INTRODUCTION
Palaeozoic stromatoporoids are a group of calcareous sponges considered to be either monophyletic (Riding and Kershaw, Reference Riding and Kershaw1977; Stearn et al. Reference Stearn, Webby, Nestor and Stock1999) or polyphyletic (Pisera, Reference Pisera2006, and references therein). Like modern sponges (Wulff, Reference Wulff2006), stromatoporoids could host numerous symbiotic organisms (Kershaw, Reference Kershaw1980; Tapanila, Reference Tapanila2006). Among the stromatoporoid symbionts were organisms of unknown biological affinities, forming tube-like structures that each have a single aperture (e.g. Stel, Reference Stel1976; Cook, Reference Cook1999; Tapanila, Reference Tapanila2005, Reference Tapanila2006). These structures are located inside stromatoporoids’ calcareous basal skeletons (referred to as coenostea), and have various morphological features. They are straight or helicoidal, some possessing their own calcareous walls; internal structures such as tabulae or diaphragms may also occur (Plusquellec, Reference Plusquellec1968; Tapanila, Reference Tapanila2005). Intergrowths between stromatoporoid skeletons and tubes demonstrate in vivo relationships (Plusquellec, Reference Plusquellec1968, p. 168; Tapanila, Reference Tapanila, Kelley and Bambach2008) and the reliability of such evidence is absolute (Taylor and Wilson, Reference Taylor and Wilson2003, p. 24). Relationships between stromatoporoids and these endobionts were most often considered as commensal (Tapanila, Reference Tapanila2005), as the symbionts were not observed to influence growth rates or anatomy.
The present paper documents that helicoidal tubes assigned to Torquaysalpinx sp., found in the skeletons of the Middle Devonian (Givetian; 392–385 Myr) stromatoporoids from the Mont d'Haurs section near Givet (Ardennes, France), were parasitic. This is the first known evidence of parasitism in Palaeozoic sponges.
MATERIALS AND METHODS
Study site
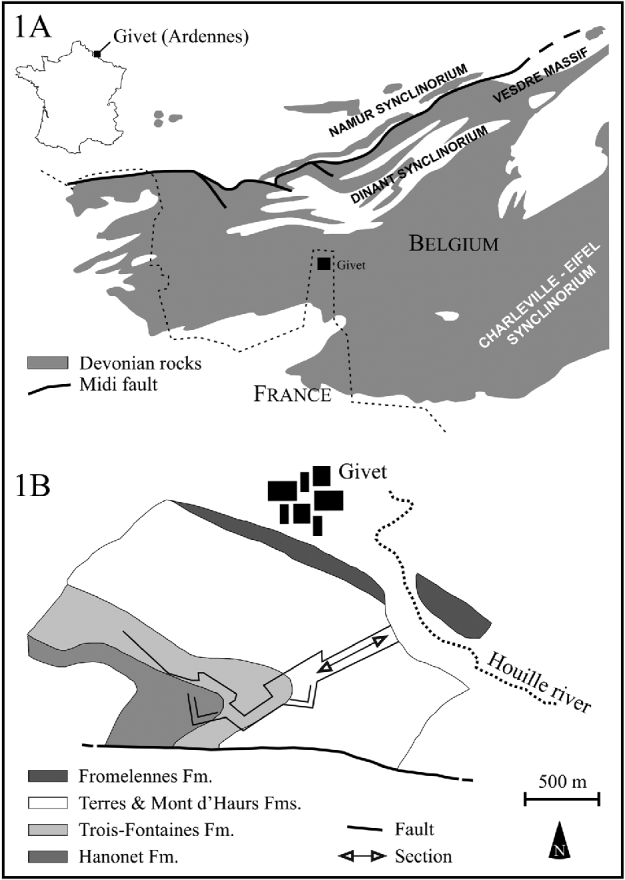
The analysed material comes from outcrops around the fortifications of Mont d'Haurs in Givet (Champagne-Ardenne, France; Fig. 1). This classic Middle Devonian (Givetian) section exposes rocks from argillaceous limestones of the Hanonet Formation to massive and biostromal limestones of the Mont d'Haurs Formation. Samples included in this study come from the upper part of the section (Mont d'Haurs Fm.), which consists of massive coral limestones, interbedded with siltstones. The microfacies are principally crinoidal mudstones and stromatoporoid-tabulate boundstones (Hubert, Reference Hubert2008, and references therein).

Fig. 1. Location of the Givet area on the map of France and the main geological structures of the Ardennes (A) and detailed location of the studied section in the Fortifications of Mont d'Haurs (B).
Preparation and repository of samples
A collection of 260 coenostea was investigated. The coenostea were analysed in thin sections, a standard method of analysis for bio-constructing organisms. Each thin section was prepared by cutting, grinding and polishing a rock sample. The sample was glued to a glass slide using epoxy adhesive. The portion glued to the glass was cut and polished, until light could pass through the rock slice. Thin sections were observed under a binocular microscope in transmitted light. In total, 29 specimens (37 thin sections) contained fossils of Torquaysalpinx sp. The analysed material is housed at the Faculté Libre des Sciences et Technologies in Lille (France), figured specimens have repository numbers GFCL 641 to 644.
The biological affinities of Torquaysalpinx
Plusquellec (Reference Plusquellec1968) created the genus Torquaysalpinx based on Devonian examples that infested a chaetetid from Torquay (UK). He stated that the microstructure of Torquaysalpinx is lamellar, with lamellae obliquely oriented to the tube surface; he concluded that it is different from serpulids. On the other hand he remarked that some serpulids (e.g. Ditrupa) show similar diaphragms closing the tube lumen. Plusquellec (Reference Plusquellec1968) concluded that these Palaeozoic endosymbionts are difficult to assign to any modern group, and suggested their classification as organisms incertae sedis.
Tapanila (Reference Tapanila2005) published an overview of fossil coral and sponge endosymbionts, in which he referred Torquaysalpinx to ichnofossils (traces of life activity). Torquaysalpinx possess their own calcareous walls and diaphragms closing their lumen. However, the presence of skeletal structures places it among body fossils, contrary to the opinion of Tapanila.
Plusquellec (Reference Plusquellec1968) stated that the microstructures of Torquaysalpinx are different from those of serpulids. Recent studies, however, show that serpulids can have extremely diversified wall structures, with 1–4 layers and various microstructures (Vinn et al. Reference Vinn, Ten Hove, Mutvei and Kirsimäe2008). The unilamellar structure of Torquaysalpinx, with lamellae obliquely oriented to the tube surface, could thus fit among the serpulids. It is necessary to point out that modern-type serpulids appeared in the Late Triassic (Vinn and Mutvei, Reference Vinn and Mutvei2009); between the youngest Torquaysalpinx and known serpulids there is a gap of at least 150 Ma. In any case, diagenetic processes can strongly change original microstructures (see discussions by Oekentorp, Reference Oekentorp2001, Reference Oekentorp2007; Zapalski, Reference Zapalski2010). Microstructural criteria must be therefore treated with caution.
The internal septa and microstructure of Torquaysalpinx resemble that of trypanoporids (O. Vinn, personal communication). Trypanoporids have recently been considered as relatives of tentaculitids (Weedon, Reference Weedon1991), which form the separate class Tentaculita (probably allied to lophophorans; Vinn and Isakar, Reference Vinn and Isakar2007).
Modern Spirobranchus polychaetes are usually associated with corals. These polychaetes are, however, an order of magnitude larger in diameter (attaining nearly 10 mm) and length (attaining nearly 100 mm; Nishi and Nishihira, Reference Nishi and Nishihira1996). Moreover, modern polychaetes do not show helicoidal coiling, in contrast with Torquaysalpinx.
The absence of Torquaysalpinx specimens with diagenetically unaltered microstructure makes any comparisons very speculative. To conclude, it must be stated that biological affinities of Torquaysalpinx are unclear and following Plusquellec (Reference Plusquellec1968) this genus must be classified as incertae sedis.
RESULTS
Hosting organisms
The stromatoporoids hosting Torquaysalpinx organisms belong to 8 genera and 13 species. Most of them are well known in the southern Ardennes and form an important element of the regional biota (Lecompte, Reference Lecompte1951, Reference Lecompte1952; Hubert et al. Reference Hubert, Zapalski, Nicollin, Mistiaen and Brice2007; Zapalski et al. Reference Zapalski, Hubert, Nicollin, Mistiaen and Brice2007a). These include: Atelodictyon sp., Actinostroma clathratum, A. crassepilatum, A. sertiforme, A. tabulatum crassum, A. verrucosum, Densastroma? sp., Stromatoporella granulata, Stromatoporella? sp., Clathrocoilona spissa, Trupetostroma? sp., Stromatopora huepschii and Atopostroma sp.
Most of these organisms had massive or low domical skeletons, with the exception of Clathrocoilona, which formed lamellar coenostea. These skeletons are composed of horizontal elements (laminae sensu lato) and vertical (pillars sensu lato); more details concerning the morphology of coenostea have been given by Stearn et al. (Reference Stearn, Webby, Nestor and Stock1999).
Infesting organisms
Torquaysalpinx endosymbionts (Figs 2 and 3) are helicoidally curved tubes of various lengths. The tubes possess their own calcareous walls, as well as small, often bent calcareous diaphragms closing the lumen (Fig. 2B, C); these features are diagnostic for the genus (Hill, Reference Hill, Moore and Teichert1981). Tube diameter increases slightly towards the top of each coenosteum (the mean diameter is 0·332 mm, ranging from 0·22 to 0·58 mm). The longest observed tubes attained 10 mm; the smallest diameter of coiling is about 0·22 mm and the largest are 1·30 mm.

Fig. 2. Stromatoporoids of the genus Actinostroma and parasites Torquaysalpinx sp. from the Givetian of Mont d'Haurs section (Champagne-Ardenne, France). (A) Actinostroma crassepilatum (specimen GFCL 641), longitudinal section. (B) Detail of Actinostroma sertiforme (specimen GFCL 642), arrow shows diaphragm in the Torquaysalpinx sp., longitudinal section. (C) Actinostroma crassepilatum (specimen GFCL 643), tangential section. A, C – Scale bars=1 mm. B – Scale bar=500 μm.
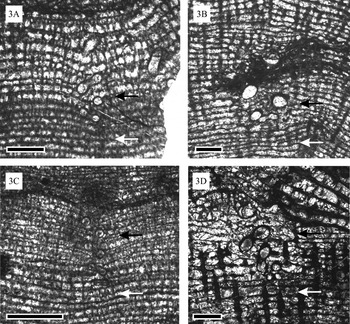
Fig. 3. Stromatoporoids of the genus Actinostroma and parasites Torquaysalpinx sp. from the Givetian of Mont d'Haurs section (Champagne-Ardenne, France). Changes in the skeletal organization of the host. (A) Actinostroma verrucosum (specimen GFCL 644), longitudinal section. Note that laminae around the host are bent down (black arrow and above), while below the parasite the skeletal organization is normal (white arrow and below). (B) Actinostroma crassepilatum (specimen GFCL 641), longitudinal section. The white arrow shows a region with normal skeletal organization, the black arrow with modified skeleton. (C) Actinostroma verrucosum (specimen GFCL 644), longitudinal section. The white arrow shows a region with normal skeletal organization, the black arrow shows a modified skeleton where laminae are bent down. (D) Actinostroma crassepilatum (specimen GFCL 643), longitudinal section. The white arrow shows a region with normal skeletal organization, the black arrow shows a modified skeleton. Note that around the parasite (black arrow) the skeletal elements are much thinner than below (white arrow). Scale bars=1 mm.
Torquaysalpinx endosymbionts were very rare in the examined material; out of the collection of 260 samples of stromatoporoids, only 29 skeletons contained these symbionts. Besides Torquaysalpinx sp. another endosymbiont, Streptindytes sp. (see Tapanila, Reference Tapanila2005) also occurred in several samples.
The positions of endosymbionts in the host skeleton
The endosymbionts are rare in coenostea, with up to 7 (usually 1–3) found in each. They do not exhibit any particular preference for placement, and are not concentrated in either peripheries or centres of coenostea. In contrast, tabulate corals occurring in the same beds contain much more abundant Torquaysalpinx, with dozens in a single corallum.
Torquaysalpinx tubes are oriented perpendicular to the laminae of the stromatoporoid. The laminae around each tube are bent slightly downward (Figs 2A and 3A–D), and pillars are often less abundant in these areas (Fig. 3D); these changes are evidence of skeletal modification of the host.
The interaction between Torquaysalpinx and its hosts
Endosymbionts are common in Palaeozoic bio-constructing colonial organisms – stromatoporoids and corals (Tapanila, Reference Tapanila2005). They are far more commonly found in corals, while in stromatoporoids they are infrequent (Tapanila, Reference Tapanila2005, Reference Tapanila2006) and for this reason analyses of coral endosymbionts have been more frequently published (e.g. Oekentorp, Reference Oekentorp1969; Stel, Reference Stel1976; Zapalski et al. Reference Zapalski, Pinte and Mistiaen2008; Reference Zapalski and KönigshofZapalski, 2009). Since the work of Sokolov (Reference Sokolov1948), the relationship between coral hosts and endosymbionts was considered as commensal (e.g. Plusquellec, Reference Plusquellec1968; Oekentorp, Reference Oekentorp1969; Tapanila, Reference Tapanila2005). An opposite opinion was expressed by Stel (Reference Stel1976); and Zapalski (Reference Zapalski2007) has shown that at least some of these organisms were parasitic in corals. The relationship between similar symbionts and stromatoporoids has remained vague until now.
The growth rates of bio-constructing organisms are reflected by changes in vertical spacing of elements; thus changes of spacing reflect changes in growth rate (Insalaco, Reference Insalaco1996; Young and Kershaw, Reference Young and Kershaw2005; Zapalski et al. Reference Zapalski, Hubert, Mistiaen, Álvaro, Aretz, Boulvain, Munnecke, Vachard and le Vennin2007b). It has been shown that endosymbionts may affect growth rate (Tapanila, Reference Tapanila2005, Fig. 1). This statement was, however, not supported by the material. Finding specimens with growth rhythm changed by the endosymbiont would solve the problem of the nature of this symbiosis (Zapalski, Reference Zapalski2007).
The fossil record is strongly biased in the terms of palaeoecological relationships. Parasites are rarely identified in the fossil record; this relationship may be identified on the basis of taxonomic position of the infesting organism and/or inferred from host modifications and symbiont placement (e.g. Bassett et al. Reference Bassett, Popov and Holmer2004; Poinar and Poinar, Reference Poinar and Poinar2004; Poinar and Boucot, Reference Poinar and Boucot2006). Tapanila (Reference Tapanila, Kelley and Bambach2008) proposed tests permitting the recognition of various kinds of symbioses in fossil records. He stated that parasitism could be recognized on the basis of reduction of the host's growth rate, as this demonstrates a negative influence on the host. Such a reduction of growth rate is clearly visible in some of our material: laminae around the endosymbionts are bent downwards, and pillars are less abundant (Fig. 3). Settling of the endosymbiont and decrease of growth rates around its spiral tube in successive growth stages is schematically shown in Fig. 4. The inhibition of growth observed in the Torquaysalpinx-stromatoporoid association resembles that in the modern demosponge Verongia gigantea infested by Haplosyllis polychaetes. In the latter association the reduction of host growth rates is significant (Reiswig, Reference Reiswig1973).
Stromatoporoids, like corals, show growth banding. In our material the seasonal growth bands are not clearly visible, but Young and Kershaw (Reference Young and Kershaw2005) have shown that annual growth band thickness may vary from 3 to 8 mm (but there is no unequivocal evidence that stromatoporoid bands are annual, see Young and Kershaw, Reference Young and Kershaw2005, p. 645). It can therefore be estimated that the duration of the Torquaysalpinx–stromatoporoid association was 1–2 years. Long-term relationship is a feature of parasitism (Combes, Reference Combes2001). Moreover, modification of normal growth bands by Torquaysalpinx can be treated as a phenotypic modification; this is another indicator of parasitism (Dawkins, Reference Dawkins1982; Combes, Reference Combes2001).
Modern Haplosyllis infesting Verongia can be considered as a model for the relationship between Torquaysalpinx and stromatoporoids. In the recent association the polychaete feeds directly upon the host by inserting its chitinized proboscis through canal walls and sucking up the soft sponge tissues (Reiswig, Reference Reiswig1973, p. 214). A similar situation might have occurred in the discussed case, although one must keep in mind that there is no direct evidence for a polychaete affinity for Torquaysalpinx.
Torquaysalpinx organisms occurring in stromatoporoids meet the criterion of ‘host as habitat’, typical for parasitism (Littlewood and Donovan, Reference Littlewood and Donovan2003). We can state that the infesting organism profited by gaining habitat and the food resource.
Some of the analysed specimens showed clear inhibition of growth of the sponge host around the parasite, but in other specimens this effect was not distinct. The interaction between host and individual may fluctuate during the lives of individuals (Cheney and Côté, Reference Cheney and Côté2005) or from individual to individual (a symbiont not harmful for one individual can become pathogenic for another; Casadevall and Pirofski, Reference Casadevall and Pirofski2000; Sachs and Wilcox, Reference Sachs and Wilcox2006) – such a situation is very common in modern associations (Drake, Reference Drake2008; Leung and Poulin, Reference Leung and Poulin2008).
A large variety of symbiotic organisms occurs in sponges, in spite of anti-fouling agents secreted by the latter (Krug, Reference Krug, Fusetani and Clare2006). Endosymbionts are rare in stromatoporoids – only about 10% of specimens are affected. The scarcity of these endosymbionts might indicate that such anti-fouling agents were already present during the Palaeozoic (this is, however, speculative). The fitting of ancient parasites to their hosts and vice versa might have caused the evolution of the relationship from parasitic to mutualistic.
CONCLUSIONS
Skeletal overgrowths of stromatoporoid sponges and their endosymbionts demonstrate in vivo relationships. The decrease of host growth rates indicates the negative influence of Torquaysalpinx sp. on its stromatoporoid hosts. Torquaysalpinx organisms were gaining habitat and possibly also food resources. This relationship was therefore negative for the host, and positive for the parasite. We can speculate that food transfer between the parasite and its host was similar to that in modern Verongia (demosponge) infested by Haplosyllis (polychaete). In this association the parasite feeds directly upon the tissues of the host. Moreover, the Torquaysalpinx–stromatoporoid association meets the criterion of ‘host as habitat’ typical for parasitism; it was also a long-term relationship. This is the first evidence of parasitism in Palaeozoic sponges.
Torquaysalpinx sp. shows numerous similarities to polychaetes, but is older than the oldest known serpulids by at least 150 Ma. Microstructural features place it close to tentaculitids (Vinn, personal communication), but the lack of specimens with diagenetically unaltered microstructures makes any comparisons deficient. Owing to a lack of evidence for biological affinities, these organisms are classified as incertae sedis.
ACKNOWLEDGEMENTS
Dr Graham Young (Manitoba) kindly devoted his time to correct the English of our manuscript, we are deeply indebted for his help. We are also very grateful to Dr Olev Vinn (Tartu) for discussing the problems of biological affinities of Torquaysalpinx. The remarks of two anonymous journal referees helped to significantly improve the manuscript. Warm thanks are due to Mrs Aleksandra Hołda-Michalska (Warsaw), who prepared the schematic drawings of infestation. Dr Adam T. Halamski (Warsaw) benevolently read the manuscript.