INTRODUCTION
Organisms encountering pathogens may protect themselves through either of two defensive strategies, resistance or tolerance. Resistance allows organisms to avoid, reduce or clear an infection, and thereby limit a parasite burden (Råberg et al. Reference Råberg, Graham and Read2009; Svensson and Råberg, Reference Svensson and Råberg2010; Medzhitov et al. Reference Medzhitov, Schneider and Soares2012). Tolerance allows hosts to minimize the negative impact of pathogens on their fitness after an infection has been established, despite the damage to hosts caused by a parasite burden (Simms, Reference Simms2000; Svensson and Råberg, Reference Svensson and Råberg2010; Baucom and de Roode, Reference Baucom and de Roode2011). More formally, tolerance is defined for a genotype as the rate of decrease in host fitness with an increase in parasite burden (Råberg et al. Reference Råberg, Graham and Read2009; Baucom and de Roode, Reference Baucom and de Roode2011). A large number of mechanisms may contribute to tolerance (Råberg et al. Reference Råberg, Graham and Read2009; Ayres and Schneider, Reference Ayres and Schneider2012), which may show heritable and environmental influences (Råberg et al. Reference Råberg, Graham and Read2009).
In Alaska female three-spined sticklebacks infected by the diphyllobothriidean cestode Schistocephalus solidus have the capacity to produce clutches of eggs while carrying large burdens of the parasite (Heins et al. Reference Heins, Singer and Baker1999, Reference Heins, Baker, Toups and Birden2010a ; Heins and Baker, Reference Heins and Baker2008; Heins, Reference Heins2012). These observations contrast with those in other regions where host females show greater sensitivity to infection affecting their reproductive capacity and fitness. Sticklebacks among populations in England (Arme and Owen, Reference Arme and Owen1967; Pennycuick, Reference Pennycuick1971), Scotland (Tierney et al. Reference Tierney, Huntingford and Crompton1996), and Canada (McPhail and Peacock, Reference McPhail and Peacock1983) generally do not reproduce when infected by S. solidus. Only females with small masses of parasites relative to host body mass (BM) (parasite : host biomass ratio <10%) are capable of producing egg clutches (McPhail and Peacock, Reference McPhail and Peacock1983; Milinski, Reference Milinski, Barnard and Behnke1990). Thus, populations of sticklebacks in Alaska appear to show tolerance to infections of S. solidus. Moreover, these populations exhibit two different tolerance responses to infection by S. solidus.
Notwithstanding their ability to produce clutches of eggs while carrying large parasite burdens, female sticklebacks in Walby Lake experience fecundity reduction involving a decrease in clutch size with increased parasite index (PI) (Heins et al. Reference Heins, Baker, Toups and Birden2010a ). Infected females also exhibit reduced egg mass and clutch mass, showing progressive decreases in these characteristics as parasite mass increases in relation to host BM (Heins et al. Reference Heins, Baker, Toups and Birden2010a ). These reductions in host reproductive performance are side effects of parasitism reflecting the loss of nutrients to the parasite (Schultz et al. Reference Schultz, Topper and Heins2006; Heins et al. Reference Heins, Baker, Toups and Birden2010a ). Mechanisms causing fecundity reduction, which functionally starve the host, may eventually produce a ‘castration-like effect’ when parasite burdens are very high (Baudoin, Reference Baudoin1975; Hall et al. Reference Hall, Becker and Cáceres2007; Heins et al. Reference Heins, Baker, Toups and Birden2010a ; Barber, Reference Barber2013).
In Scout Lake infected females produce larger clutches before they too lose reproductive capacity when burdens become very high (Heins, Reference Heins2012). The increase in clutch size is linked to a reduction in egg mass while clutch mass is unchanged in comparison to uninfected females (Heins, Reference Heins2012). Thus, host females trade-off increased fecundity for reduced ovum mass as a means of fecundity compensation (Heins, Reference Heins2012), which is the only tolerance mechanism having been given much attention in the animal literature (Baucom and de Roode, Reference Baucom and de Roode2011). Fecundity compensation is an inducible, phenotypically plastic reproductive tactic allowing hosts to reproduce earlier in life or to increase fecundity and thereby realize a measure of reproductive success before parasites reduce or stop reproduction (Minchella and LoVerde, Reference Minchella and LoVerde1981; Minchella, Reference Minchella1985). It may evolve as an alternative solution to parasitism because evolving and maintaining immune systems as well as mounting an immune defence is costly (Minchella, Reference Minchella1985; Parker et al. Reference Parker, Barribeau, Laughton, de Roode and Gerardo2011). Fecundity compensation is but one of several non-immunological host defences to the challenges presented by parasites (Parker et al. Reference Parker, Barribeau, Laughton, de Roode and Gerardo2011).
In this study, we survey populations of three-spined sticklebacks from nine lakes in Alaska in an effort to assess responses to infection among host populations from the region. In 2003, we demonstrated reduction in egg size associated with S. solidus infection among these same lakes, including Walby and Scout, which was hypothesized to have resulted from nutrient theft (Heins and Baker, Reference Heins and Baker2003). Subsequent studies on the host-parasite relationship in Walby and Scout lakes (Heins et al. Reference Heins, Baker, Toups and Birden2010a ; Heins, Reference Heins2012) showed that ovum mass may be decreased through either fecundity reduction or fecundity compensation. To assess the occurrence of the two responses to infection among the populations, we added females from which we could obtain counts of clutch sizes to the samples used in the Heins and Baker (Reference Heins and Baker2003) study. The additional specimens could not be used in the earlier study because they did not provide estimates of egg mass; they were reproductively mature females with a clutch but not ripe, fully formed and ovulated eggs. In some cases, we were also able to include females from additional collections taken during the same year to increase our sample sizes. Herein, we also provide new analyses and re-assessments of data from the earlier reports by Heins and Baker (Reference Heins and Baker2003), Heins et al. (Reference Heins, Baker, Toups and Birden2010a ) and Heins (Reference Heins2012).
MATERIALS AND METHODS
Study sites and samples
Collections of Gasterosteus aculeatus were obtained from nine lakes in the Matanuska–Susitna (Mat–Su) Valley and the Kenai Peninsula (Kenai) in southcentral Alaska from 1994 to 1999. The lakes are Big Beaver(1994; Mat–Su; 61·5838N, 149·8288W), Cornelius (1999; Mat–Su; 61·6318N, 149·2538W), Duck (1990; Kenai; 60·6828N, 151·2248W), Lampert (1995; Kenai; 59·6458N, 151·4668W), Loberg (1995; Mat–Su; 61·5608N, 149·2588W), Scout (1997; Kenai; 60·5358N, 150·8298W), Tern (1998; Kenai; 60·5338N, 149·5508W), Walby (1996; Mat–Su; 61·6198N, 149·2118W) and Willow (1997; Mat–Su; 61·7448N, 150·0578W). The data for Scout and Walby lakes were taken, in part, from Heins and Baker (Reference Heins and Baker2003), Heins et al. (Reference Heins, Baker, Toups and Birden2010a ) and Heins (Reference Heins2012). Although the lakes in this investigation were each sampled in one year, a long-term study from Scout (8 years) and Walby (18 years) lakes showed that the responses to infection in each lake were consistent across years despite variation in infection levels and environmental conditions (D. Heins et al. unpublished data). Thus, we do not expect that the results of this study are dependent upon annual variation in the focal populations.
The Mat–Su Valley lies north of the Cook Inlet and encompasses the Matanuska and Susitna river valleys and the intervening area. The lakes sampled there (Big Beaver, Cornelius, Loberg, Walby and Willow) are among the many lakes and ponds that dot the glacial moraine in the Valley. Lakes in the Mat–Su Valley usually are covered with ice from October to May (Woods, Reference Woods1985). The Kenai Peninsula borders the southeastern shores of the Cook Inlet. The lakes sampled there (Duck, Lampert, Scout and Tern) are located in the lowland of the northern area of the peninsula.
Samples were taken using 3- to 6-mm wire-mesh minnow traps that were set near the shore, as is the common practice for a number of studies of sticklebacks in Alaska and elsewhere. Fish were anaesthetized until quiescent in MS222 before fixation and storage in 10% formalin until examination, with the exception of a small number of fish from Duck Lake we obtained from M.A. Bell that had been fixed in formalin and stored in isopropyl alcohol. Collections were made in late May–June during the approximate 6-week spawning season that occurs after ice-out in Alaska (Heins et al. Reference Heins, Singer and Baker1999). Females examined in this study were 2-year-old fish that had been infected earlier in life (Heins et al. Reference Heins, Singer and Baker1999).
Specimen examination
In order for our general readers to understand the process of clutch production and the basis for our metrics of clutches, we briefly outline the reproductive biology of female three-spined sticklebacks. Ovaries of three-spined stickleback females may be classified into developmental stages following Baker et al. (Reference Baker, Foster, Heins, Bell and King1998) and Heins et al. (Reference Heins, Singer and Baker1999): latent (LA); early maturing (EM); late maturing (LM); mature (MA); ripening (MR); and ripe (RE). Females in the latter three stages (MA, MR and RE) have readily discernible clutches of developing oocytes or ripe eggs. Ripe females bear clutches of fully formed, ovulated eggs whereas MA and MR females have developing oocytes still in their follicles. As the reproductive season approaches, females progress through LA, EM and LM stages leading up to the formation and oviposition of the first clutch of the season. During the spawning season, adult females produce and spawn multiple clutches as they repeatedly cycle through the LM, MA, MR and RE stages of the ‘clutch-production cycle’ (Heins and Baker, Reference Heins and Baker1993; Brown-Peterson and Heins, Reference Brown-Peterson and Heins2009).
Specimens of the three-spined stickleback were dissected to remove any S. solidus plerocercoids and to determine sex and reproductive condition. Oocytes or eggs in each clutch were counted to determine egg number (clutch size, CS). Spawning females ovulate all oocytes in each clutch and then oviposit all of the ripe eggs in the clutch during a single spawning bout (Wootton, Reference Wootton1976; Bakker and Mundwiler, Reference Bakker and Mundwiler1994; Brown-Peterson and Heins, Reference Brown-Peterson and Heins2009); they are not fractional spawners. Thus, our counts of the number of eggs in clutches represent the actual clutch sizes. A small number of fish with extreme pathologically small ‘clutches’ in their follicles were not used in subsequent analyses of clutch characteristics. These females were barely able to produce a ‘clutch’ and may not have been able to spawn the few developing oocytes in the ovaries. They could have been classified as ‘non-reproductive’ because they were clearly debilitated in comparison to all other parasitized females with clutches. We describe them here, however, in the interest of repeatability. Carcasses of eviscerated fish (all contents of body cavity removed, excepting kidneys) were weighed to the nearest 0·001 g after they were blotted with a paper towel to measure somatic BM. Specimens from Duck Lake were measured with digital calipers to the nearest 0·1 mm standard length (SL) because storage in alcohol would have reduced BM.
Plerocercoids from each host were weighed together to the nearest milligram after they were blotted. We estimated the mass of each un-weighable parasite, of weight less than 1 mg, to be 0·5 mg, based on measurements of the individual masses of a number of small parasites on a more sensitive, precise balance. The combined parasite : host biomass ratio (PI; expressed as a percentage) for each host was calculated using the formula PI = PM/BM, where PM is the total weight of all parasites and BM is the mass of the eviscerated carcass (Arme and Owen, Reference Arme and Owen1967; LoBue and Bell, Reference LoBue and Bell1993; Tierney et al. Reference Tierney, Huntingford and Crompton1996). For the females from Duck Lake stored in alcohol, we assumed that any reductions in masses of parasites and hosts were proportional. PI was used as a metric for severity of infection because parasite biomass should be related to nutrient theft, and the ratio should be related to pathology arising from nutrient loss (Hurd, Reference Hurd2001). Moreover, trophically transmitted parasites such as S. solidus should show intensity-independent effects on the host, with the full extent of pathology expected in single infections as well as in multiple ones (Lafferty and Kuris, Reference Lafferty and Kuris2002; Kuris, Reference Kuris2003; Fogelman et al. Reference Fogelman, Kuris and Grutter2009).
Study design and statistical analyses
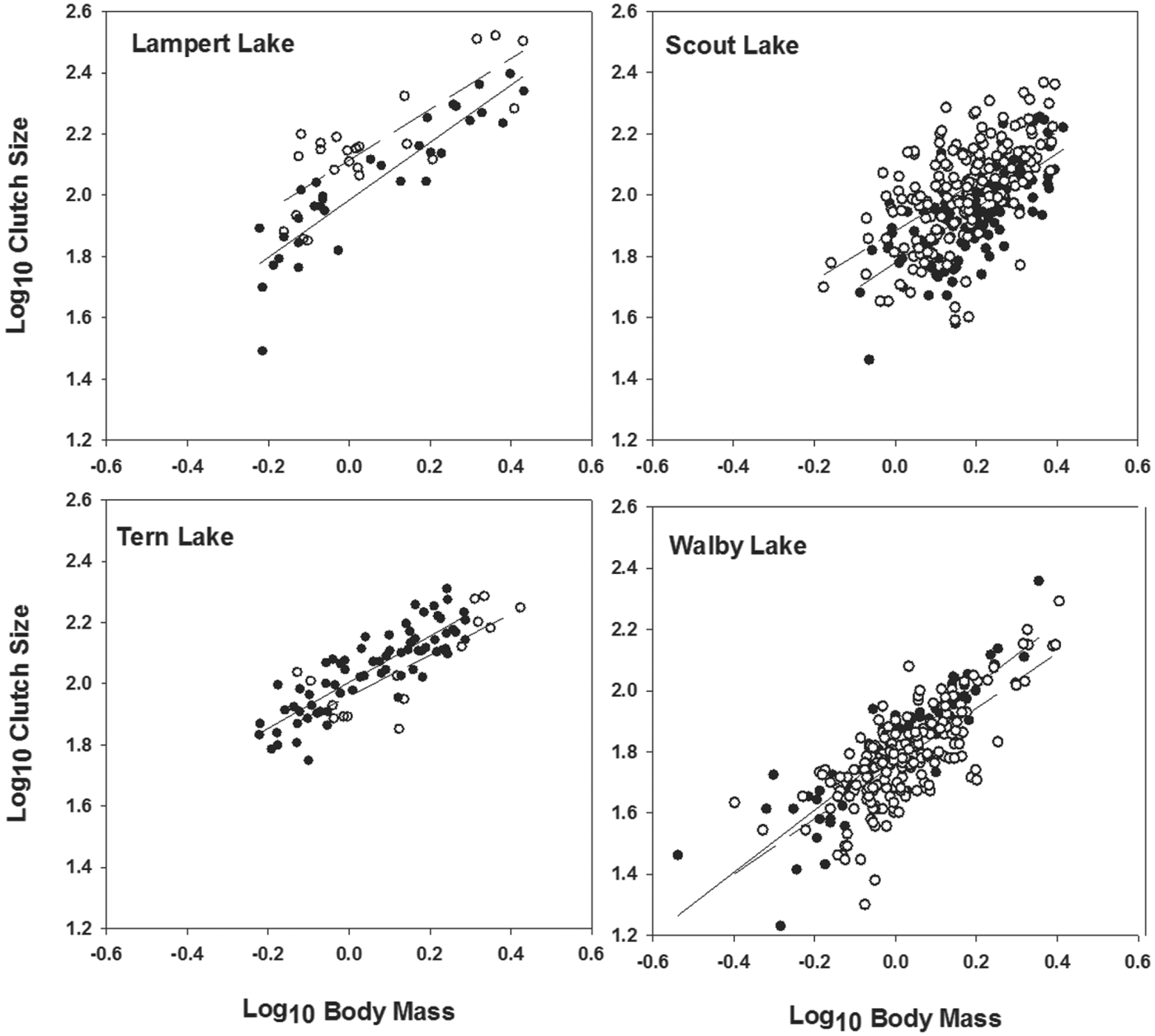
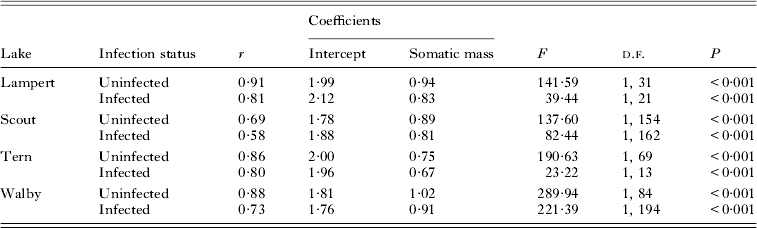
We transformed data for BM, SL and CS to log10 values and used least-squares regressions to analyse the relationship between either measure of body size and CS. Both uninfected and infected females show significant, positive relationships between BM or SL and CS (Heins et al. Reference Heins, Baker, Toups and Birden2010a ; Heins, Reference Heins2012; Heins and Baker, personal observation). Thus, comparisons of CS between uninfected and infected females for each lake, except for Duck Lake, included an adjustment for BM using analysis of covariance (ANCOVA), notwithstanding some significant interactions between BM and infection status in the analyses. We justified the use of BM as a covariate because the covariate explained a significant level of variation, the interactions involved slopes influenced by pathology, and simulation tests have established that ANCOVA is sufficiently robust to violations of this assumption under many circumstances (Wu, Reference Wu1984; Reist, Reference Reist1985; Sullivan and D'Agostino, Reference Sullivan and D'Agostino2002). We used SL as a covariate for Duck Lake because any effects of storage in isopropyl alcohol on measurements of body size should be minimal for SL, and log10-transformed values for BM and SL are highly correlated among fish preserved in formalin. Residuals from log10 CS-BM regressions were used in regression analyses to analyse the relationship between size-adjusted CS and PI among infected females.
The computer program SYSTAT (Systat Software Inc.) was used for the statistical computations. Analyses of CS were performed after CS, BM and SL were converted to log10 values to meet the assumptions of the statistical analyses. Similarly, we used arcsine-transformed PI values in the statistical analyses.
RESULTS
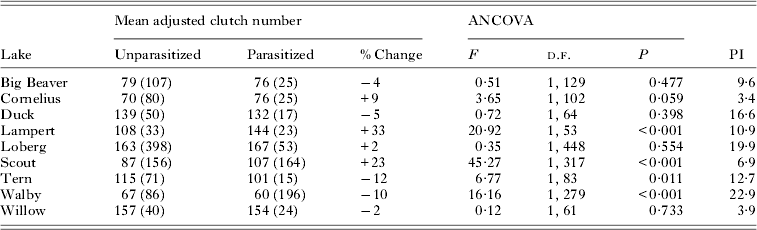
Adjusted mean clutch size among females parasitized by S. solidus was significantly lower in Tern and Walby lakes (Table 1), which amounted to reductions of 12 and 10%, respectively. Parasitized females in these populations also showed significant decreases in mean egg mass (Heins and Baker, Reference Heins and Baker2003): tern (uninfected, 669 μg; infected, 454 μg; −32%, P<0·001), Walby (uninfected, 657 μg; infected, 524 μg; −20%; P<0·001). There were no significant differences in mean adjusted clutch size among infected females from five of the lake populations, including Big Beaver, Cornelius, Duck, Loberg and Willow (Table 1). Parasitized females in each of these populations showed reductions in mean egg mass between 8 and 22%, all of which were statistically significant (Heins and Baker, Reference Heins and Baker2003): Big Beaver (uninfected, 612 μg; infected, 566 μg; −8%; P<0·05), Cornelius (uninfected, 757 μg; infected, 648 μg; −14%; P<0·001), Duck (uninfected, 631 μg; infected, 526 μg; −17%; P<0·001), Loberg (uninfected, 568 μg; infected, 483 μg; −15%; P<0·001), Willow (uninfected, 517 μg; infected 406 μg; −22%; P<0·001). Two of the nine populations (Lampert Lake and Scout Lake) showed a significant increase in clutch size among infected females when compared with uninfected females (Table 1). Infected females in Lampert and Scout lakes also showed significant decreases in mean egg mass (Heins and Baker, Reference Heins and Baker2003): Lampert (uninfected, 673 μg; infected 556 μg; −17%; P<0·001), Scout Lake (uninfected, 700 μg; infected 550 μg; −21%; P<0·001).
Table 1. Results of analysis of covariance (ANCOVA) comparing log10 mean female clutch size adjusted for log10 female body mass (standard length, Duck Lake) of uninfected and infected three-spined stickleback females from each population. The antilogs of mean female clutch number adjusted for female body mass (number of specimens) are presented, along with the per cent increase or decrease in mean clutch size of infected females and the mean parasite : host body mass ratio (PI) of infected females
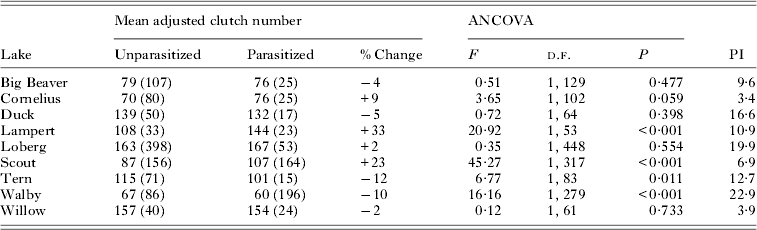
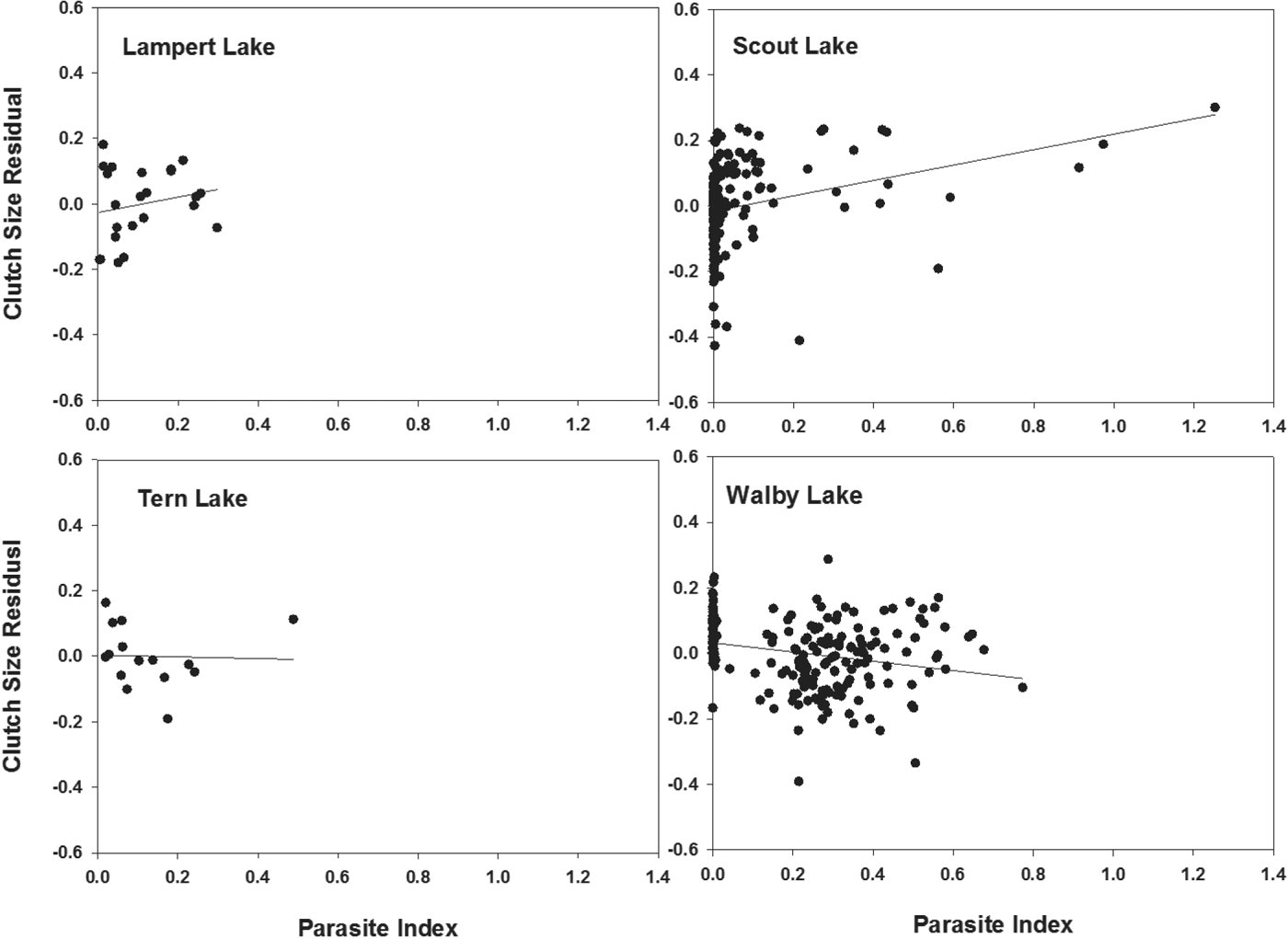
Among those populations showing significant differences in CS between uninfected and infected females, CS residuals (from regressions of log10 CS on log10 BM, Table 2, Fig. 1) were not significantly related to PI among infected females from either Lampert Lake (r = 0·20; F = 0·856; d.f. = 1,21; P = 0·365) or Tern Lake (r = −0·03; F = 0·015; d.f. = 1, 13; P = 0·904), showing that size-adjusted CS did not vary significantly with PI in these samples (Fig. 2). CS residuals were significantly and negatively related to PI in Walby Lake (r = −0·24; F = 12·205; d.f. = 1, 194; P = 0·001; Heins et al. Reference Heins, Baker, Toups and Birden2010a ), revealing that size adjusted CS decreased with increasing PI. In Scout Lake, the CS residuals were significantly and positively related to PI (r = 0·24; F = 10·20; d.f. = 1, 161; P = 0·002), showing an increase in size adjusted CS with increasing PI.
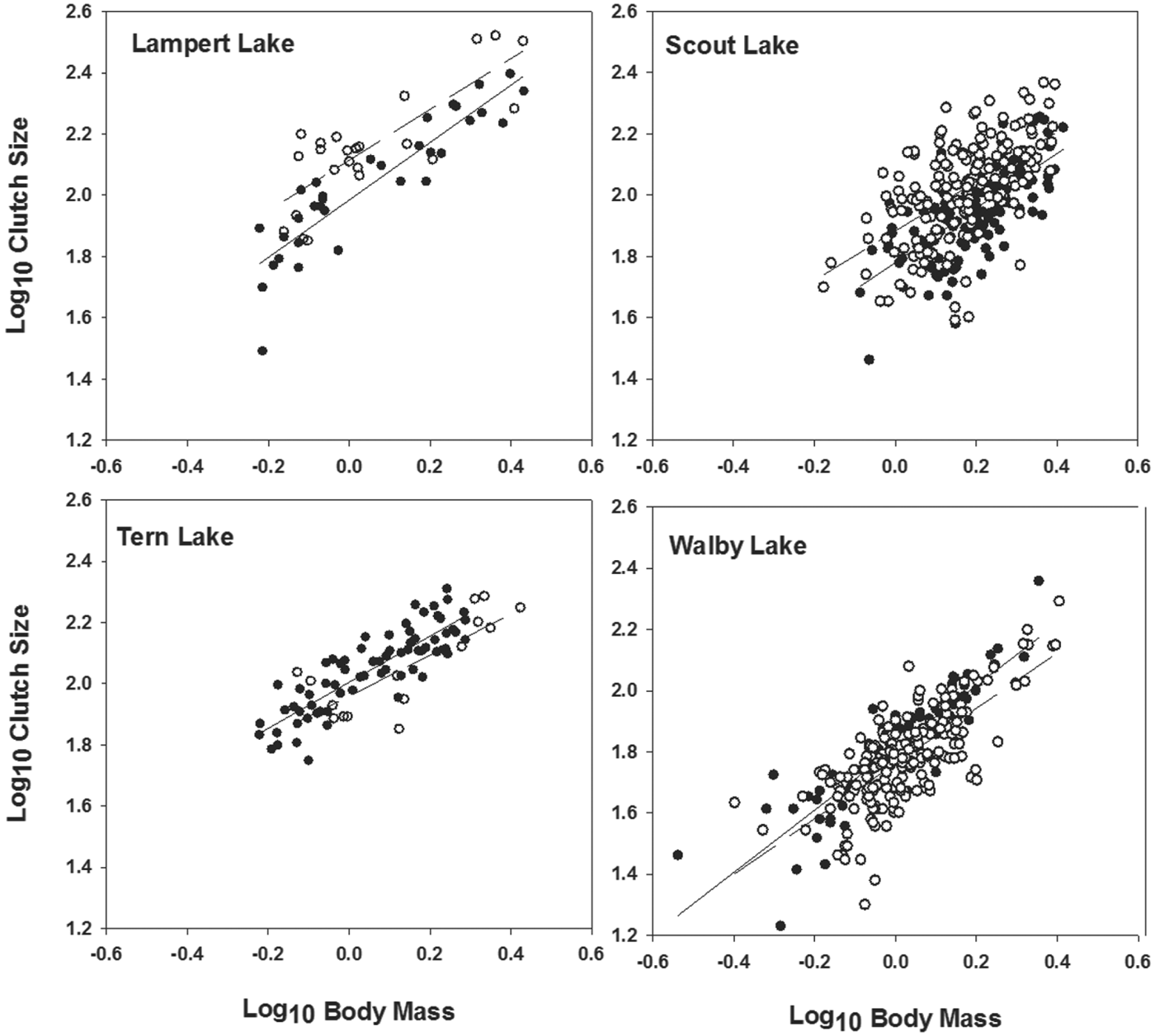
Fig. 1. Relationship between log10 clutch size and log10 body mass of female three-spined stickleback fish, Gasterosteus aculeatus, from Lampert and Scout lakes (upper row, fecundity compensation) and Tern and Walby lakes (lower row, fecundity reduction) of south-central Alaska that were uninfected (filled circles, solid lines) or infected (open circles, dashed lines) by Schistocephalus solidus. Data and plots for Scout and Walby lakes were taken from Heins et al. (Reference Heins, Baker, Toups and Birden2010a ) and Heins (Reference Heins2012).
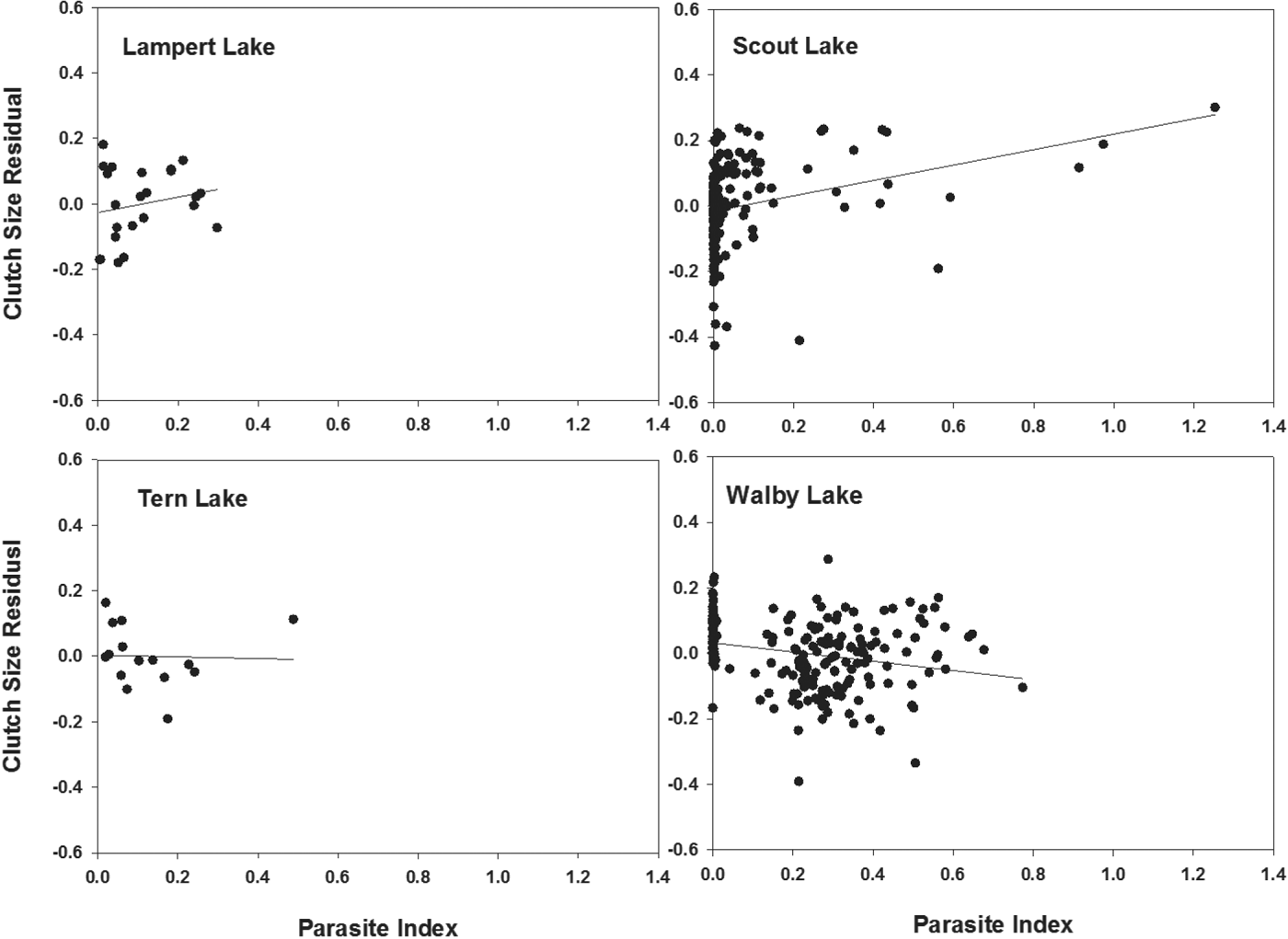
Fig. 2. Relationship between residual from regression of log10 clutch size and log10 body mass with the parasite :host mass ratio (parasite index) of female threespine stickleback fish, Gasterosteus aculeatus, infected by Schistocephalus solidus. Plots show results from Lampert and Scout lakes (upper row, fecundity compensation) and Tern and Walby lakes (lower row, fecundity reduction) of south-central Alaska. Data for Scout and Walby lakes were taken from Heins et al. (Reference Heins, Baker, Toups and Birden2010a ) and Heins (Reference Heins2012).
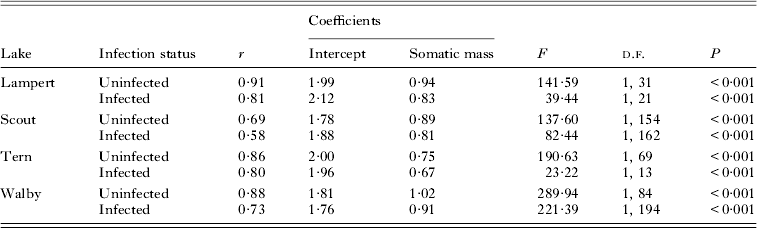
Table 2. Statistics for ordinary least squares regressions of log10 clutch size on log10 somatic mass for infected and uninfected three-spined stickleback females from Lampert, Scout, Tern and Walby lakes, Alaska, which are the four lakes showing significant reductions in clutch size
DISCUSSION
This investigation, coupled with the studies by Heins and Baker (Reference Heins and Baker2003), Heins et al. (Reference Heins, Baker, Toups and Birden2010a ) and Heins (Reference Heins2012), have revealed two apparent tolerance responses to infection by S. solidus among female three-spined sticklebacks in Alaska: fecundity reduction and fecundity compensation. In each case, female hosts are capable of clutch production despite heavy parasite burdens (Heins et al. Reference Heins, Baker, Toups and Birden2010a ; Heins, Reference Heins2012), in contrast to the response seen in other regions where females are only capable of reproduction when carrying light burdens (Arme and Owen, Reference Arme and Owen1967; Pennycuick, Reference Pennycuick1971; McPhail and Peacock, Reference McPhail and Peacock1983; Tierney et al. Reference Tierney, Huntingford and Crompton1996).
Fecundity reduction
Fecundity reduction in parasitized females results from nutrient theft by S. solidus (Schultz et al. Reference Schultz, Topper and Heins2006; Heins et al. Reference Heins, Baker, Toups and Birden2010a ). Our data suggest that the phenomenon involves two levels of host response. One level involves a significant reduction in ovum mass without a significant reduction in clutch size (Big Beaver, Cornelius, Duck, Loberg and Willow lakes), and another level involves a significant reduction in both ovum mass and clutch size (Tern and Walby lakes). Our observations, therefore, suggest that ovum mass is more readily influenced by parasitism and is decreased before clutch size is reduced as PI increases. Here below we consider this possibility using previously published studies on the reproductive biology and the influence of feeding ration on reproduction in stickleback females.
In the three-spined stickleback, clutch size appears to be largely a function of female size (Wootton, Reference Wootton, Bell and Foster1994; Fletcher and Wootton, Reference Fletcher and Wootton1995; Ali and Wootton, Reference Ali and Wootton1999). Although nutrients for clutch production are derived from ingested food between spawning episodes, there is little direct influence on clutch size from food consumed during the spawning season because somatic tissues may be depleted if food is insufficient (Wootton, Reference Wootton1973; Wootton, Reference Wootton1977; Fletcher, Reference Fletcher1984; Wootton, Reference Wootton, Bell and Foster1994). Moreover, the number of eggs in a clutch is not adjusted to energy income from food consumed after the clutch has been formed from the recruitment stock of oocytes (Wootton, Reference Wootton, Bell and Foster1994; Heins and Brown-Peterson, Reference Heins and Brown-Peterson2010). This deduction from feeding trials has been confirmed for uninfected and infected three-spined sticklebacks through histological studies (Brown-Peterson and Heins, Reference Brown-Peterson and Heins2009; Heins and Brown-Peterson, Reference Heins and Brown-Peterson2010). Egg size is little influenced by reduced food rations until energy input is reduced to very low levels for an extended time (Fletcher and Wootton, Reference Fletcher and Wootton1995; Ali and Wootton, Reference Ali and Wootton1999). How then do we explain the two levels of response to infection in stickleback females? Inferences based on published studies provide two non-exclusive hypotheses.
Three-spined sticklebacks have been shown to have hierarchical reproductive responses to differing food rations, involving spawning rate and seasonal totals for fecundity and egg mass (Fletcher and Wootton, Reference Fletcher and Wootton1995). Our results suggest that hierarchical responses extend to clutch size and egg mass of individual females. Infections of S. solidus appear functionally to starve the host fish (Schultz et al. Reference Schultz, Topper and Heins2006; Hall et al. Reference Hall, Becker and Cáceres2007; Heins et al. Reference Heins, Baker, Toups and Birden2010a ). Sticklebacks typically become infected soon after birth at age 0+ but do not become sexually mature until they are 2 years old (Heins et al. Reference Heins, Singer and Baker1999, Reference Heins, Baker and Green2011). Thus, females have already experienced a prolonged drain of nutrients to the parasite when they begin producing clutches. If conclusions from short-term feeding trials can be extended to the long-term effect of S. solidus (i.e. nutrient theft has the same influence as food reduction), we may expect to see ovum mass reduced without an effect on clutch size in females having low parasite burdens and already depleted resources from the prolonged effects of parasitism. Once the commitment to a clutch of a certain size has been made based on a female's size, egg mass may be reduced simply because the full demands of ovum development and the parasite growth cannot be met in a host female with reduced somatic energy. Alternatively, a female may perceive that she is getting a particular ration without detecting the loss of nutrients to the parasite following assimilation and reduces the egg mass after the commitment to a clutch of a certain size has been made. For whatever reason, then, we expect that ovum mass should be more sensitive to the effect of nutrient theft than clutch size, and egg size should be reduced by S. solidus parasitism before clutch size.
Our results and those of Heins et al. (Reference Heins, Baker, Toups and Birden2010a ) showing greater reductions in clutch size with increasing PI are more difficult to resolve with what is known from published studies and suggest that female size alone does not determine clutch size. If female size were the sole determinant of clutch size, we should only expect decreases in egg mass in all infections. Thus, females seem to perceive the energy level that will support a clutch of a given size, thereby reducing clutch size as energy levels decline with increasing PI. But why, then, are both clutch size and egg size reduced and not clutch size alone? If a female does not accurately detect her energetic state or does not appropriately take into account the energy loss to the parasite as she recruits a new clutch, then we would expect both traits to show decreases as the parasite burden increases. The effects observed in these life-history traits should reflect both the long-term loss of energy to the parasite over nearly 2 years and the more immediate demands of the pathogen during the spawning season. Thus, we should expect the alterations of host life history to have some relationship to PI.
Consistent with these expectations, samples showing significant reductions in both clutch size and egg mass generally seem to have higher PIs than those samples showing reductions in ovum mass without significant change in clutch number. A tally of available PIs from those samples used here (Table 1) and additional ones from Walby Lake (24.4, 1993; 4.1, 2002; 15.2, 2003; Heins et al. Reference Heins, Baker, Toups and Birden2010a ), showed that four of the five PIs were >10·0 in the former group, whereas two of five were >10·0 in the latter group. Among samples showing significant reductions in clutch size and ovum mass, the mean PI was 15·9 (n = 5), whereas the mean for those samples with significant reductions in ovum mass alone was 10·7 (n = 5). Although the difference was in the direction we would expect, there was no significant difference in arcsine-transformed means between the two groups (ANOVA: F = 1·10; d.f. = 1, 8; P = 0·33), possibly because of the small sample sizes available and variation in PIs within each group. Moreover, ecological conditions experienced by the hosts may have had unknown influences on the host response to parasitism (Hall et al. Reference Hall, Becker and Cáceres2007; Smith, Reference Smith2007). Our limited understanding of the influence of PI and ecological conditions on clutch size and ovum mass warrants further investigation of these dynamics in the interplay between host and parasite. In turn, these symbiotic interactions and the environmental influences on them may also provide new insights into the life-history strategy of the three-spined stickleback.
Although there may be years when the populations for which we observed low PIs and the absence of significant differences in mean clutch size experience epizootics leading to greater levels of infection (Heins et al. Reference Heins, Birden and Baker2010b , Reference Heins, Baker and Green2011), long-term observations (D. Heins, personal observation) suggest that infections typically vary at low levels in those lakes where we observed low PIs. Thus, females in these lakes may often show only reductions in ovum mass. At present, we do not know whether ecological factors limit infections by acting at different stages of the parasite's life cycle or whether these populations of sticklebacks have evolved resistance mechanisms (Scharsack et al. Reference Scharsack, Kalbe, Harrod and Gisep2007; de Roij et al. Reference de Roij, Harris and MacColl2011). These populations, therefore, present interesting opportunities for understanding the mechanisms maintaining low levels of infection that appear to have little effect on clutch size.
The impact of parasitism on host fitness through effects of reduced ovum mass is unknown. In experimental feeding trials, differences in food ration did not cause proportional changes in the constituents of eggs (Fletcher and Wootton, Reference Fletcher and Wootton1995; Ali and Wootton, Reference Ali and Wootton1999). This suggests that smaller eggs in infected females may simply have less of each component, if food limitation provides the appropriate model for the effect of the parasite on ovum production in host females. In that case, the impact on host fitness would be expected to result directly from offspring size which is related to ovum mass.
Although infected female sticklebacks in Tern Lake showed a significant decrease in mean size-adjusted clutch size, they did not show significant relationships between residual clutch size and PI, showing that clutch size adjusted for female BM did not vary with parasite burden. Residual clutch size of host females in Walby Lake decreased significantly with PI, revealing a decrease in size-adjusted clutch size with parasite burden. The differences between samples may have been influenced by the sample size and range of PI values in parasitized females. Although female sticklebacks exhibiting fecundity reduction may often show a significant decrease in clutch size as PI increases, they do not always show a significant decrease even when sample sizes and ranges of PI are large (Heins et al. Reference Heins, Baker, Toups and Birden2010a ), perhaps reflecting unknown ecological influences.
Fecundity compensation
Fecundity compensation should be more common among host–parasite systems where parasites cause a complete reduction in reproductive capacity (Minchella and LoVerde, Reference Minchella and LoVerde1981; Krist, Reference Krist2001). It should also occur in cases where parasites increase mortality or reduce fitness late in life (Polak and Starmer, Reference Polak and Starmer1998), as appears to be the case in the stickleback populations studied in Alaska. Evolutionary theory predicts that where parasitism decreases the future reproductive potential of hosts, they should increase their current reproductive output (Williams, Reference Williams1966; Minchella and LoVerde, Reference Minchella and LoVerde1981; Minchella, Reference Minchella1985; Agnew et al. Reference Agnew, Koella and Michalakis2000). Fecundity compensation has been demonstrated in a number of taxa (Heins, Reference Heins2012) and may occur at individual and population levels (Granovitch et al. Reference Granovitch, Yagunova, Maximovich and Sokolova2009). At the individual level, fecundity compensation involves trade-offs in an infected organism's energy budget, whereas ‘population fecundity compensation’ occurs among uninfected hosts, reflecting an evolutionary trade-off in heavily infested populations (Hall et al. Reference Hall, Becker and Cáceres2007; Granovitch et al. Reference Granovitch, Yagunova, Maximovich and Sokolova2009). Fecundity compensation is not a generalized phenomenon (Moore, Reference Moore2002). Therefore, we expect to find populations that did not evolve the non-immunological defence to infection. Investigations in Alaska have demonstrated the phenomenon in two of nine populations surveyed thus far. Thus, fecundity compensation appears to be uncommon among those populations and rare among populations of three-spined sticklebacks throughout the northern hemisphere, whereas fecundity reduction, simply involving diminished reproductive performance as PI increases, seems to be common at least among populations of the three-spined stickleback in Alaska.
Our analyses did not reveal a significant relationship between residual clutch size and PI for Lampert Lake, showing that clutch size adjusted for female BM did not vary with parasite burden. We would not have expected a significant relationship between residual clutch size and PI among host females showing fecundity compensation if females do not continue to elevate CS as the parasite burden increases. In Scout Lake, however, infected females appear to have elevated CS as the parasite burden increased, consistent with the trade-off between clutch size and ovum mass observed by Heins (Reference Heins2012). The difference in our results for the two lakes may reflect the sample characteristics for Lampert Lake (small sample size and restricted range of PI).
ACKNOWLEDGEMENTS
We thank the students from both Clark University and Tulane University who assisted with this study in the field and the laboratory. Mike Bell made specimens from Duck Lake available to us. This investigation was supported by grants from the Newcomb College Institute of Tulane University. We thank a number of landowners who have allowed us safe and convenient access to lakes, especially Scott Willis.