INTRODUCTION
A parasite species, even host-opportunistic, varies in its abundance and/or prevalence among different host species (Combes, Reference Combes2001). The variation in parasite abundance and prevalence among hosts has led to a classification of hosts by the pattern of their relationships with a parasite (e.g., Marshall, Reference Marshall1981). As a result, a host species could be defined as being a ‘true’, ‘primary’, ‘accidental’, ‘exceptional’, ‘preferred’, ‘normal’ or ‘secondary’ host for a particular parasite. However, despite attribution of a particular host into the category of ‘true’ or ‘primary’ host for a particular flea species, abundance or prevalence of this flea on this host often appeared to be low (e.g., Stanko et al. Reference Stanko, Miklisova, Goüy de Bellocq and Morand2002). Furthermore, in most cases, the slotting of a host to one of these categories was based solely on the personal impression of the researcher who studied these associations, so strict adherence to this classification system is usually not followed. Instead, a simpler classification of hosts is used in which a host with the highest abundance and/or prevalence of a parasite is considered to be its principal host, while other hosts exploited by the parasite are considered auxiliary hosts (Dogiel et al. Reference Dogiel, Petrushevski and Polyanski1961). By definition, abundance and/or prevalence of a parasite are lower in an auxiliary host species than in a principal host species (Marshall, Reference Marshall1981).
Differences in parasite abundance and distribution between the principal and the auxiliary host species are related to differential exploitative or reproductive performance of a parasite on these hosts (Krasnov et al. Reference Krasnov, Khokhlova, Oguzoglu and Burdelova2002a, Reference Krasnov, Sarfati, Arakelyan, Khokhlova, Burdelova and Degen2003). However, parasite abundance and/or prevalence often vary greatly among auxiliary hosts (Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova and Poulin2004; Poulin, Reference Poulin2005). One of the reasons suggested for the uneven distribution of a parasite among auxiliary hosts is the degree of similarity between the principal host and various auxiliary hosts (Poulin, Reference Poulin2005). In other words, parasites are expected to be more abundant and/or prevalent in auxiliary hosts that are more similar to the principal hosts. Phylogenetic relatedness among species is a good reflection of their overall life-history and physiological and ecological similarity (Brooks and McLennan, Reference Brooks and McLennan1991; Harvey and Pagel, Reference Harvey and Pagel1991). Consequently, parasite abundance and/or prevalence in an auxiliary host are expected to decrease with an increase in phylogenetic distance between the host and the principal host of a parasite. This hypothesis was tested using 2 different parasite-host associations, namely flea parasites of small mammals (Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova and Poulin2004) and metazoan parasites of teleost fish (Poulin, Reference Poulin2005). Strong support for the hypothesis was found in the former but not in the latter study (see Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova and Poulin2004 for the detailed explanation of this contradiction).
The results of Krasnov et al. (Reference Krasnov, Shenbrot, Khokhlova and Poulin2004) suggest that every time a flea adds a new host to its host spectrum, the phylogenetic affinity of this new host is important, that is, it is advantageous for a flea species to exploit species that are closely related to the principal host species. In other words, when a flea exploits an auxiliary host, its exploitation success would likely depend on phylogenetic proximity of this host with a principal host. This has never been tested experimentally, although assorted data on flea feeding patterns in different host species are presented across a number of sources (see Krasnov, Reference Krasnov2008).
In this study, we focused on exploitative performance of fleas and asked if and how the success of a flea in exploitation of an auxiliary host is affected by the phylogenetic distance between this host and the principal host. To answer this question, we investigated the feeding of 2 flea species (Parapulex chephrenis and Xenopsylla ramesis) on a principal and 8 auxiliary host species. We measured feeding performance via (a) the proportion of fleas that took a bloodmeal from a host during 2 days of continuous access to a host and (b) the size of a flea's bloodmeal taken during a timed period of feeding. We predicted that fleas will perform better, that is a higher proportion of fleas will feed and take larger bloodmeals, if fleas fed on (a) a principal host compared to an auxiliary host and (b) an auxiliary host phylogenetically close to a principal host compared to an auxiliary host phylogenetically distant from a principal host.
Both flea species in this study are common rodent parasites in the central Negev Desert. Parapulex chephrenis is a host specialist found mainly on the Egyptian spiny mouse Acomys cahirinus, while it was recorded on a congeneric golden spiny mouse Acomys russatus less often and on gerbils (Meriones crassus and Gerbillus dasyrus) only occasionally (Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Vashchenok and Khokhlova1997, Reference Krasnov, Hastriter, Medvedev, Shenbrot, Khokhlova and Vashchenok1999). In contrast, Xenopsylla ramesis is a host generalist and parasitizes a variety of rodents. The highest abundance of this flea has been recorded on the gerbils Psammomys obesus and Meriones crassus and highest prevalence on the latter host (Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Vashchenok and Khokhlova1997, Reference Krasnov, Hastriter, Medvedev, Shenbrot, Khokhlova and Vashchenok1999). Given the difference in the degree of host specificity, we predicted that the difference in flea feeding performance between the principal and the auxiliary hosts would be manifested stronger in host-specific P. chephrenis than in host-opportunistic X. ramesis, while the opposite would be true for difference in flea feeding performance among the auxiliary hosts.
MATERIALS AND METHODS
Fleas
We used fleas from our laboratory colonies started in 1999 from field-collected specimens either on M. crassus, P. obesus and G. dasyurus (X. ramesis) or A. cahirinus (P. chephrenis). Fleas were maintained on rodents kept individually in plastic cages with a wire mesh floor over a pan with a mixture of sand and dried bovine blood at 25°C air temperature with a photoperiod of 12:12 (L:D) h. Xenopsylla ramesis was maintained on M. crassus and G. dasyurus, while P. chephrenis was maintained on A. cahirinus. Every 2 weeks, all substrate and bedding material from the rodent's nest box and pan were collected into plastic boxes with perforated lids and transferred to an incubator (FOC225E, Velp Scientifica srl, Milano, Italy; 2°C air temperature and 75% relative humidity) where the fleas developed. Further details on breeding and maintenance of fleas can be found elsewhere (e.g., Krasnov et al. Reference Krasnov, Khokhlova, Oguzoglu and Burdelova2002a, Reference Krasnov, Sarfati, Arakelyan, Khokhlova, Burdelova and Degen2003; Khokhlova et al. Reference Khokhlova, Serobyan, Krasnov and Degen2009a, Reference Khokhlova, Serobyan, Krasnov and Degenb, Reference Khokhlova, Serobyan, Degen and Krasnov2010). All fleas used in experiments were selected randomly from colonies.
Rodents
We used 9 rodent species, namely 5 gerbils (M. crassus, G. dasyurus, Gerbillus andersoni, Gerbillus pyramidum, and Gerbillus nanus), 2 spiny mice (A. cahirinus and A. russatus), the house mouse (Mus musculus) and the golden hamster (Mesocricetus auratus). Meriones crassus, G. dasyurus, G. nanus and both spiny mice were from laboratory colonies started in 1997–1999 and 2009 (G. nanus) (see details in Krasnov et al. Reference Krasnov, Khokhlova, Oguzoglu and Burdelova2002a, Reference Krasnov, Sarfati, Arakelyan, Khokhlova, Burdelova and Degen2003; Khokhlova et al. Reference Khokhlova, Serobyan, Krasnov and Degen2009a, Reference Khokhlova, Serobyan, Krasnov and Degenb, Reference Khokhlova, Serobyan, Degen and Krasnov2010). Gerbillus andersoni and G. pyramidum as well as feral house mice were captured in the wild in the Negev Desert. All ectoparasites were removed using a toothbrush and the rodents were maintained in a separate room during 2 months prior to experiments. Golden hamsters are commercially available.
Rodents were maintained individually or in pairs in plastic cages (60×50×40 cm) at 25°C air temperature with a photoperiod of 12:12 (L:D) h, and with sawdust and dried grass as bedding material. They were offered millet seed and fresh alfalfa (Medicago sp.) ad libitum daily. No water was available as the alfalfa supplied enough for their needs. Acomys cahirinus and A. russatus were offered commercial cat chow once a week. When bred in the laboratory, a male and a female rodent born from different parents were placed in each cage initially. Young were transferred to a new cage at 1 month of age. In this study, we used adult males that were maintained individually for at least 1 month prior to experiments and had been previously exposed to flea parasitism.
Experimental design and procedures
Fleas were randomly selected from plastic boxes where they developed (see above). An individual rodent was placed in a plastic cage (40 cm×30 cm×10 cm) with a floor with 3–5 mm of clean sand and covered by a wire mesh (5 mm×5 mm). Twenty to 50 newly emerged (24–48 h old) female P. chephrenis or X. ramesis were placed on a rodent, together with 10–30 conspecific males (dependent on rodent's size). After 2 days of uninterrupted stay in a rodent's cage with continuous host availability, fleas were collected from both the rodent's body and cage substrate. To collect fleas from the rodent's body, we brushed them out over a white plastic pan with a toothbrush. The hair of the rodent was brushed several times, until no fleas were recovered. We counted recovered fleas (males and females separately and those recovered from the host's body and cage substrate separately). Then, we examined the fleas under light microscopy (40X magnification) and counted the number of fleas with blood in their midguts as those that had taken a bloodmeal. Fleas were transferred into an incubator (FOC225E, Velp Scientifica srl, Milano, Italy) and maintained at 25°C air temperature and 90% relative humidity for 24 h.
To measure bloodmeal size after a timed period of feeding, we placed each individual rodent in a wire mesh (5 mm×5 mm) tube (15 cm length and 5 cm diameter for M. crassus, spiny mice and hamsters or 10 cm in length and 2 cm in diameter for other gerbils and house mice) that limited movement and did not allow self-grooming. Tubes with rodents were placed in individual white plastic baths. Fleas (P. chephrenis or X. ramesis) collected from the same individual rodent were weighed (males and females separately, ±0·01 mg, 290 SCS Precisa Balance, Precisa Instruments AG, Switzerland) and then released into the hair of this rodent. After feeding on a host for 2 (X. ramesis) or 6 (P. chephrenis) h, fleas were collected as described above until all fleas were recovered. Then, we examined fleas under light microscopy, selected those with at least 60% of midgut filled with blood and re-weighed them (males and females separately) as described above. The difference in flea's body mass before and after feeding was taken as blood consumption. Each experimental treatment with each flea species and each host species was replicated 5–12 times. Each group of fleas and each individual rodent were used in experiments only once.
Data analyses
Total blood consumed by a flea (=mean bloodmeal size) that had a bloodmeal was calculated as the difference in total mass of fleas after and before feeding divided by the number of fleas. Mass of fleas that did not feed was subtracted. To calculate mass-specific intake, we divided total blood consumed by the mass of fleas that took a bloodmeal.
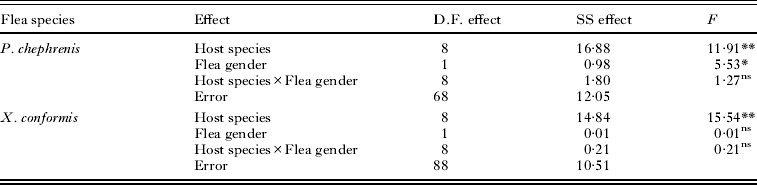
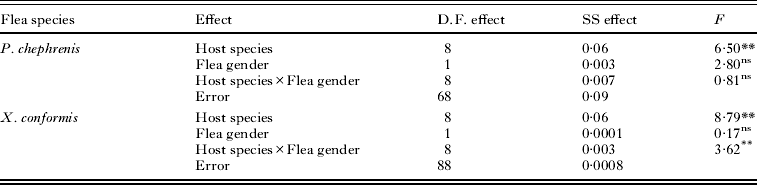
We analysed the proportion of fleas that took a bloodmeal during 2 days of uninterrupted host availability and the bloodmeal size using 2-way ANOVAs with host species and flea gender as independent variables separately for P. chephrenis and X. conformis. We used univariate tests of significance for planned comparisons to compare dependent variables between flea genders within host species and Fisher Least Significant Difference (LSD) tests to compare dependent variables within flea sex (when necessary; see Results) among host species.
To test for the relationships between flea feeding performance and the phylogenetic distance between an auxiliary and a principal host, we calculated (a) mean proportion of fleas that fed during 2 days of uninterrupted host availability and (b) mean bloodmeal size for each host and flea species separately. We also calculated phylogenetic distances between a principal host (A. cahirinus for P. chephrenis and M. crassus for X. ramesis) and each of the remaining 8 host species. Phylogenetic distances between hosts were calculated from branch length of a phylogenetic tree using package ‘ape’ (Paradis et al. Reference Paradis, Claude and Strimmer2004) implemented in the R 2.13.0 software environment (R Development Core Team 2011). The phylogenetic tree (topology and/or branch length) for 9 rodent species was based on Jansa and Weksler (Reference Jansa and Weksler2004), Chevret and Dobigny (Reference Chevret and Dobigny2005), Bininda-Emonds et al. (Reference Bininda-Emonds, Cardillo, Jones, MacPhee, Beck, Grenyer, Price, Vos, Gittleman and Purvis2007) and Abiadh et al. (Reference Abiadh, Chetoui, Lamine-Cheniti, Capanna and Colangelo2010) and is presented in Fig. 1.

Fig. 1. Phylogenetic tree for 9 rodent species used in the study.
Then, we regressed a variable describing flea performance on an auxiliary host against phylogenetic distance of this host from a principal host of a given flea species. The latter variable obviously included phylogenetic information. Consequently, there was no need for further phylogenetic correction. Prior to analyses, dependent variables (proportion of fleas that fed and bloodmeal size) were angular- or log-transformed, respectively. The transformations produced distributions that did not deviate significantly from normality (Kolmogorov-Smirnov tests; P>0·15 for both). Figures represent non-transformed data.
RESULTS
In both flea species, the proportion of fleas that took bloodmeals during 2 days of uninterrupted host availability depended significantly on host species, while a significant effect of flea gender was found in P. chephrenis only (Table 1). However, the latter effect was mainly due to the substantial difference in feeding activities of male and female fleas on G. andersoni where a higher proportion of females than males refused to feed (F=12·53, P<0·001; Fig. 2). No significant gender difference in the proportion of fed fleas was found in the remaining hosts (F=0–1·39, P>0·20 for all), although trends of lower feeding activity of female than male fleas can be envisaged from Fig. 2 for G. dasyurus, G. nanus and M. musculus. In general, all P. chephrenis readily fed on spiny mice (A. cahirinus and A. russatus), G. pyramidum, M. crassus and the hamster (M. auratus), while some fleas refused to feed on the remaining gerbil species and the majority of fleas avoided feeding on the house mouse (M. musculus) (Fisher's LSD tests among host groups A. cahirinis/A.russatus/G. pyramidum/M. crassus/M. auratus versus G. andersoni/G. dasyurus/G. nanus/G. andersoni versus M. musculus; P<0·05 for all; Fig. 2). Almost all X. ramesis took blood from all gerbils as well as from the hamster, while far fewer fleas fed on A. russatus and only a few individuals took bloodmeals from A. cahirinus and M. musculus (Fisher's LSD tests among host group gerbils/hamster versus A. russatus versus A. cahirinis/M. musculus; P<0·001 for all; Fig. 3).

Fig. 2. Mean (±S.D.) proportion of female and male Parapulex chephrenis that took bloodmeals during 2 days of uninterrupted host availability. Abbreviations of host species names are: Ac, Acomys cahirinus; Ar, Acomys russatus; Ga, Gerbillus andersoni; Gd, Gerbillus dasyurus; Gn, Gerbillus nanus; Gp, Gerbillus pyramidum; Ma, Mesocricetus auratus; Mc, Meriones crassus; Mm, Mus musculus.

Fig. 3. Mean (±S.D.) proportion of Xenopsylla ramesis that took bloodmeals during 2 days of uninterrupted host availability. See Fig. 2 for abbreviations of host species names.
Table 1. Summary of ANOVAs of the effects of host species and flea gender on the proportion of fleas Parapulex chephrenis and Xenopsylla ramesis that took bloodmeals during 2 days of uninterrupted host availability
The mass-specific size of a single bloodmeal depended on host species in both fleas, while significant interaction between host species and flea gender was found in X. ramesis only (Table 2). Parapulex chephrenis took significantly larger bloodmeals from G. pyramidum and G. dasyurus than from the remaining host species (Fisher's LSD tests, P<0·05 for all; Fig. 4). No difference in the mass-specific amount of blood taken by a flea was found among the remaining host species, (Fisher's LSD tests, P>0·05 for all; Fig. 4). Female X. ramesis took larger bloodmeals from both species of spiny mice, the hamster, the house mouse and G. pyramidum than from the other hosts (Fisher's LSD tests, P<0·05 for all; Fig. 5), while in male X. ramesis, bloodmeals taken from spiny mice and the house mouse were larger than those taken from gerbils and the hamster (Fisher's LSD tests, P<0·05 for all; Fig. 5).

Fig. 4. Mean (±S.D.) mass-specific size of a bloodmeal (mg * mg−1) taken by Parapulex chephrenis from different host species. See Fig. 2 for abbreviations of host species names.

Fig. 5. Mean (±S.D.) mass-specific size of a bloodmeal (mg * mg−1) taken by female and male Xenopsylla ramesis from different host species. See Fig. 2 for abbreviations of host species names.
Table 2. Summary of ANOVAs of the effects of host species and flea gender on mass-specific size of a single bloodmeal in fleas Parapulex chephrenis and Xenopsylla ramesis that took bloodmeals during 2 days of uninterrupted host availability
We found no significant relationship between the proportion of fleas that chose to feed on an auxiliary host during 2 days of uninterrupted host availability and the phylogenetic distance between this host and the principal host in both P. chephrenis (r 2=0·04, F 1,6=0·29 for female fleas and r 2=0·06, F 1,6=0·41 for male fleas; P>0·54 for both) and X. ramesis (r 2=0·08, F 1,6=0·54, P>0·49). The same was true for the mass-specific size of a single bloodmeal for P. chephrenis and male X. ramesis (r 2=0·08, F 1,6=0·50 and r 2=0·11, F 1,6=0·74; respectively; P>0·40 for both). However, the relationship between mass-specific size of a bloodmeal taken from an auxiliary host by a female X. ramesis and phylogenetic distance between the auxiliary and the principal host was significantly positive (r 2=0·54, F 1,6=7·23, P=0·04). These fleas took larger bloodmeals from auxiliary hosts phylogenetically distant from their principal host, M. crassus (Fig. 6).

Fig. 6. Relationship between mass-specific size of a bloodmeal (mg * mg−1) taken by female Xenopsylla ramesis from an auxiliary host species, and phylogenetic distance of this host from the principal host (Meriones crassus).
DISCUSSION
Results of this study did not unequivocally support our predictions that better feeding performance would be observed in fleas feeding on a principle host rather than an auxiliary host and that feeding performance between the principal and the auxiliary hosts would be manifested more strongly in the host-specific P. chephrenis than in the host-opportunistic X. ramesis. Although feeding performance of fleas differed among different hosts, we found that: (1) fleas did not always perform better on a principal than on an auxiliary host; and (2) flea performance on an auxiliary host did not negatively correlate with phylogenetic distance of this host from a principal host. In fact, it some cases, fleas fed better on hosts that were phylogenetically distant from their principal host than on hosts that were close. Moreover, the two measures of feeding performance demonstrated opposite trends. Feeding performance in terms of proportion of fleas that fed during 2 days of uninterrupted host availability indicated better performance on auxiliary hosts that were phylogenetically close to the principal host. This was better manifested in the host-opportunistic X. ramesis than in the host-specific P. chephrenis. In contrast, feeding performance in terms of the size of a bloodmeal indicated better performance on auxiliary hosts that were phylogenetically distant from the principal host. This was equally manifested in both fleas.
Feeding performance of a parasite is not purely a function of the parasite, but rather a net result of a parasite's responses to a host and a host's responses to a parasite's attack. Indeed, a host defends itself against a parasite by a variety of mechanisms including behavioural and immunological responses. In our experiments, both these groups of defences seemed to act. When fleas had uninterrupted access to a host, host behavioural defences (e.g., grooming) could diminish the number of fleas that consumed a bloodmeal (Eckstein and Hart, Reference Eckstein and Hart2000) although, even the best groomer, is able to remove only a small proportion of fleas (Hinkle et al. Reference Hinkle, Koehler and Patterson1998). Nevertheless, host grooming has been shown to decrease flea feeding success even when it does not decrease the number of fleas substantially (Hawlena et al. Reference Hawlena, Abramsky, Krasnov and Saltz2007). In addition, immunological defences of a host that could suppress flea feeding (e.g., Jones, Reference Jones and Wikel1996) undoubtedly acted. When we measured the size of a bloodmeal in restrained rodents with restricted grooming ability, the bloodmeal size resulted from flea responses to the host and host immunological responses to the flea.
Different hosts possess different species-specific abilities to defend themselves against any ectoparasite. Regarding behavioural defences, hosts with higher manipulating abilities seem to be more effective groomers than hosts with lower abilities. For example, Nikitina and Nikolaeva (Reference Nikitina and Nikolaeva1979) demonstrated that among several rodent species infested with different flea species, the field mouse Apodemus agrarius was the most efficient groomer, whereas the vole Microtus arvalis was the least efficient groomer. Similarly, Zhovty and Vasiliev (Reference Zhovty and Vasiliev1962) showed high grooming abilities in the hamster Phodopus sungorus but low grooming abilities in the ground squirrel Spermophilus dauricus. In this study, the proportion of fed fleas after 2 days of host availability was the lowest on house mice, which likely has the highest manipulative abilities among the 9 rodent species. High grooming dexterity in house mice is supported indirectly by the fact that in nature they are mainly parasitized by Leptopsylla segnis. This flea is usually found on the skull vertex and the middle of the back of a rodent, that is, areas that are difficult to reach by a rodent's limbs (Traub, Reference Traub, Traub and Starke1980). Moreover, it possesses special spine arrangements referred to as ‘crowns of thorns’ that allow it to hook onto the hairs of the host and remain in this position for several hours or even days (Farhang-Azad et al. Reference Farhang-Azad, Traub and Wisseman1983; Traub, Reference Traub, Traub and Starke1980).
Regarding immunological defences, mounting of immune responses and investment in immune defences has been suggested to depend on the pattern of parasite pressure (Combes, Reference Combes2001), including the frequency and probability of parasite attacks (Martin et al. Reference Martin, Møller, Merino and Clobert2001; Tella et al. Reference Tella, Scheuerlein and Ricklefs2002). Indeed, large energy expenditure towards immunological defence would be of little advantage if encounters with the parasite are rare (Poulin et al. Reference Poulin, Brodeur and Moore1994). Consequently, if frequency and/or probability of attacks by parasites are low, then a host can limit its allocation of energy for immune responses by the development of the responses only after being attacked by the parasite (‘post-invasive’). If, however, frequency and/or probability of parasitism are high, a continuous maintenance of a certain level of immune ‘readiness’ in the host is advantageous. For example, 2 gerbils, G. dasyurus and G. andersoni, differ sharply in their natural species richness of flea assemblages and prevalence of infestation with the former being parasitized by several flea species but with relatively low prevalence and intensity of infestation, while the latter is parasitized mainly by a single flea species but with 95–100% prevalence (Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Vashchenok and Khokhlova1997, Reference Krasnov, Hastriter, Medvedev, Shenbrot, Khokhlova and Vashchenok1999; Hawlena et al. Reference Hawlena, Abramsky and Krasnov2005). Results of the study by Khokhlova et al. (Reference Khokhlova, Spinu, Krasnov and Degen2004) demonstrated that G. dasyurus was characterized mainly by ‘post-invasive’ immune responses, while G. andersoni showed permanent immune ‘readiness’ even if not parasitized. Göuy de Bellocq et al. (Reference Goüy de Bellocq, Krasnov, Khokhlova and Pinshow2006) demonstrated species-specific differences in mounting of an immune response measured via phytohaemagglutinin injection assay among 10 rodent species that differed markedly in species richness of their natural flea assemblages as well as in flea prevalence and abundance. Bird species parasitized by many flea species mount stronger immune responses than bird species parasitized by a few flea species (Møller et al. Reference Møller, Christe and Garamszegi2005).
Fleas, in turn, may have different species-specific abilities to withstand the defence efforts of hosts. For example, variation among flea species in their ability to cope with host grooming has been demonstrated (e.g., Nikitina and Nikolaeva Reference Nikitina and Nikolaeva1981). This variation seemed to stem from the among-flea variation in behaviour and morphology. From a behavioural perspective, distribution of some but not other fleas across a body of a host is often characterized by preference to those body areas that are the least reachable by paws or teeth of a host (e.g., Ma, Reference Ma1983). From a morphological perspective, some but not other fleas possess sclerotinized helmets, ctenidia, spines and setae that allow them to attach to the host's hairs and to resist the host's grooming (e.g., Amin, Reference Amin1982). However, it is unknown whether different flea species have different abilities to cope with host immune responses.
The relationship between a parasite response to a host and a host response to a parasite undoubtedly depends on the history and ‘tightness’ of a host-parasite association. During co-evolution of a particular parasite and a particular host, natural selection will favour those parasites that are able to successfully extract resources from hosts, while it will favour those hosts that are able to diminish the harm incurred by parasites. From the parasite perspective, one of the resulting scenarios may be that a parasite can extract only a small amount of the resource (e.g., blood) from a host but it can be compensated by efficient use of this resource. Indeed, in our study, P. chephrenis consumed less blood from A. cahirinus than from G. dasyurus and G. pyramidum, while X. ramesis consumed less blood from M. crassus than from spiny mice. However, Krasnov et al. (Reference Krasnov, Sarfati, Arakelyan, Khokhlova, Burdelova and Degen2003) and Sarfati et al. (Reference Sarfati, Krasnov, Ghazaryan, Khokhlova, Fielden and Degen2005) showed that when P. chephrenis fed on A. cahirinus as compared to G. dasyurus, it filled its gut faster, digested blood for a shorter time with less energy spent for digestion and survived longer when starved while feeding on the former host. Furthermore, a host that does not usually encounter a given flea species might lack immune defences against this flea despite a well-known phenomenon of cross-resistance (e.g., Rechav and Dauth Reference Rechav and Dauth1987). For example, Studdert and Arundel (Reference Studdert and Arundel1988) reported a severe allergic reaction in cats which hunted rabbits infested with the rabbit flea Spilopsyllus cuniculi. The severity of these symptoms indicated that cats had a much higher response to rabbit fleas than to Ctenocephalides felis with which they were normally infested. As a result, a parasite might easily extract resources from a new host species due to the lack of defence mechanisms of this host against the parasite. In a previous study (Krasnov et al. Reference Krasnov, Korine, Burdelova, Khokhlova and Pinshow2007), we fed P. chephrenis and X. ramesis on their preferred hosts as well as on the Egyptian fruit bat Rousettus aegyptiacus, an alien host to both fleas. It appeared that the feeding performance on an alien bat host measured as a proportion of fleas that took a bloodmeal did not differ from that on a preferred rodent host. In the present study, we found a similar trend, albeit in another feeding variable. Although X. ramesis feed more readily on gerbilline than on murine hosts, they took less blood from the former than from the latter. Females of this species demonstrated an increase in the size of a bloodmeal taken from an auxiliary host with an increase of phylogenetic distance from the principal host. Parapulex chephrenis took larger bloodmeals from G. pyramidum and G. dasyurus than from any other hosts, but did not show any clear-cut relationship between feeding performance and phylogenetic position of a host.
In addition, ‘tightness’ of a host-flea association may be determined by ecological rather than evolutionary mechanisms. For example, many host-specific fleas produce only 1 generation per year with adult (that is, blood feeding) stage lasting a very short time (Haitlinger, Reference Haitlinger1973). As a result, a given individual of a specific host of this flea may rarely encounter it and, thus, may not develop a specific immunological response. In contrast, many host-opportunistic fleas reproduce and occur as adults all year round (Darskaya Reference Darskaya1970; Haitlinger Reference Haitlinger1973), so they attack continuously many host species which, in turn, may acquire resistance against these fleas (Fielden et al. Reference Fielden, Rechav and Bryson1992). This notion, however, cannot be invoked for explanation of our results because both fleas in our study occur as imago continuously (e.g., Krasnov et al. Reference Krasnov, Burdelova, Shenbrot and Khokhlova2002b for X. ramesis).
The above suggests that variation in flea feeding performance among host species results from interplay between (a) inherent species-specific defence abilities of host species, (b) inherent species-specific flea abilities to withstand host defences and (c) evolutionary tightness of association between a particular host species and a particular flea species. Furthermore, feeding patterns of fleas on different species (specifically, feeding success and the size of a bloodmeal) might not be the best proxy for comparative assessment of their general performance and evolutionary success on these species, although these patterns proved to be good proxy for reproductive success of a flea in comparisons within the same host species (Khokhlova et al. Reference Khokhlova, Serobyan, Krasnov and Degen2009b). Indeed, the efficiency of processing the bloodmeal, i.e. the above-mentioned lower energy expenditure of P. chephrenis for digestion of A. cahirinus blood as compared to G. dasyurus blood (Sarfati et al. Reference Sarfati, Krasnov, Ghazaryan, Khokhlova, Fielden and Degen2005) could be the main reason for the higher reproductive success of the flea on the former than on the latter host (Krasnov et al. Reference Krasnov, Khokhlova, Oguzoglu and Burdelova2002a). In other words, the quantity of blood taken from a host does not necessarily correspond to its quality. For example, a flea may take large bloodmeals from a host due to the lack of adequate immune response. However, this blood may be of low quality, that is, deficient in some nutritional components (Lehane, Reference Lehane2005) and/or require higher energy expenditure for digestion (Sarfati et al. Reference Sarfati, Krasnov, Ghazaryan, Khokhlova, Fielden and Degen2005). resulting in a smaller number of offspring and/or offspring of lower quality than would be the case if a flea takes a smaller amount of better quality blood (richer in nutrients and/or easier to digest). This suggests that the question about the relationships between parasite performance on auxiliary and principals hosts and the importance of phylogenetic position of these hosts relative to the principal host requires further investigations. These investigations should involve other than feeding measures such as, for example, energy costs of bloodmeal digestion and reproductive output.
ACKNOWLEDGEMENTS
We thank two anonymous referees for their helpful comments on earlier version of the manuscript. This study was supported by the Bi-National Science Foundation (Grant no. 2008142 to BRK, ISK and LJF). The experimental procedures complied with the laws of the State of Israel. This is publication no. 742 of the Mitrani Department of Desert Ecology.










