanthamoeba at the genus level is relatively easy due to the presence of characteristics such as acanthopodia in trophozoites and double wall of cysts (Visvesvara, Reference Visvesvara, Tanowitz and Brutto2013). Pussard and Pons (Reference Pussard and Pons1977) divided the genus into three groups according to the size and shape of cysts; however, this classification is uncertain because the morphology of the cysts can modify according to the culture conditions. The most accepted methodology for classifying Acanthamoeba spp. based on the smaller subunit sequences of the 18S rDNA gene, so that the genus can be divided into genotypes, which would correspond to species. Each genotype exhibits 5% or more of divergent sequences between different genotypes (Schroeder et al., Reference Schroeder, Booton, Hay, Niszl, Seal, Markus, Fuerst and Byers2001; Trabelsi et al., Reference Trabelsi, Dendana, Sellami, Sellami, Cheikhrouhou, Neji, Makni and Ayadi2012). Currently, Acanthamoeba spp. differentiate into 21 genotypes (T1–T21) (Corsaro et al., Reference Corsaro, Walochnik, Köhsler and Rott2015; Reference Corsaro, Köhsler, Di Filippo, Venditti, Monno, Di Cave, Berilli and Walochnik2017). Several studies use tolerance assays to predict the pathogenic potential of Acanthamoeba environmental isolates (Khan et al., Reference Khan, Jarroll and Paget2002; Al-Herrawy et al., Reference Al-Herrawy, Bahgat, Mohammed, Ashour and Hikal2013).
Due to the opportunistic nature of Acanthamoeba spp. and its possible role as a reservoir of pathogens of importance in health services infections, the monitoring of this protozoan in hospital environments becomes important and could be used as a quality biomarker in hospitals for the improvement of air quality in hospital settings, because in these places people are more debilitated and susceptible to infections and cysts of Acanthamoeba spp. are resistant to several disinfection systems, remaining in the environment for years, becoming a source of dissemination of pathogens (Ooi et al., Reference Ooi, Mak, Chen and Ambu2017). In this sense, the present study investigated the occurrence of FLA in air-conditioners of a public hospital in the city of Florianópolis, SC, Brazil, with a particular focus on isolation and genotyping of Acanthamoeba isolates.
Materials and methods
Samples
Fifty-four dust samples were collected from filters, flaps and diffuser of air conditioners of fifteen environments of a public hospital in the city of Florianópolis, SC, Brazil, between March 2014 and March 2015. The collection environments were: chemotherapy unit (CU), emergency (EM), gynecology (GN), haemodialysis unit (HU), ICU, medical clinic I (MCI), medical clinic II (MCII), obstetrical centre (OC), ophthalmology (OPT), outpatient surgical centre (OSC), paediatrics (PED), SC, surgical clinic I (SCI), surgical clinic II (SCII) and sterilization room (ST). Samples were collected using sterile swabs, which were placed in contact with 10 mL of Page saline solution (2.5 mm NaCl, 1 mm KH2PO4, 0.5 mm Na2HPO4, 40 mm CaCl2 and 20 mm MgSO4) for 30 min to promote the detachment of amoebic forms, when presents. After, the samples were centrifuged at 500 × g for 5 min, the supernatant was discarded and the pellet was resuspended in 200 μL of Page saline solution.
Isolation of free-living amoebae
The suspension obtained from each pellet was inoculated in the centre of 1.5% non-nutrient agar (NNA) plates, overlaid with layers of Escherichia coli (ATCC 25922) previously heat-inactivated (for 2 h at 56°C). The plates were sealed with Parafilm® and incubated at 30°C for up to 25 days. Three plates were prepared for each dust sampled. Each plate was examined daily under optical microscopy (at 100×) to verify the presence of amoebic forms. When the presence of cysts or trophozoites was observed, it was performed subculture from the transference of a small piece of agar containing the amoebic forms to a new plate in order to isolate it from other microorganisms. Subsequently the isolates were axenised in PYG growth medium [0.75% (w/v) proteose peptone, 0.75% (w/v) yeast extract and 1.5% (w/v) glucose with antibiotics] and incubated at 30°C. When necessary, PYG medium supplemented with 10% foetal bovine serum was used to promote amoebic development.
Morphological studies
The cysts and trophozoites of the FLA isolated from dust of air conditioners were morphologically characterized (Pussard and Pons, Reference Pussard and Pons1977; Page, Reference Page1988).
The size of the amoebic forms was estimated using calibrated ocular micrometre. For each isolate, 10 cysts and 10 trophozoites were measured. The results were expressed as mean ± s.d..
Molecular identification of isolates
Extraction of total DNA from each isolate (containing 106 trophozoites/mL) was performed using the QIAamp® DNA mini kit (Qiagen, Hilden, Germany), according to the manufacturer's recommendations. The polymerase chain reaction (PCR) was performed with the genus-specific primers JDP1 and JDP2 according to Schroeder et al. (Reference Schroeder, Booton, Hay, Niszl, Seal, Markus, Fuerst and Byers2001). The positive control included DNA from the strain Acanthamoeba castellanii Neff (ATCC 30010) and the negative control as a substitute for DNA template included DNA free water. The amplicons were separated by electrophoresis on 1.5% agarose gel, stained with 1μg/mL ethidium bromide and observed under a UV-light transilluminator. The PCR products were purified using the PureLink® PCR Purification Kit (Invitrogen, Carlsbad USA) according to the manufacturer's instructions. The purified amplicons were sequenced in both senses using the amplification primers and BigDye® sequencing kit in an ABI 3730 automated sequencer (Applied Biosystems, EUA).
To determine the genotypes, sequencing data was aligned with Acanthamoeba genotype sequences available in the GenBank database based on the DF3 using Basic Local Alignment Search Tool (BLAST) program of the US National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST) to search for the most similar sequences. The sequences obtained in this study were deposited in the GenBank database under accession numbers MF076628 to MF076666. Sequence alignments were performed using CLUSTAL W for pairwise alignments and phylogenetic tree was constructed with MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018) using the neighbour joining method, with the bootstrap based on 1000 random replicates.
Tolerance (physiological) assays
Thermotolerance and osmotolerance assays were performed as previously described (Caumo et al., Reference Caumo, Frasson, Pens, Panatieri, Frazzon and Rott2009). Briefly, for the osmotolerance assay 103 trophozoites were inoculated onto 1.5% NNA plates containing mannitol 1.0 M, each with overlaid with layers of heat-killed E. coli (ATCC 25922). NNA plates under the same conditions without the addition of mannitol were used as a control. All plates were incubated at 30°C for 10 days, after this incubation period, growth was evaluated in optical microscopy (at 100×). For this, the number of cysts or trophozoites visualized in about 20 mm from the inoculum site (previously demarcated) of the each plate, in five microscope fields were counted and classified, with counts of zero (−), 1–15 (+), 16–30 (++), >30 (+++).
For the thermotolerance assay, 103 trophozoites were inoculated onto 1.5% NNA plates, each with overlaid with layers of heat-killed E. coli. Plates were incubated at 30 and 40°C for 10 days. The plates, submitted to 30°C, were used as control in growth assessment. The growth evaluation after the incubation period was done as in the osmotolerance assay. All assays were carried out in triplicates.
The isolates were classified into three groups according to their growth in the tolerance tests. Isolates that were able to develop in the hyperosmolar medium and the temperature of 40°C were classified as potentially pathogenic. Isolates that developed in one of the tests were classified as being of low pathogenic potential and when it was not able to develop against none of the adverse conditions were classified as probably non-pathogenic.
Results
Of the 54 dust samples obtained from air conditioners of hospital environments, 42 (77.8%) were positive for FLA. Of these three (7.2%) were not able to develop in axenic medium. Therefore, 39 (92.9%) axenic isolates of FLA were obtained for morphological and genotypic studies. All the amoeba isolates in this study were identified morphologically (Table 1) as belonging to the genus Acanthamoeba. The trophozoites presented acanthopodia, and a nucleus with well-defined central nucleolus (Fig. 1a). Thirty-seven isolates presented characteristics compatible with group II (Fig. 1b), and two isolates to morphological group III. No isolate presented group I characteristics. All the measurements of cysts and trophozoites presented size expected for genus according to Pussard and Pons (Reference Pussard and Pons1977) and Page (Reference Page1988).
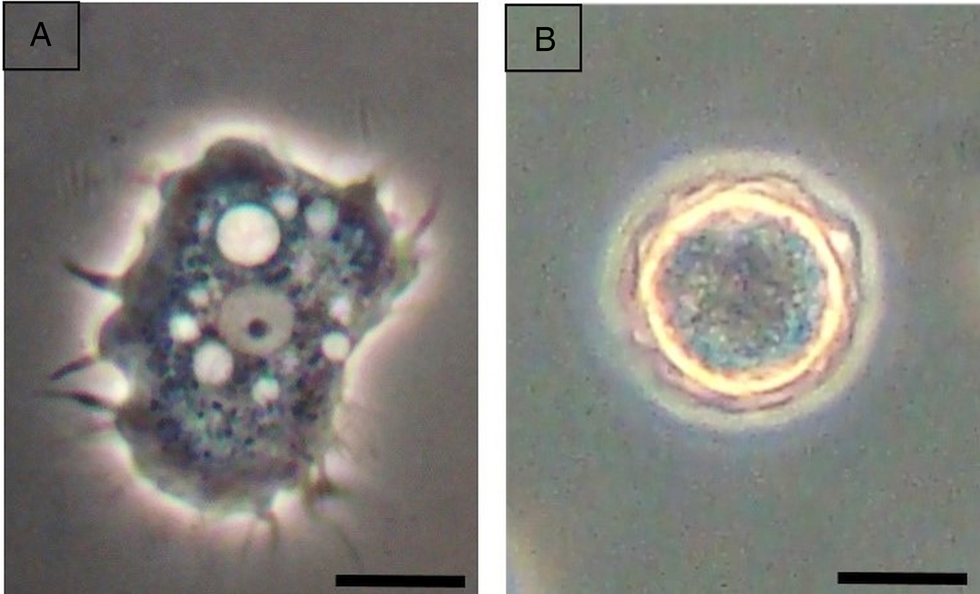
Fig. 1. Trophozoite of Acanthamoeba spp. presenting acanthopodia, and a nucleus with well-defined central nucleolus (a) and cyst compatible with group II (b). The bars represent 10 μ m.
Table 1. Morphological identification of Acanthamoeba isolates obtained from dust from air conditioners of a public hospital in Florianópolis

Central filter (CF); diffuser (D); filter (F); flaps (Fp).
The PCR using genus-specific primers (JDP1 and JDP2) confirmed that the 39 isolates from the study belonged to the genus Acanthamoeba. The expected amplification product (ASA.S1 18S rDNA) of ~500 bp was observed (Fig. 2). Sequencing of PCR products revealed that 19 (48.7%) isolates belonged to the genotype T4, 16 (41.0%) to the T5 genotype and 4 (10.3%) to genotype T11 (Table 2) when compared to the reference sequences deposited at GenBank. The percentage of identity between the sequences of this study and those used as reference ranged from 97 to 100%.

Fig. 2. Neighbour-joining 18S rDNA tree of genotype Acanthamoeba spp. (MEGA X program). Test isolates including reference strains representing T1–T20 genotypes. Numbers at the nodes are percentage-bootstrapping values on 1000 replicates. Balamuthia mandrillaris was used as the outgroup. Bar 0.02 substitutions per nucleotide position.
Table 2. Genotypic identification of Acanthamoeba isolates obtained from dust from air conditioners of a public hospital in Florianópolis

Central filter (CF); diffuser (D); filter (F); flaps (Fp).
The sequences from Acanthamoeba spp. isolates were used to construct the phylogenetic tree to illustrate the relationships between the isolates obtained and reference sequences of Acanthamoeba genotypes T1–T20 retrieved from GenBank. The relationships among these isolates were examined by using the neighbour-joining method as showed in Fig. 2. The tree showed that 19 isolates are strictly related with Acanthamoeba T4 genotype chosen as references with 98% of identity, 16 isolates T5 genotype with 100% of identity with the T5 sequence references. Four of the 39 isolates analysed showed a strict correspondence with the deposited sequences for the genotype T11, with 100% of identity. The association of obtained isolates in this study with individual genotypes was supported by significant bootstrap values (Fig. 2).
Of the 39 isolates of Acanthamoeba submitted to the osmo and thermotolerance assays, seven (18.0%) isolates were considered potentially pathogenic, because it had concomitant growth in hyperosmolar medium and at elevated temperature of 40°C. Isolates that developed only at elevated temperature 25 (64.1%) and only in hyperosmolar 2 (5.2%) were classified as low pathogenic potential. Among the isolates, 5 (12.8%) presented no growth at 1.0 M mannitol and at 40°C and were considered probably non-pathogenic isolates (Table 3).
Table 3. In vitro growth of the Acanthamoeba isolates in the osmotolerance and thermotolerance assays

Central filter (CF); diffuser (D); filter (F); flaps (Fp).
*Scores: without growth (–); 1–15 cysts and/or trophozoites (+); 16–30 (++) cysts and/or trophozoites and >30 cysts and/or trophozoites (+++). The assays were performed in triplicate; for each replicate, cysts and/or trophozoites were counted in five microscope fields (at 100×).
Discussion
Studies of FLA isolation in hospital environments are scarce, despite the importance of these microorganisms as potential causers of opportunistic infections and as vehicles and reservoirs of pathogens. Some reports of isolation of these amoebas in hospital environments have been described from water systems (Trabelsi et al., Reference Trabelsi, Dendana, Neji, Sellami, Cheikhrouhou, Makni and Ayadi2016; Muchesa et al., Reference Muchesa, Leifels, Jurzik, Hoorzook, Barnard and Bartie2017), dust and biofilm (Silva and Rosa, Reference Silva and Rosa2003; Carlesso et al., Reference Carlesso, Artuso, Caumo and Rott2010; Costa et al., Reference Costa, Castro, Ferreira, Furst, Crozeta and Thomaz-Soccol2010). Reports of FLA isolation from air conditioners have been described in some countries such as Chile (Astorga et al., Reference Astorga, Lorenzo-Morales, Martín-Navarro, Alarcón, Moreno, González, Navarrete, Piñero and Valladares2011) and Malaysia (Chan et al., Reference Chan, Mak, Low, Koh, Ithoib and Mohamed2011), however, in hospital environments the presence of these amoebae in air conditioners is still poorly investigated. In the present study, a high culture rate of FLA was observed, being higher than 70%, indicating the high prevalence of these amoebae in air conditioning units in the investigated hospital.
All isolates were characterized as belonging to the genus Acanthamoeba. The morphological identification of the isolates of the present study showed the presence of double-walled cysts with characteristics compatible with group II and III. The morphological group II harbours Acanthamoeba species commonly isolated from environmental and clinical samples, described as responsible for most cases infection in humans, such as amoebic keratitis and GAE (Walochnik et al., Reference Walochnik, Obwaller and Aspöck2000).
Currently molecular methods for the detection of Acanthamoeba spp. are being increasingly used due to the high sensitivity and specificity of these methods (Visvesvara et al., Reference Visvesvara, Moura and Schuster2007). The PCR using primers that amplify a conserved region of the 18S rDNA gene is the most used, since the sequencing of the fragment obtained in the PCR allows the determination of the genotype (Fuerst et al., Reference Fuerst, Booton and Crary2015). The three genotypes of Acanthamoeba spp. identified in this study (T4, T5 and T11) have a wide environmental distribution, being reported the isolation of these from samples from water (Sente et al., Reference Sente, Erume, Naigaga, Magambo, Ochwo, Mulindwa, Namara, Kato, Sebyatika, Muwonge and Ocaido2016), soil (Todd et al., Reference Todd, Reyes-Batlle, Martín-Navarro, Dorta-Gorrín, Lopez-Arencibia, Martínez-Carretero, Piñero, Valladares, Lindo and Lorenzo-Morales2015) and dust (Niyyati et al., Reference Niyyati, Lorenzo-Morales, Rahimic, Motevalli-Haghia, Martín-Navarro, Farnia, Valladares and Rezaeian2009). The prevalence of the T4 genotype in environmental samples, reported in other studies (Geisen et al., Reference Geisen, Fiore-Donno, Walochnik and Bonkowski2014). Rahdar et al. (Reference Rahdar, Niyyati, Salehi, Feghhi, Makvandi, Pourmehdi and Farnia2012) verified the predominance of this genotype in isolates obtained from soil and water from a province of Iran. Similarly, Geisen et al. (Reference Geisen, Fiore-Donno, Walochnik and Bonkowski2014) reported the predominance of the T4 genotype in Acanthamoeba isolates from soil samples from three distinct locations, the Netherlands, Sardinia and Tibet. This is the genotype most associated with cases of keratitis and amoebic encephalitis, as well as other opportunistic infections caused by this protozoan (Siddiqui and Khan, Reference Siddiqui and Khan2012).
The T5 genotype was the second most found. In the study by Booton et al. (Reference Booton, Visvesvara, Byers, Kelly and Fuerst2005), which included 200 isolates of Acanthamoeba, this genotype was identified as the second most prevalent among environmental isolates, as well as second in the study by Ledee et al. (Reference Ledee, Iovieno, Miller, Mandal, Diaz, Fell, Fine and Alfonso2009) that included isolates of amoebic keratitis. The T5 genotype is associated with cases of amoebic keratitis and encephalitis (Siddiqui and Khan, Reference Siddiqui and Khan2012).
Some studies relate the T11 genotype to cases of amoebic keratitis (Hajialilo et al., Reference Hajialilo, Behnia, Tarighi, Niyyati and Rezaeian2016; Jercic et al., Reference Jercic, Aguayo, Saldarriaga-Córdoba, Muiño, Chenet, Lagos, Osuna and Fernández2019). This was one of the genotypes described as causing this infection in a large research carried out in Austria that included cases of Acanthamoeba infections in the last 20 years (Walochnik et al., Reference Walochnik, Scheikl and Haller-Schober2015).
Studies that evaluated the presence of Acanthamoeba in dust and soil samples showed similar results to the present study, reporting the presence of T4, T5 and T11 genotypes, with T4 genotype predominating (Niyyati et al., Reference Niyyati, Lorenzo-Morales, Rahimic, Motevalli-Haghia, Martín-Navarro, Farnia, Valladares and Rezaeian2009; Todd et al., Reference Todd, Reyes-Batlle, Martín-Navarro, Dorta-Gorrín, Lopez-Arencibia, Martínez-Carretero, Piñero, Valladares, Lindo and Lorenzo-Morales2015). Acanthamoeba isolation studies from air conditioners have been performed in some countries such as Chile (Astorga et al., Reference Astorga, Lorenzo-Morales, Martín-Navarro, Alarcón, Moreno, González, Navarrete, Piñero and Valladares2011) and Malaysia (Chan et al., Reference Chan, Mak, Low, Koh, Ithoib and Mohamed2011), showing the presence of T3, T4, T5 and T11.
There are few reports of isolation of Acanthamoeba in hospital settings, despite the importance of these microorganisms as causing opportunistic infections, as well as vehicles and pathogen dispersers (Kocazeybek, Reference Kocazeybek2015). Carlesso et al. (Reference Carlesso, Artuso, Caumo and Rott2010) described the presence of T4 genotype Acanthamoeba in dust samples and T5 genotype in biofilm samples, both from a hospital environment in Porto Alegre, Rio Grande do Sul, Brazil. An investigation conducted in Austria for the presence of AVL and bacteria in refrigeration systems following a legionellosis outbreak in and around a hospital reported the presence of nine Acanthamoeba isolates belonging to the T4 genotype, which had amoeba resistant bacteria in its interior, emphasizing the importance of these amoebas as bacterial vehicles (Scheikl et al., Reference Scheikl, Tsao and Horn2016).
All genotypes identified in our study are associated with cases of human infections (Siddiqui and Khan, Reference Siddiqui and Khan2012; Jercic et al., Reference Jercic, Aguayo, Saldarriaga-Córdoba, Muiño, Chenet, Lagos, Osuna and Fernández2019). These results deserve special attention from the hospital community, considering the isolation environment, the characteristic of many patients in a hospital environment, as they may be immunologically susceptible to infections, as well as the opportunistic nature of Acanthamoeba spp. However, further sequencing is required to obtain a better understanding of the spread of amoebae throughout the studied hospital.
Several authors report that osmo and thermotolerance assays can determine the pathogenicity of an Acanthamoeba isolate, since isolates capable of adapting physiologically and resisting to adverse conditions, such as growth in hyperosmolar medium and at elevated temperatures, are more adapted and can cause infections in the man and animals (Khan et al., Reference Khan, Jarroll and Paget2002).
In this study, pathogenic isolates and with low pathogenic potential were obtained from hospital settings, such as EM room, OSC, SC and surgical clinic, which are environments where patients with severe health conditions and those susceptible to opportunistic infections are found. Although most of the isolates were not classified as pathogenic, they still have significant epidemiological importance, since they can serve as vehicles and reservoirs of pathogenic microorganisms in health service settings.
Financial support
This study was supported by grants from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflict of interest
None.
Ethical standards
Not applicable.








