INTRODUCTION
The metazoan phylum Myxozoa today comprises more than 2000 known species. Most of them are endoparasites of fish (Lom and Dyková, Reference Lom and Dykova2006), although some species parasitize amphibians, reptiles (Eiras, Reference Eiras2005), birds (Bartholomew et al. Reference Bartholomew, Atkinson, Hallett, Lowenstine, Garner, Gardiner, Rideout, Keel and Brown2008) and mammals (Friedrich et al. Reference Friedrich, Ingolic, Freitag, Kastberger, Hohmann, Skofitsch, Neumeister and Kepka2000; Prunescu et al. Reference Prunescu, Prunescu, Pucek and Lom2007). Myxozoans are characterized by transmission via multicellular spores that possess nematocyst-like polar capsules which establish attachment to the host after chemical activation and mechanical stimulation (Kallert et al. Reference Kallert, El-Matbouli and Haas2005). The life cycles that have been resolved for most myxozoans involve both vertebrate and invertebrate hosts. The latter are typically annelids for the class Myxosporea (Bütschli, Reference Bütschli1881) and freshwater Bryozoa for the class Malacosporea (Canning et al. Reference Canning, Curry, Feist, Longshaw and Okamura2000). The systematic position of the Myxozoa is still controversial; the most recent molecular phylogenetic study provides strong evidence for affinities to the Cnidaria (Jiménez-Guri et al. Reference Jiménez-Guri, Philippe, Okamura and Holland2007a).
In contrast to the numerous Myxosporea, only 3 malacosporean species have been described. They are found as worm or sac-like parasites in freshwater bryozoans. One of them is Tetracapsuloides bryosalmonae, causing Proliferative Kidney Disease (PKD) in salmonid fish and the other two are Buddenbrockia plumatellae (Schröder, Reference Schröder1910), parasitizing various species of freshwater bryozoans, and the recently described B. allmani, which seems to be host specific to the rare bryozoan Lophopus crystallinus (Tops et al. Reference Tops, Curry and Okamura2005; Hill and Okamura, Reference Hill and Okamura2007; Canning et al. Reference Canning, Curry, Hill and Okamura2007). The existence of further Buddenbrockia species can be assumed according to 18S rDNA and protein encoding sequence data (Tops et al. Reference Tops, Curry and Okamura2005; Jiménez-Guri et al. Reference Jiménez-Guri, Okamura and Holland2007b) differing considerably from the sequences of the 3 species known to date. Differences in spore size and morphology found in 2 worm-shaped Buddenbrockia species from the bryozoan Plumatella repens (Morris et al. Reference Morris, Morris and Adams2002; McGurk et al. Reference McGurk, Morris and Adams2006) and a sac-shaped Buddenbrockia sp. parasitizing Cristatella mucedo (Canning et al. Reference Canning, Okamura and Curry1996) substantiate this assumption.
Among the Malacosporea, the vertebrate host is known only for T. bryosalmonae (Anderson et al. Reference Anderson, Canning and Okamura1999; Feist et al. Reference Feist, Longshaw, Canning and Okamura2001; Morris and Adams Reference Morris and Adams2006). Occasional reports about developmental stages with characteristics of malacosporeans found in pillar cells and endotheliocytes of gills and in cells of the blood vessel endothelium of brain and kidney of common carp (Cyprinus carpio) (Voronin, Reference Voronin1993; Voronin and Chernysheva, Reference Voronin and Chernysheva1993) point out the possible existence of further fish-infecting malacosporeans.
Hill and Okamura (Reference Hill and Okamura2007) found colonies of the bryozoan L. crystallinus raised in the laboratory from dormant sages (statoblasts) to be PCR-positive for malacosporean DNA, indicating possible vertical transfer of these parasites via statoblasts. In contrast, no parasite DNA could be detected in statoblasts from the freshwater bryozoan Fredericella sultana collected from colonies infected with T. bryosalmonae (Grabner and El-Matbouli, Reference Grabner and El-Matbouli2008). It can be assumed that different life-cycle strategies exist within the class Malacosporea and not all malacosporeans may require a vertebrate host. In the present study, we conducted a laboratory infection experiment to identify possible fish hosts for representatives of the genus Buddenbrockia.
MATERIALS AND METHODS
Bryozoa
A variety of bryozoan colonies attached to pieces of wood was collected in October 2008 from a pond (Bronner Weiher, Bavaria, Germany; 48°01′00·00″ N, 10°46′59·65″ E) and kept in a laboratory culture system according to Morris et al. (Reference Morris, Morris and Adams2002) and Grabner and El-Matbouli (Reference Grabner and El-Matbouli2008) at a water temperature of 15°C, which was the same as in the pond at the time of sampling. Macroinvertebrates associated with the bryozoan colonies were removed as far as possible (mostly larvae of caddies flies, chironomids, flatworms, beetle larvae and aquatic mites). Colonies growing side by side on submerged pieces of wood were examined with a dissecting microscope to detect malacosporean infections. By using the key of Wood and Okamura (Reference Wood and Okamura2005), 3 bryozoan species were identified as Plumatella fruticosa, P. repens and C. mucedo. In spite of careful examination of the collected substrata, no other bryozoan species were detected. P. fruticosa was the least abundant species with 4 small colonies of approximately 2–3 cm diameter. Colonies of C. mucedo were also small (1 cm length and 0·5 cm width), but about 10 of them were present on the sampled material. P. repens showed the largest colonies with approximately 2–10 cm in diameter. Individual colonies were often growing together and therefore their number can only be estimated to about 5–8. Overt infection with wormlike malacosporeans was found only in P. repens. One infected zooid containing 2 worms was sampled and frozen at −20°C for DNA extraction.
Floating statoblasts from the P. repens colony containing the malacosporeans were collected and approximately 100 were frozen for subsequent DNA extraction. Approximately 50 statoblasts were germinated to produce new colonies by pipetting them under 2 inverted Petri-dishes floating in a small box with dechlorinated tap water at 20°C to stimulate hatching. Colonies started to grow after about 1 week. Attempts to germinate statoblasts from P. fruticosa and C. mucedo failed.
Fish
Specific pathogen-free (SPF) carp (C. carpio) (6–8 cm) were raised from eggs in our laboratory. SPF young of the year brown trout (Salmo trutta) (5–6 cm) were obtained from a local hatchery and minnow (Phoxinus phoxinus) (4–5 cm) were purchased from a pet shop. All fish were kept in aquaria with flow-through dechlorinated tap water and fed with a commercial diet of fish pellets (BioMar). As the minnow were not certified SPF, liver, kidney and spleen of 6 specimens were prepared for PCR to test for existing malacosporean infections. Additionally, carp from 2 natural ponds (fish farm Wielenbach 47°53′5·00″ N, 11°09′17·00″ E and fish farm Seeshaupt 47°49′4·00″ N, 11°17′14·00″ E) were obtained to test if malacosporeans are also present in other areas.
Fish infection and sampling
Fish (6 carp, 3 minnows and 3 brown trout) were exposed to the above-mentioned collection of bryozoan species including the overtly infected P. repens in a 50 L aquarium. Water was changed (1/3) ever second day. After 1 week, the carp, minnow and trout were separated from the bryozoans and transferred to separate 20 L aquaria with flow-through dechlorinated tap water. During and after the exposure, temperature was adjusted to 18°C ±1°C, and the fish were fed daily with fish pellets (BioMar).
One fish from each species was sampled at 3, 4 and 5 weeks post-exposure (p.e.) and the remaining carp were sampled at 8, 10 and 18 weeks p.e. The fish were euthanized with MS222 anaesthetic (Tricaine-methane-sulphonate; Sigma-Aldrich) and kidney, spleen and liver of all fish sampled after 3 weeks p.e. were excised and frozen at −20°C for DNA extraction. At the remaining time-points, samples of the kidney were taken for DNA extraction and pieces of all inner organs were fixed in 10% neutral buffered formalin for histology. Small pieces of liver, spleen and kidney were fixed in 2·5% glutaraldehyde for electron microscopy. Blood smears and squash preparations of the kidney were prepared from all fish and examined for parasite stages.
After 7 weeks p.e. of fish to field-collected bryozoans, statoblast-raised P. repens were cohabitated for 8 h per day with the exposed carp. This time-point was chosen, as sporogony can be expected according to previous results from T. bryosalmonae (Kent and Hedrick, Reference Kent and Hedrick1986; Morris and Adams, Reference Morris and Adams2008). For the remaining time the colonies were transferred back to the laboratory culture to allow feeding. These colonies were visually examined every second day for the presence of malacosporean stages with a dissection microscope and samples from the tip of a growing branch, comprising 4 to 5 zooids were taken for PCR at 3, 4, 6 and 8 weeks p.e. to the fish. Two carp from each natural pond were dissected and kidney samples were taken for molecular analysis.
DNA Extraction and PCR
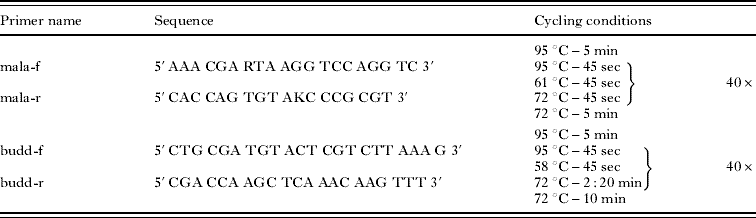
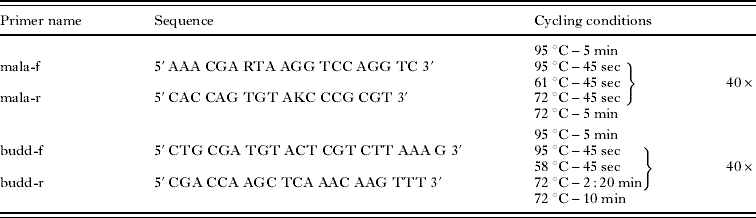
Tissue and statoblast samples were homogenized using a Tissue Lyser® (QIAGEN) and DNA was extracted with a QIAamp® DNA Mini Kit (QIAGEN) following the manufacturer's protocol. For detection of malacosporean parasites, specific primers amplifying a product of about 640 bp of the malacosporean 18S rDNA (mala-f/mala-r; Table 1) were designed by comparison of a variety of myxozoan 18S rDNA sequences from GenBank. Specificity of the mala-f/mala-r primer pair was tested by a BLAST search (Primer-Blast, National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/tools/primer-blast) for homologies with DNA sequences of other species in the database and by PCR with DNA of uninfected fish tissue, DNA from laboratory raised Bryozoa (F. sultana) and DNA of several myxozoans (T. bryosalmonae, B. plumatellae, Myxobolus cerebralis, Henneguya sp., Sphaerospora renicola). For sequencing, a primer-pair (budd f/budd r; Table 1) amplifying a 1784 bp fragment of the malacosporean 18S rDNA was designed. Amplification with both primer pairs was carried out in 20 μl reaction volume containing 10 μl of 2x ReddyMix PCR Master Mix (ABGene), 0·5 μmol of each primer, 1 μl of DNA and 7 μl of distilled water. All PCR-cycling-conditions are given in Table 1. All primers were obtained from Eurofins MWG Operon. For sequencing, the PCR-products were purified with a QIAquick PCR-purification Kit (QIAGEN) and sent to GATC Biotech. Sequences were compared to known sequences in GenBank by a BLAST search (blastN, http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Table 1. Primer-sequences and PCR programs
Phylogenetic analysis
The partial 18S rDNA sequences of malacosporeans were aligned with Clustal W (Thompson et al. Reference Thompson, Gibson, Plewniak, Jeanmougin and Higgins1997), together with the sequences of an unknown malacosporean from F. sultana (GenBank Accession no. AJ937879), of B. allmani (AJ937880), of a sac-like (AJ937881) and a worm-like B. plumatellae (AY074914) and a sequence of T. bryosalmonae (U70623). The resulting multiple alignment was corrected visually for inconsistencies using the BioEdit software v7.0.9 resulting in a final alignment of 1055 bp. Phylogenetic analysis was conducted with the Bayesian inference software MrBayes v3.1.2 (Ronquist and Huelsenbeck, Reference Ronquist and Huelsenbeck2003). Modelgenerator v0.85 (Keane et al. Reference Keane, Creevey, Pentony, Naughton and McInerney2006) was used to determine the evolutionary model for this analysis. A general time reversible model (GTR+I+Γ) with rate variation across sites estimated by a gamma-distribution (4 rate categories) was found to be most appropriate. Two independent runs were conducted with 4 chains and 1 000 000 generations. Trees were sampled every 100 generations. The first 25% of the samples were discarded (burn-in-phase) and a 50% majority-rule consensus tree was created. The resulting tree was visualized with MEGA v4 (Tamura et al. Reference Tamura, Dudley, Nei and Kumar2007).
Light and electron microscopy
The formalin-fixed samples were embedded in paraffin (Tissue-Tek VIP, Sakura Bayer Diagnostics). Sections were cut at 5 μm (1140 Autocut, Reichert-Jung) and either stained with haematoxylin and eosin (H&E) or processed for immunohistochemistry (IHC). For IHC staining, the T. bryosalmonae-specific monoclonal antibody P01 (Aquatic Diagnostics LTD) was used according to the manufacturer's instructions. Sections were counter-stained with haematoxylin. Kidney samples from a SPF carp and a PKD-infected rainbow trout were used as negative and positive control respectively.
Samples for electron microscopy were fixed in 2·5% glutaraldehyde in Soerensen buffer pH 7·4, post-fixed in 1% osmium tetroxide and embedded in Epon resin. Semi- and ultra thin sections were cut with a Reichert-Jung Ultracut microtome. Semi-thin sections were stained with methylene blue. Ultra-thin sections were contrasted with lead citrate and uranyl acetate and analysed on a Zeiss EM 10. Blood smears were stained with Giemsa.
RESULTS
Primer specificity
The specificity tests with the mala-f/r primers amplified the intended template of the 18S rDNA of T. bryosalmonae and Buddenbrockia sp. and did not result in amplification of DNA from uninfected fish, bryozoans or the myxozoans tested. No unintended matches were found in BLAST-search. The budd f/r primer pair successfully amplified the desired parasite DNA sequence and did not produce any unspecific bands.
Bryozoa
Two days after transfer to the laboratory, an overt malacosporean infection was observed in 3 zooids of P. repens in a colony comprising several hundred individual zooids. The morphology of the parasite sacs was similar to those described previously for worm-shaped B. plumatellae (Canning et al. Reference Canning, Tops, Curry, Wood and Okamura2002; Morris et al. Reference Morris, Morris and Adams2002; McGurk et al. Reference McGurk, Morris and Adams2006). The worms showed fast bending and coiling movements. After 2 weeks in the laboratory most of the bryozoans used for fish infection died and no more malacosporean stages were found in the remaining zooids by visual inspection with a dissection microscope. The DNA extracted from the infected zooid was amplified with the budd f/r primer pair. BLAST search of the resulting product (1674 bp; GenBank Accession no. FJ939289) showed highest similarity (99·5%) to B. plumatellae (AY074914).
No malacosporean DNA could be detected by PCR with the mala f/r-primers in the statoblasts collected from the infected colony. About 50% of the P. repens statoblasts germinated and colonies developed. The sample from those colonies was PCR positive after cohabitation with exposed carp for 3 weeks. All further samples taken from the exposed bryozoan colonies at 4, 6 and 8 weeks p.e. to the carp gave a negative result with PCR. No overt infection of the exposed bryozoans could be observed visually within this period.
Fish infection
The PCR and sequencing results are summarized in Table 2. No malacosporean DNA was detected in any of the 6 minnows tested before the infection experiment. None of the fish cohabitated with infected Bryozoa showed any external symptoms or any abnormities in inner organs during the course of the trials. DNA from liver, spleen and kidney of the carp sampled after 3 weeks p.e. showed the expected band on an agarose gel after PCR-amplification with the mala f/r primers. Among the 3 organs, the strongest band was detected in the kidney sample. Of the samples taken from brown trout and minnow after 3 weeks p.e., only the kidney samples were PCR positive. For the carp sampled after 3 weeks p.e., the PCR-product from liver tissue was also sequenced to test if the parasite found there was identical to that in the kidney. All further kidney samples from carp were PCR positive and all sequences obtained from this fish species were 100% identical. The minnow sampled at 4 weeks p.e. was also tested positive by PCR, while the kidney of the minnow sampled 1 week later was negative. Sequencing showed that the two sequences from minnow were 100% identical. The remaining brown trout were tested negative by PCR. Surprisingly, the sequenced segments of the 18S rDNA of the parasites from carp (1626 bp; FJ939290) and minnow (1679 bp; FJ939291) differed considerably (3·7%). The sequence obtained from kidney of minnow was 99·5% similar to a B. plumatellae 18S rDNA sequence from France (AY074914), and 100% similar to the sequence from the Malacosporea-infected P. repens collected in the present study. The sequence of the parasite from carp was most similar to the sequence AJ937880 of B. allmani (97·8%) and identical to the sequence obtained from the carp-exposed P. repens.
Table 2. Sequencing and histology results of sampled fish
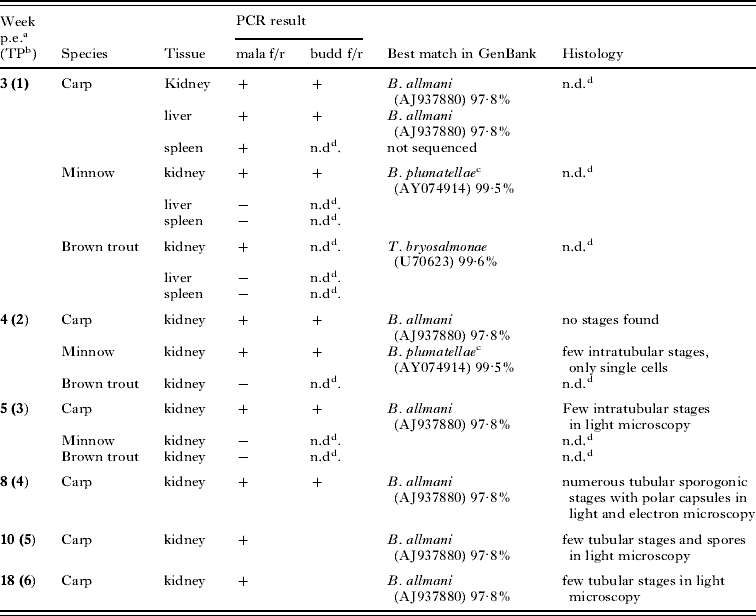
a Weeks post-exposure to infected bryozoans.
b TP: time-point.
c Sequences obtained from minnow were 100% identical to that from the Buddenbrockia sp. in P. repens sampled in the present study.
d n.d.: not determined.
+ Positive PCR-result.
− Negative PCR-result. Note: no parasite stages were detected by histology in spleen and liver of fish sampled after 3 weeks p.e.
The sequence (569 bp) obtained from brown trout (FJ939294) was 99·6% similar to a sequence of T. bryosalmonae in GenBank (U70623), indicating that some of the bryozoans collected in the present study must have been infected with T. bryosalmonae, which was transmitted to exposed brown trout. Of the 4 carp from the natural ponds, 3 tested positive for Malacosporea by PCR (pond 1; 989 bp: FJ939292; pond 2; 1087 bp: FJ939293). The sequences of these isolates were 100% similar to those obtained from the laboratory-infected carp.
Phylogenetic analysis
The 6 Buddenbrockia-sequences used for phylogenetic analysis were very similar. Therefore, the number of phylogenetically informative sites was low and the placement of the different species was not resolved properly (Fig. 1). Nevertheless, the genetic separation of sac-like and worm-like B. plumatellae was supported by high posterior probability. The worm-like B. plumatellae from France (GenBank Accession no. AY074914) and the worm-like malacosporean from P. repens infecting minnows found in the present study were grouped together closely. In this consensus-tree, the carp-parasite clusters with the B. plumatellae clade, but with low support of only 0·59. Also, the placement of the unknown Buddenbrockia sp. from F. sultana (AJ937879) and B. allmani (AJ937880) is not reliable and therefore these branches were collapsed (Fig. 1).

Fig. 1. Molecular phylogeny of the genus Buddenbrockia. Results of Bayesian analysis are shown in a 50% majority-rule consensus tree with T. bryosalmonae as an outgroup. Posterior probabilities are given at the nodes. Respective GenBank Accession numbers are given behind the species names in parentheses. Asterisks indicate species from the present study. If it was necessary to distinguish the Buddenbrockia species, the respective host or an explanation is given behind the genus name.
Light and electron microscopy
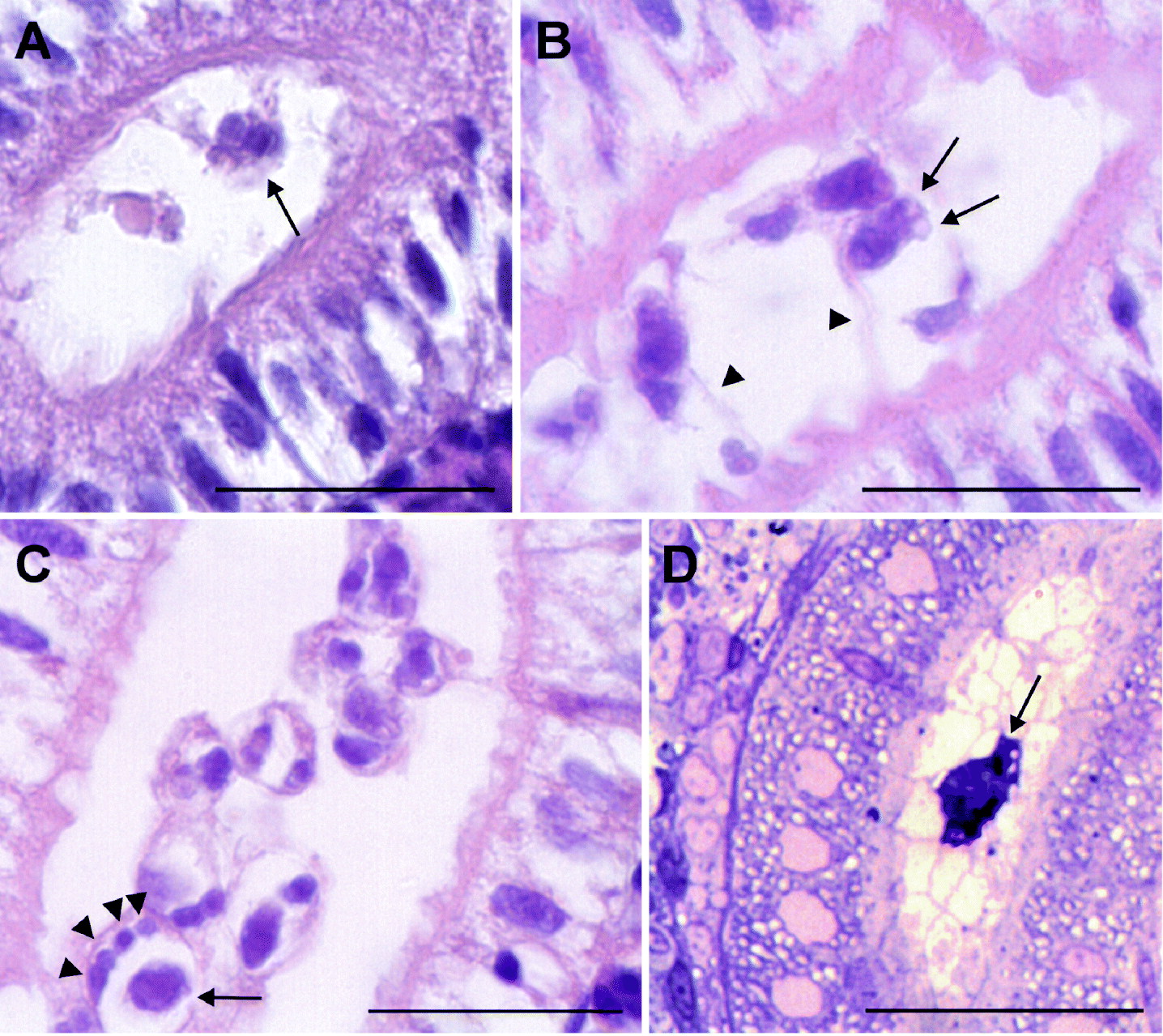
Malacosporean stages were first observed in H&E-stained paraffin and Epon semi-thin sections of organ samples from carp at 5 weeks p.e., when several intratubular stages were visible in the kidney-sections of the carp (Fig. 2A). At 8 weeks p.e., sporogonic stages were seen in light and electron microscopy of the carp kidney including maturing, elongated spores with 2 polar capsules (Fig. 2B). Longitudinal sections of infected kidney tubule segments revealed numerous adjacent groups of parasite cells in different stages of sporogony. Up to 4 small and 1 larger cell could be found within one primary cell (Fig. 2C). Processes were observed that seemed to attach the parasite cell via the tubular epithelium (Fig. 2B). In the carp sampled 10 and 18 weeks p.e., only a few stages similar to those seen at 8 weeks p.e. were found in the kidney tubules.
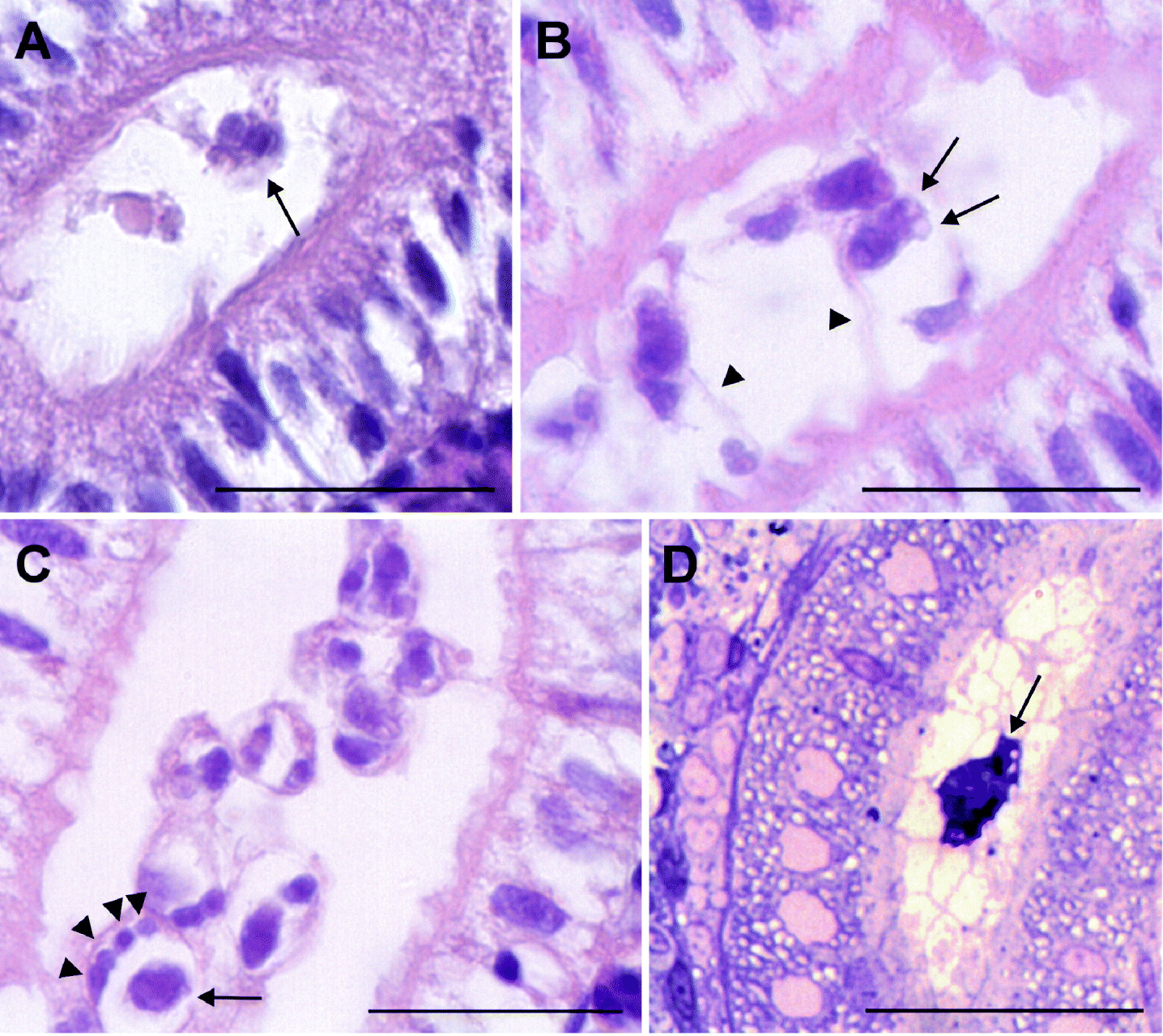
Fig. 2. Light microscopy of parasite stages in kidneys of carp and minnow. (A) Paraffin section of sporogonic stage (arrow) in kidney tubule of carp sampled at 5 weeks p.e. H&E stain. (B) Paraffin section of sporogonic stages in kidney tubule of carp sampled 8 weeks p.e. One maturing spore with developed polar capsules is shown (arrows). The parasite cells are connected to the tubular epithelium by cytoplasmic processes (arrowheads). H&E stain. (C) Paraffin section of sporogonic stages in kidney tubule of carp sampled at 8 weeks p.e. Four small cells (arrowheads) and one larger cell (arrow) can be seen in one of the primary cells. H&E stain. (D) Semi-thin section of parasite stage in kidney tubule of minnow sampled 4 weeks p.e. (arrow). Methylene blue stain. Scale bar=25 μm.
Only samples that tested positive by PCR for malacosporeans were examined histologically (4 weeks p.e.). Very few intraluminal, single cell stages were observed in semi-thin sections of kidney samples (Fig. 2D). Pre-sporogonic parasite stages outside the kidney tubules or in samples of liver and spleen of both carp and minnow were not observed. No IHC staining of Buddenbrockia-stages using the monoclonal antibody specific for T. bryosalmonae could be achieved. Parasite stages were not detected in squash preparations or in blood smears.
In electron microscopy, sporogonic stages were found in kidney tubule sections of the carp sampled 8 weeks p.e. Stages of different maturation were seen within kidney tubules consisting of a primary cell enclosing the sporogonic cells thereby forming a pseudoplasmodium. The sporogonic cells comprised one S-T doublet (secondary and tertiary cell) and a group of secondary cells without tertiary cells (Fig. 3A). Analogous to the light microscopic observations, the greatest number of secondary cells seen in electron microscopy was 5 (Fig. 3B). Developing parasite cells seemed to anchor to microvilli of tubular epithelial cells by pseudopodia-like processes (Fig. 3C). Two capsulogenic cells forming 1 polar capsule each developed surrounded by a secondary cell (Fig. 3D). In the most mature spore stage found, 1 sporoplasm cell bearing sporoplasmosomes and 1 of the capsulogenic cells could be seen. Valvogenic cells surrounded almost the entire spore (Fig. 3E). Maturing polar capsules with polar filament were visible in some sections (Fig. 3F).

Fig. 3. Electron micrographs of malacosporean stages in kidney tubules of carp at 8 weeks p.e. (A) Pseudoplasmodium: primary cell (P) enclosing secondary (S) and tertiary (T) cells. Scale bar=2 μm. (B) Pseudoplasmodium with 5 secondary cells (S) visible within the primary cell (P). Scale bar=2 μm. (C) Pseudoplasmodium with primary (P) and secondary cell (S) showing pseudopodia-like processes reaching between the microvilli of the tubular epithelium (arrows). Scale bar=2 μm. (D) Two capsulogenic cells (C) with polar capsules (arrows) inside a secondary cell (S). Two more secondary cells without tertiary cells. Scale bar=3 μm. (E) Maturing spore with sporoplasm cell (SP) and one capsulogenic cell (C). The polar capsule has broken out of the section (asterisk). Valve cells (V) surrounding the sporoplasm and a part of the capsulogenic cell. Scale bar=2 μm. (F) Capsulogenic cells (C) with polar capsules; turns of the polar filament are visible. Scale bar=1 μm.
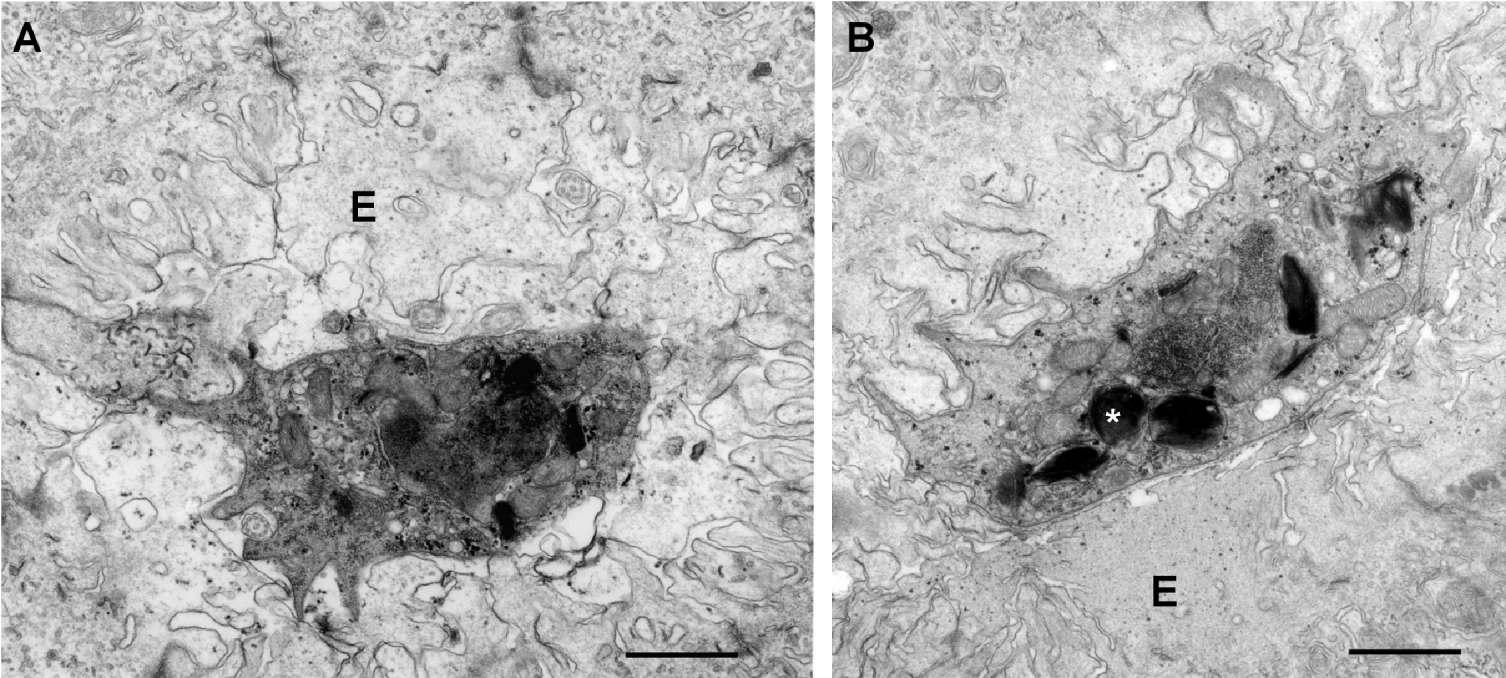
In contrast to those from carp, stages from minnow were only detectable up to 4 weeks p.e. At this time only a few stages, apparently early sporogonic stages, were present in kidney tubules. No secondary cells were observed (Fig. 4A). Some of these cells contained numerous vacuoles containing bundles of electron-dense material. Cytoplasmic processes of parasite cells seemed to enclose host material (Fig. 4B). For both carp and minnow, no pre-sporogonic stages in the kidney interstitium or in liver and spleen were observed. The results obtained by histological examination are summarized in Table 2 for each individual fish.

Fig. 4. Electron micrographs of malacosporean stages in kidney tubules of minnow. (A) Tubular single-cell parasite stage. Pseudopodia-like processes enclose host material. E: epithelial cells of kidney tubule. Scale bar=1 μm. (B) Tubular stages with vacuoles filled with stacks of electron-dense material (asterisk). E: epithelial cells of kidney tubule. Scale bar=1 μm.
DISCUSSION
Developmental stages of Buddenbrockia species have only been described from the bryozoan host, though Voronin (Reference Voronin1993) and Voronin and Chernysheva (Reference Voronin and Chernysheva1993) provided some indication that malacosporeans, other than T. bryosalmonae, parasitize fish by reporting malacosporean-like developmental stages in endothelial and pillar cells of gills, brain and kidney of common carp. The laboratory infection experiments conducted in the present study provide evidence that cyprinid fish can act as a vertebrate host for 2 species of the genus Buddenbrockia. Our infection experiment and molecular analyses indicate that the worm-like parasite in the bryozoan P. repens was able to infect minnow and is genetically identical to B. plumatellae. As the attempts to transmit this parasite from infected to non-infected bryozoans in the laboratory had failed previously (Tops et al. Reference Tops, Baxa, Mcdowell, Hedrick and Okamura2004), the finding of a susceptible fish host for B. plumatellae might be the key to elucidate the whole life cycle of this parasite. Nevertheless, it has to be noted that the minnows used for the present study were not SPF. Although the 6 fish of this population tested for malacosporean infections before the experiments were all negative, the chance remains that some of the experimental fish might have been infected before. Additionally, the number of minnows and brown trout used was low, and allows only preliminary conclusions.
The sequences obtained from infected carp differed considerably from those detected in minnows and the malacosporean sequences in GenBank. Most similar was B. allmani (97·8% 18S rDNA sequence identity), although this species is known to parasitize the bryozoan L. crystallinus (Canning et al. Reference Canning, Curry, Hill and Okamura2007), which was not present in the sample of bryozoans used for fish infection in our study. This indicates that the malacosporean parasite from carp is likely an undescribed species.
While T. bryosalmonae is capable of infecting a wide variety of salmonids (Hedrick et al. Reference Hedrick, MacConnell and de Kinkelin1993), the Buddenbrockia spp. investigated in the present study seemed to be more specific for their fish hosts, though only 2 minnows were infected and a mixed infection of the fish with more than one malacosporean can not be ruled out completely. Nevertheless, detection of the same malacosporean in all 6 exposed carp and not in minnow or brown trout provides at least some evidence that this malacosporean is more infective for carp than for the other 2 species tested. The infection with the same malacosporean detected in carp from 2 other locations and the late sporogonic stages found in the kidney tubules of infected carp provide evidence that this fish species might be the appropriate host for the undescribed Buddenbrockia sp. found in the present study. A bryozoan-derived stage of the carp-infective Buddenbrockia sp. was not observed, probably because of low prevalence of overt infections in the bryozoans collected from the field. Buddenbrockia spp. were found repeatedly in colonies of P. repens and morphological and size differences between spores from worm-like malacosporeans observed in P. repens have been reported. Morris et al. (Reference Morris, Morris and Adams2002) found ornamented spores with a mean size of 19·0 μm, whereas the spores observed in the study of McGurk et al. (Reference McGurk, Morris and Adams2006) were spherical and measured 17·7 μm in diameter. These findings indicate the presence of at least 2 more Buddenbrockia species parasitizing this bryozoan. Therefore it was tested in the present study, if this bryozoan might be the host for the carp-malacosporean. However, no successful transmission from infected carp to bryozoans could be proven so far. Statoblast-raised P. repens were tested positive for malacosporean DNA by PCR after 3 weeks of cohabitation with infected carp. This shows, that either parasite DNA or possibly spores were released from fish and attached to the outer surface of bryozoans, were taken up with food particles, or penetrated the zooids. As the carp-cohabitated bryozoans sampled at later time-points were all negative by PCR and no overt malacosporean infection developed in the exposed colonies, it is most likely that the Buddenbrockia sp. infectious for carp is specific to another species of bryozoans. Spores either could not enter P. repens or penetrated stages were destroyed by host defence mechanisms. Although 100 statoblasts collected from an infected colony of P. repens were tested by PCR for dormant stages of malacosporeans, it might still be possible that a cryptic infection was present in the statoblasts used for germination. P. fruticosa or C. mucedo were also found in the respective pond. Malacosporean infections had already been detected in the latter species as sac-like Buddenbrockia sp. (Canning and Okamura, Reference Canning and Okamura2004; Tops et al. Reference Tops, Curry and Okamura2005). To our knowledge, no malacosporeans have been found in colonies of P. fruticosa so far. One of those species might be the host for the malacosporean detected in carp. Also, some aquatic invertebrates were present on substrata and between the bryozoan colonies and could not be removed completely. It was shown that T. bryosalmonae requires only bryozoans and fish for completion of its life-cycle, but it is possible that other invertebrates may be required as intermediate hosts in the life-cycles of other malacosporeans. Further investigations are needed to find the invertebrate-host for the carp-malacosporean.
The findings of our study show that at least 3 malacosporean species coexist in a single pond, as T. bryosalmonae, worm-shaped B. plumatellae and the undescribed Buddenbrockia sp. infecting carp were present. Taking into account the worldwide distribution of freshwater bryozoans and the various species in which malacosporean infections have already been detected (Canning and Okamura, Reference Canning and Okamura2004), it can be speculated that a substantially higher number of unknown malacosporean parasites must exist.
The sporogonic stages found in histological sections of infected carp kidneys were similar to stages described previously for T. bryosalmonae (see Hedrick et al. Reference Hedrick, MacConnell and de Kinkelin1993 for review). Also, the ultrastructure of the luminal stages and the formation of spores in a primary cell (pseudoplasmodium) resembled, to a great extent, the development of T. bryosalmonae in fish kidney (Kent and Hedrick, Reference Kent and Hedrick1986; Morris and Adams, Reference Morris and Adams2008). It was observed that the capsulogenic cells form surrounded by a secondary cell, but the fate of the further 4 secondary cells found in the pseudoplasmodia is not clear. Morris and Adams (Reference Morris and Adams2008) reported similar cells in pseudoplasmodia of T. bryosalmonae where they form the valves of the spore and it is likely that this is also the case for the carp parasite. The origin of the sporoplasm cell remains unknown. Morris and Adams (Reference Morris and Adams2008) observed a sporoplasmogenic cell developing from the cell doublet to be forming the capsulogenic cells. This was not found in the present study as no intermediate stages were observed within this part of the developmental sequence. Few intratubular stages in kidney of carp were found already after 5 weeks p.e. in the present study, but the peak of intratubular development and sporogony seemed to be at 8 weeks p.e. This timing is similar to what was described previously for T. bryosalmonae. Kent and Hedrick (Reference Kent and Hedrick1986) detected first intratubular stages of T. bryosalmonae in rainbow trout kept at 15–18°C at 7 weeks p.e. and also in brown trout numerous intratubular stages were found at 7 weeks p.e. (Morris and Adams, Reference Morris and Adams2008).
Little information could be obtained about the development of the minnow parasite, as only a few single parasite cells were found. Further studies are needed to determine whether minnows are appropriate hosts for B. plumatellae and to describe the development of this parasite in the fish host in more detail. Surprisingly, pre-sporogonic stages were not observed by light or electron microscopy in organs of Buddenbrockia-infected carp or minnow, therefore the proliferative phase of these parasites remains enigmatic. The malacosporean-like intracellular stages in common carp observed by Voronin (Reference Voronin1993) and Voronin and Chernysheva (Reference Voronin and Chernysheva1993) might represent such early stages of a Buddenbrockia sp. that were not observed in the present study. An aim of future studies will be to describe the complete development of this carp malacosporean in fish, including the pre-sporogonic stages and the cellular details of sporogony.
Phylogenetic analysis did not fully clarify the relationship of the different Buddenbrockia species. However, the results support the findings of Tops et al. (Reference Tops, Curry and Okamura2005) and Jiménez-Guri et al. (Reference Jiménez-Guri, Okamura and Holland2007b) that sac-shaped and worm-shaped B. plumatellae belong to 2 different clades. The placement of the undescribed Buddenbrockia sp. from F. sultana and B. allmani (Tops et al. Reference Tops, Curry and Okamura2005; Canning et al. Reference Canning, Curry, Hill and Okamura2007) remained unclear. Our carp malacosporean placed as a sister-group to the B. plumatellae-clade, but the low node-probability makes this placement questionable. As common carp were spread by aquaculture activities in Europe in medieval times (Balon, Reference Balon1995), the parasite might have been spread too, or a European Buddenbrockia sp. adapted to the new fish host. A screening of indigenous cyprinids for malacosporean infections and phylogeographic studies are required to clarify the history and origin of this parasite.
The results of the present study suggest that like T. bryosalmonae, malacosporean parasites of the genus Buddenbrockia possess a life cycle involving a fish host. According to the sequence data, there may exist a further Buddenbrockia species to those already described, further enhancing diversity of the class Malacosporea. An aim of future studies will be to find the susceptible fish species that can transmit malacosporean parasites of the genus Buddenbrockia to bryozoans.
ACKNOWLEDGEMENTS
The authors thank Edit Eszterbauer for help with the phylogenetic analysis and Dennis Kallert and Sho Shirakashi for revising the manuscript.