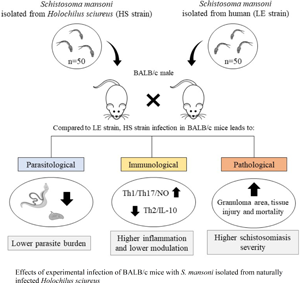
Introduction
Schistosomiasis is a neglected tropical disease that affects approximately 250 million people worldwide, causing severe morbidity and mortality in humans living in endemic areas, and Schistosoma mansoni is one of its main aetiologic agents (Gryseels et al., Reference Gryseels, Polman, Clerinx and Kestens2006; Weerakoon et al., Reference Weerakoon, Gobert, Cai and McManus2015; McManus et al., Reference McManus, Dunne, Sacko, Utzinger, Vennervald and Zhou2018; WHO, 2020).
Parasite burden and host immune response dictate the pathology of schistosomiasis (De Jesus et al., Reference De Jesus, Silva, Santana, Magalhães, de Jesus, de Almeida, Rêgo, Burattini, Pearce and Carvalho2002; Abath et al., Reference Abath, Morais, Montenegro, Wynn and Montenegro2006; Colley and Secor, Reference Colley and Secor2014). It is known that during the first 4 weeks of infection, characterized as a pre-patent acute phase, the Th1 immune response is predominant, which is associated with increased production of pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-2, IL-6, IL-12, tumour necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) that stimulate the classic activation of macrophages to an M1 profile (Coulson et al., Reference Coulson, Smythies, Betts, Mabbott, Sternberg, Wei, Liew and Wilson1998; Pearce and Macdonald, Reference Pearce and MacDonald2002; Dunne and Cooke, Reference Dunne and Cooke2005; Colley and Secor, Reference Colley and Secor2014). The severity of the disease at this stage depends on the intensity of the inflammatory response (Brunet et al., Reference Brunet, Finkelman, Cheever, Kopf and Pearce1997; Hoffmann et al., Reference Hoffmann, Cheever and Wynn2000). After the adult worms begin oviposition (postural acute phase), the immune response is strongly polarized towards a Th2 profile, mainly via the stimulation by soluble egg antigens (SEA). This new immunological environment leads to an increase in the production of IL-4, IL-5, IL-9, IL-13 and chemokines such as CCL11 and CCL24, which stimulate eosinophilia, differentiation of alternatively activated macrophages (M2), and production of immunoglobulin (Ig)-E (Dunne et al., Reference Dunne, Butterworth, Fulford, Kariuki, Langley, Ouma, Capron, Pierce and Sturrock1992; Cheever et al., Reference Cheever, Hoffmann and Wynn2000; Pearce and Macdonald, Reference Pearce and MacDonald2002; Pearce et al., Reference Pearce, Kane, Sun, Taylor, McKee and Cervi2004; Burke et al., Reference Burke, Jones, Gobert, Li, Ellis and McManus2009). The Th2 immune response can balance the pro-inflammatory immune profile of the early stages of the disease, and is responsible for the formation of granulomas around the parasite eggs trapped in the host tissues (Brunet et al., Reference Brunet, Finkelman, Cheever, Kopf and Pearce1997; Hams et al., Reference Hams, Aviello and Fallon2013).
The granuloma response is essential for sequestering parasite antigen and repairing the tissue damage caused by lytic substances secreted from the parasite eggs; however, if the granulomas are not well modulated by the regulatory immune response of the host, they may evolve into large areas of fibrosis, leading to severe cases of chronic schistosomiasis (Lenzi et al., Reference Lenzi, Kimmel, Schechtman, Pelajo-Machado, Romanha, Pacheco, Mariano and Lenzi1998; Cheever et al., Reference Cheever, Hoffmann and Wynn2000; Hams et al., Reference Hams, Aviello and Fallon2013; Schwartz and Fallon, Reference Schwartz and Fallon2018). In this chronic phase, the increase in host modulatory immune response reduces type 1 and 2 responses, which can minimize the severity of the disease because it decreases the initial damage produced by the type-1 response and also reduces the granuloma size and fibrosis detected later in the infection (Booth et al., Reference Booth, Mwatha, Joseph, Jones, Kadzo, Ireri, Kazibwe, Kemijumbi, Kariuki, Kimani, Ouma, Kabatereine, Vennervald and Dunne2004; Hesse et al., Reference Hesse, Piccirillo, Belkaid, Prufer, Mentink-Kane, Leusink, Cheever, Shevach and Wynn2004; Lundy and Lukacs, Reference Lundy and Lukacs2013). However, the modulatory response also favours parasite survival in the host circulatory system (Hesse et al., Reference Hesse, Piccirillo, Belkaid, Prufer, Mentink-Kane, Leusink, Cheever, Shevach and Wynn2004; Angeles et al., Reference Angeles, Mercado and Rivera2020). Therefore, the balance of protective or harmful role of Schistosoma-induced granulomas can influence the morbidity of schistosomiasis, and different parasite strains can disrupt this homoeostasis and affect disease severity.
The occurrence of new strains of S. mansoni may be facilitated by the ability of this parasite to infect different vertebrate hosts in addition to humans. Non-human primates (Nelson, Reference Nelson1960; Standley et al., Reference Standley, Mugisha, Verweij, Adriko, Arinaitwe, Rowell, Atuhaire, Betson, Hobbs, van Tulleken, Kane, van Lieshout, Ajarova, Kabatereine and Stothard2011), cattle (Barbosa et al., Reference Barbosa, Barbosa and Arruda1962; Modena et al., Reference Modena, dos Santos Lima and Coelho2008) and some wild rodent species have already been found to be naturally infected with this parasite (Théron et al., Reference Théron, Pointier, Morand, Imbert-Establet and Borel1992; Rey, Reference Rey1993; Alarcón de Noya et al., Reference Alarcón de Noya, Pointier, Colmenares, Théron, Balzan, Cesari, González and Noya1997; Hanelt et al., Reference Hanelt, Mwangi, Kinuthia, Maina, Agola, Mutuku, Steinauer, Agwanda, Kigo, Mungai, Loker and Mkoji2010; Miranda et al., Reference Miranda, Miranda, Rodrigues, Lira, Nogueira, Viegas-Melo and Silva-Souza2017; Catalano et al., Reference Catalano, Sène, Diouf, Fall, Borlase, Léger, Bâ and Webster2018). Successive infections in these animals may allow the emergence of new parasite strains with different levels of adaptability and virulence (D'Andrea et al., Reference D'Andrea, Maroja, Gentile, Cerqueira, Maldonado Júnior and Rey2000; Colley and Loker, Reference Colley and Loker2018). Indeed, S. mansoni strains and genotypes have been found in parasites isolated from naturally infected Rattus rattus rodents on the island of Guadeloupe (Barral et al., Reference Barral, Morand, Pointier and Théron1996; Sire et al., Reference Sire, Durand, Pointier and Théron1999). In addition, the same S. mansoni genotypes were isolated from both humans and naturally infected wild rodents from areas endemic for schistosomiasis in Africa, indicating the occurrence of zoonotic transmission (Catalano et al., Reference Catalano, Léger, Fall, Borlase, Diop, Berger, Webster, Faye, Diouf, Rollinson, Sène, Bâ and Webster2020). However, even with these important findings, the possible impact of S. mansoni strains or genotypes on the pathology of schistosomiasis is still mostly unknown.
In Brazil, wild rodents such as Holochilus sciureus and Nectomys squamipes are known to be naturally infected by S. mansoni (Rey, Reference Rey1993; Gentile et al., Reference Gentile, Barreto, Gonçalves, Soares, D'Andrea and Rokni2012; Miranda et al., Reference Miranda, Miranda, Rodrigues, Lira, Nogueira, Viegas-Melo and Silva-Souza2017), but the role of these animals in the epidemiology and transmission of schistosomiasis remains unclear. Previous studies have shown that S. mansoni isolates obtained from H. sciureus have high pathogenicity in Biomphalaria snails (Bastos et al., Reference Bastos, Silva, de Souza, Lemos Neto and Piedrabuena1982). Moreover, there is evidence of antigenic variation in parasites isolated from infected H. sciureus (Carneiro et al., Reference Carneiro, Bastos, Neto, Liance, Picot and Houin1991). However, the impact of S. mansoni strains isolated from wild rodents on the severity of schistosomiasis remains poorly investigated in the human population and mice models. In this study, we used experimentally infected BALB/c mice to comparatively evaluate the parasitological and immunopathological aspects of the infection with S. mansoni isolated from the naturally infected H. sciureus and from an infected patient.
Materials and methods
Parasite strains and mice infection
The Holochilus sciureus strain (HS strain) of S. mansoni was originally isolated between 2017 and 2018 from naturally infected H. sciureus rodents captured from the municipalities of São Bento and Peri Mirim, State of Maranhão, Northeastern Brazil (Do Carmo-Silva et al., Reference do Carmo-Silva, Teles-Reis, Silva-Soares, Rodrigues, Lira, Nogueira, Viegas-Melo, Cardoso, Miranda and Silva-Souza2019), and this parasite strain has maintained through successive infections (3–4 generations) of BALB/c mice and B. glabrata snails of SB strain (collected in the same capture area of H. sciureus). The human strain of S. mansoni, designated as the LE strain, was isolated in 1968 from a chronically infected patient living in the city of Belo Horizonte, State of Minas Gerais, Southeastern Brazil (Pellegrino and Katz, Reference Pellegrino, Katz and Dawes1968). This parasite strain has been maintained through successive infections in hamsters (Mesocricetus auratus) and B. glabrata snails from the BH strain since then (the exact number of generations is unknown). Both parasite strains are routinely maintained at the Schistosomiasis and Immunohelminthology Laboratory of the Institute of Biological Sciences (ICB) at the Federal University of Minas Gerais (UFMG).
Male BALB/c mice, aged 6–8 weeks (~20 g), acquired from an established colony at the University's mouse facility (CEBIO-ICB, UFMG, Brazil), were used as a vertebrate experimental model of S. mansoni infection in the current experiments. During the experiment, animals were kept in microisolators accommodated in ventilated racks (Alesco, São Paulo, Brazil) and provided with standard chow (Presence, Primor, Brazil) and tap water ad libitum. A total of 50 viable cercariae obtained from B. glabrata snails that were previously infected with the LE or HS strain, diluted in a maximum volume of 500 μL of saline solution (0.9% NaCl), were inoculated subcutaneously into each animal in the experimental groups. The experimental procedures were approved by the Ethics Committee on Animal Use (protocols n°46/2019 and n°368/2018) of the Federal University of Minas Gerais (UFMG, Brazil).
Experimental design
In each experimental procedure, we evaluated 3 groups: the control group, composed of non-infected BALB/c mice; the LE group, comprising BALB/c mice infected with S. mansoni from the LE strain; and the HS group, with BALB/c mice infected with S. mansoni from the HS strain. For each experimental group and each time point, at least 7–10 mice were evaluated, and the experiments were repeated at least once to confirm the results. The experimental animals were examined weekly for 12 weeks to monitor variations in weight and mortality and to quantify the number of eggs eliminated in the feces (infected groups only). For detailed parasitological evaluation, 7–10 infected mice from each experimental group were euthanized at 8 (acute schistosomiasis) and 12 (chronic schistosomiasis) weeks after the infection. At these time points, before euthanasia, the feces of each infected mouse were individually collected and processed for the detection of occult blood and quantification of parasite eggs. Then, each mouse was intraperitoneally anaesthetized (ketamine, 80 mg kg−1; xylazine, 10 mg kg−1) to draw blood samples from the axillary vascular plexus with anticoagulants. Whole blood was used to measure the haemoglobin concentration and red blood cell count, while the plasma of these blood samples was used to estimate transaminase activity. After exsanguination and cervical dislocation, each animal had the portal circulation perfused to recover adult worms, and the lungs, liver, spleen and intestines were removed and processed to estimate the number of eggs retained in each organ.
Since the parasitological analyses destroy the host tissues, a second experiment was performed in parallel, using the same experimental groups and infection doses, to evaluate immunological and pathological alterations. For the immunopathological analysis, we also used 7–10 mice/group/time and the experimental procedure was repeated at least once. The experimental animals were also evaluated weekly for weight and mortality assessment and euthanized after 8 and 12 weeks of infection. Just before euthanasia, fecal samples were collected from each mouse to confirm the infection and blood samples to realize another evaluation of haemogram and transaminase activity. For the immunopathological analysis, the liver of each experimental animal was removed and its lobes were separated and processed, as follows: the left lobe was preserved in a fixative solution for histopathological analysis, while the bilobed and caudate lobes were stored at −20°C and used to estimate the tissue levels of cytokine, nitric oxide and the enzymatic activity of eosinophil peroxidase (EPO), myeloperoxidase (MPO), N-acetyl-β-d-glucosaminidase (NAG) and arginase, as indirect markers of cellular recruitment and activation. The quadrate lobe was also preserved at −20°C and used to estimate the hydroxyproline content, as an indicative marker of collagen deposition. The small intestine was also recovered and divided into a proximal portion (duodenum) to quantify hydroxyproline content; a medial portion (jejunum) to measure enzymatic activity; and a distal portion (ileum). The distal portion was divided in half: the first half was used to assess cytokine concentration and the other was used for histopathological evaluation.
Clinical evaluation
Weight variation and mortality
Each mouse from each group had its individual bodyweight measured weekly using an analytical electronic scale (Analytical Balance Scale, New Jersey, USA). Variations in weight (gain/loss) were calculated by subtracting each mouse's weight on day 0 (before infection) from its current weight (Finlay et al., Reference Finlay, Liu, Ermel and Adamson2015). For mortality curve assessment, groups of infected mice and controls were observed weekly for 12 weeks. Animals that showed extreme cachexia, dyspnoea, piloerection and signs of apathy were euthanized and included in the mortality curve.
Red blood cells and haemoglobin
After anaesthesia, the whole blood of each animal was collected from the axillary plexus vessels using a Pasteur pipette embedded in EDTA (Gibco, Invitrogen Corporation, New York, USA) and placed in 5 ml tubes (K3 – KASVI, Paraná, Brazil). Blood samples were analysed using an automatic blood counter (Bio-2900 Vet, Bioeasy, USA) to assess the number of red blood cells (mm3) and haemoglobin concentration (g dL−1).
Parasite burden
Fecal samples were processed and the number of eggs per gram of feces was estimated according to Negrão-Corrêa et al. (Reference Negrão-Corrêa, Souza, Pinho, Barsante, Souza and Teixeira2004), as described below. The fecal material of each experimentally infected mouse was weighed (Bioprecisa, FA2104N, Curitiba, Brazil) and homogenized in 5 mL of saline solution containing 10% formaldehyde. For counting, two 200-μL aliquots of each sample were analysed using an optical microscope (Nikon Eclipse E200), and the average number of eggs per stool aliquot was extrapolated to the total number of eggs per gram of feces.
Adult worms present in the hepatic portal circulatory system of mice infected with S. mansoni (LE or HS) were recovered and counted using the method described by Pellegrino and Siqueira (Reference Pellegrino and Siqueira1956). Briefly, the abdominal cavity of each euthanized animal was exposed, the distal region of the small intestine was tied with a thread, and the portal vein was sectioned. Then, a needle attached to an infusion pump (Automatic Pippeting Brewer Machine, model 60453, B.D) was introduced into the thoracic aorta for perfusion of the circulatory system, using 0.85% saline solution containing 80 U L−1 of heparin. The solution containing adult worms flowed through the portal vein from each animal and was collected in an individual beaker. Worms recovered from each infected mouse were morphologically classified as either males or females (Neves et al., Reference Neves, Pereira, De Oliveira, Gomes and Machado-Silva1998) and counted using a stereomicroscope (Zeiss Stemi Dv4). As described by Freire et al. (Reference Freire, Rodrigues-Silva, Machado-Silva and Rey2003), the infectivity rate or recovery rate (%) of each schistosome strain was calculated based on the total number of worms recovered with respect to the number of cercariae inoculated.
For the recovery and counting of eggs retained in different tissues of the infected animals, the tissue was digested as was previously described (Cheever, Reference Cheever1968). After perfusion, the lungs, liver, spleen and intestine (small and large) were removed, triturated and individually digested in potassium hydroxide solution (5% KOH) at 37°C for 4 h. The product of the tissue digestion of each animal was centrifuged (1500 g for 5 min), and the sediment containing the eggs was washed 5 times and resuspended in 5 mL of saline solution containing 10% formaldehyde. For counting, two 200-μL aliquots of each sample were analysed using an optical microscope, and the average number of eggs per aliquot of digested tissue suspension was expressed in eggs/organ (Maggi et al., Reference Maggi, Rocha, Camelo, Fernandes and Negrão-Corrêa2021).
To estimate the fecundity of S. mansoni, we used the sum of the number of eggs retained in all tissues evaluated and eliminated in feces, which was then divided by the number of female worms recovered from each animal infected with either parasite strain (Boulanger et al., Reference Boulanger, Reid, Sturrock, Wolowczuk, Balloul, Grezel, Pierce, Otieno, Guerret, Grimaud, Butterworth and Capron1991).
Tissue immune response
The sandwich ELISA technique was used to measure the levels of TNF-α, IFN-γ, IL-12, IL-17, IL-4, IL-5, IL-13, IL-33 and IL-10 in the liver and small intestine homogenate of mice from the experimental and control groups, using commercially available kits (DuoSet, R&D Systems) according to the manufacturer's instructions. Initially, 100 mg of the bilobed liver lobe and the distal portion of the small intestine were processed in a tissue homogenizer (Power General 125; Fisher Scientific, Pittsburgh, PA, USA), with 1 mL of phosphate buffer solution containing 0.5% Tween 20, 0.5% serum bovine albumin, 0.1 mm phenylmethylsuphonyl fluoride, 0.1 mm benzetonic chloride, 10 mm EDTA and 20 IU aprotinin (Sigma, St. Louis, MO, USA). The obtained homogenate was centrifuged for 20 min at 4000 × g at 4°C, and the supernatant was collected and used for cytokine quantification, as has been detailed by Rezende et al. (Reference Rezende, Moreira, Fernandes, Rodrigues and Negrão-Corrêa2020). The precipitate was further processed to indirectly estimate eosinophil and neutrophil infiltration/activation using EPO (Strath et al., Reference Strath, Warren and Sanderson1985) and MPO (Ivey et al., Reference Ivey, Williams, Collins, Jose and Williams1995) activity assays, respectively, as has been adapted by Fernandes et al. (Reference Fernandes, Pereira, Eschenazi, Schilter, Sousa, Teixeira and Negrão-Corrêa2008).
For the indirect estimation of macrophage recruitment and activation, 100 mg of the caudate liver lobe or the medial portion of the small intestine was homogenized in buffer solution (0.1 M NaCl, 0.02 M Na3PO4 and 0.015 M Na2EDTA, pH 4.7). After erythrocyte lysis and disruption of intracellular vesicles using 0.1% Triton X-100 (Sigma) saline solution, the supernatant was used to measure the activity of NAG according to Barcelos et al. (Reference Barcelos, Talvani, Teixeira, Vieira, Cassali, Andrade and Teixeira2005) and expressed in arbitrary units. Samples from the same tissue homogenate were also used to estimate the activity of arginase (expressed in mm of urea), a marker of M2 activation, as was previously described by Corraliza et al. (Reference Corraliza, Campo, Soler and Modolell1994), and nitrite concentration (μM), the NO end-product of M1 activation, using the Griess reaction (Tsikas, Reference Tsikas2007).
Tissue pathology
Liver and intestine damage
For the indirect assessment of liver damage, the activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were quantified from plasma samples of animals of the different experimental groups using the colorimetric test commercially available from Labtest®, following the manufacturer's instructions. The microplates were read in a microplate reader at 505 nm and the results were expressed in U L−1.
To indirectly assess intestinal damage, an immunochromatographic test for the detection of occult blood (iFOBT, Labtest®) was performed using 10 mg of feces collected from each experimental mouse, following the manufacturer's recommendations. The results were expressed as the frequency of animals with positive or negative test results for each experimental group.
Quantification of collagen content in tissue samples
For the indirect determination of collagen deposition (Reddy and Enwemeka, Reference Reddy and Enwemeka1996), we quantified the hydroxyproline content by taking 100 mg of the square liver lobe or proximal portion of the small intestine of each experimental animal, which was then homogenized in saline solution and lyophilized (Liotop K105, Brazil). The lyophilized product was subjected to alkaline hydrolysis (1 mL of 2 N NaOH) and autoclaved at 120°C for 20 min. Thereafter, 50 μL of the hydrolysed material was added to 450 μL of the chloramine T oxidizing reagent (0.056 M chloramine T, 10% N-propanol in pH 6.5 acetate/citrate buffer) and incubated for 20 min. A standard curve plotting the range 1.5–25 μg of hydroxyproline (Alfa Aesar, Massachusetts, USA) was prepared in the same way. The colorimetric reaction was initiated by adding Ehrlich's reagent (1 M p-dimethylaminobenzaldehyde diluted in N-propanol/perchloric acid 2:1, v/v). The samples were then centrifuged (1500 × g for 10 min at 4°C). The absorbance of the supernatant samples from the standard curve and processed tissue was measured at 550 nm using a Spectra Max microplate reader (Molecular Devices, Sunnyvale, CA, USA), and the results were expressed as μg mL−1 of hydroxyproline.
Histopathology
The left lobe of the liver and final portion of the ileum of non-perfused mice were used for histopathological analysis. The area of the granuloma induced by experimental infections using LE or HS strains was analysed. These organs were gently removed and processed separately as follows: the liver was washed with 0.85% saline (pH 7.2 at 37°C) and fixed in PBS containing 10% formalin for 24 h; the intestines were opened longitudinally and washed with 0.85% saline solution. After removing the debris, the intestinal samples were transferred to a filter paper with the mucosal surface facing upwards, and the tissue was soaked in PBS containing 10% formalin. The material was wrapped around a small wooden stick and tied in a roll shape, which was then fixed for 24 h in a new buffered 10% formalin solution. Subsequently, the liver and intestine samples were washed, dehydrated in increasing series of alcohol (70°GL to absolute), clarified in increasing dilutions of xylol and embedded in paraffin blocks for histopathological analysis. Sections of individual host tissues (5-μm sections) from different experimental groups were stained with haematoxylin–eosin (H&E) (Luna, Reference Luna1968) and analysed under an optical microscope (Olympus BX41). Images of the liver and intestines were captured using a digital camera (Olympus DP12) coupled to the microscope and analysed using Image Pro-plus software (version 4.0) at 20 × magnification to measure the area of granulomas. Only granulomas from the exudative–productive phase that surrounded a single egg with a visible miracidium were considered suitable for evaluation (Rezende et al., Reference Rezende, Moreira, Fernandes, Rodrigues and Negrão-Corrêa2020). In the liver parenchyma, at least 35 randomly selected granulomas were measured from each experimental group, while in the small intestine at least 15 granulomas per group were measured.
Statistical analysis
The normality of the data was initially analysed using the Kolmogorov–Smirnov test. Two-way analysis of variance (ANOVA) followed by the Bonferroni post-test was used to compare the means of the parametric data between more than 2 groups, and the Student's t-test was used to compare between 2 groups. The comparison of medians from non-parametric data between the 2 groups was performed using the Mann–Whitney test. Data related to the survival curve were analysed using the log-rank test. Categorical data frequencies were compared using Fisher's exact test. Statistical significance was set at P < 0.05. GraphPad Prism version 8 (Prism Software, Irvine, California, USA) and STATA version 11 (Stata Corp., College Station, TX, USA) were used to perform these tests and construct graphs.
Results
Parasite burden
As shown in Fig. 1, the total number of adult worms (Fig. 1A) recovered from the circulatory system of mice infected with the HS strain of S. mansoni was lower than that obtained from mice infected with the LE strain, mainly in the chronic phase of schistosomiasis. Therefore, the number of worms recovered in relation to the dose of inoculated cercariae (infectivity rate) for the LE strain was approximately 24–25%, and for the HS strain, this rate was 16.5% in the acute phase and 8.4% in the chronic phase. A similar reduction was observed in the number of both male and female worms in mice infected with the HS strain (Fig. 1B and, C). However, the sex ratio of adult worms (male: female) was closest to equity (1.4:1) in infections with the HS strain when compared to the LE strain (1.8:1), especially in the chronic phase.
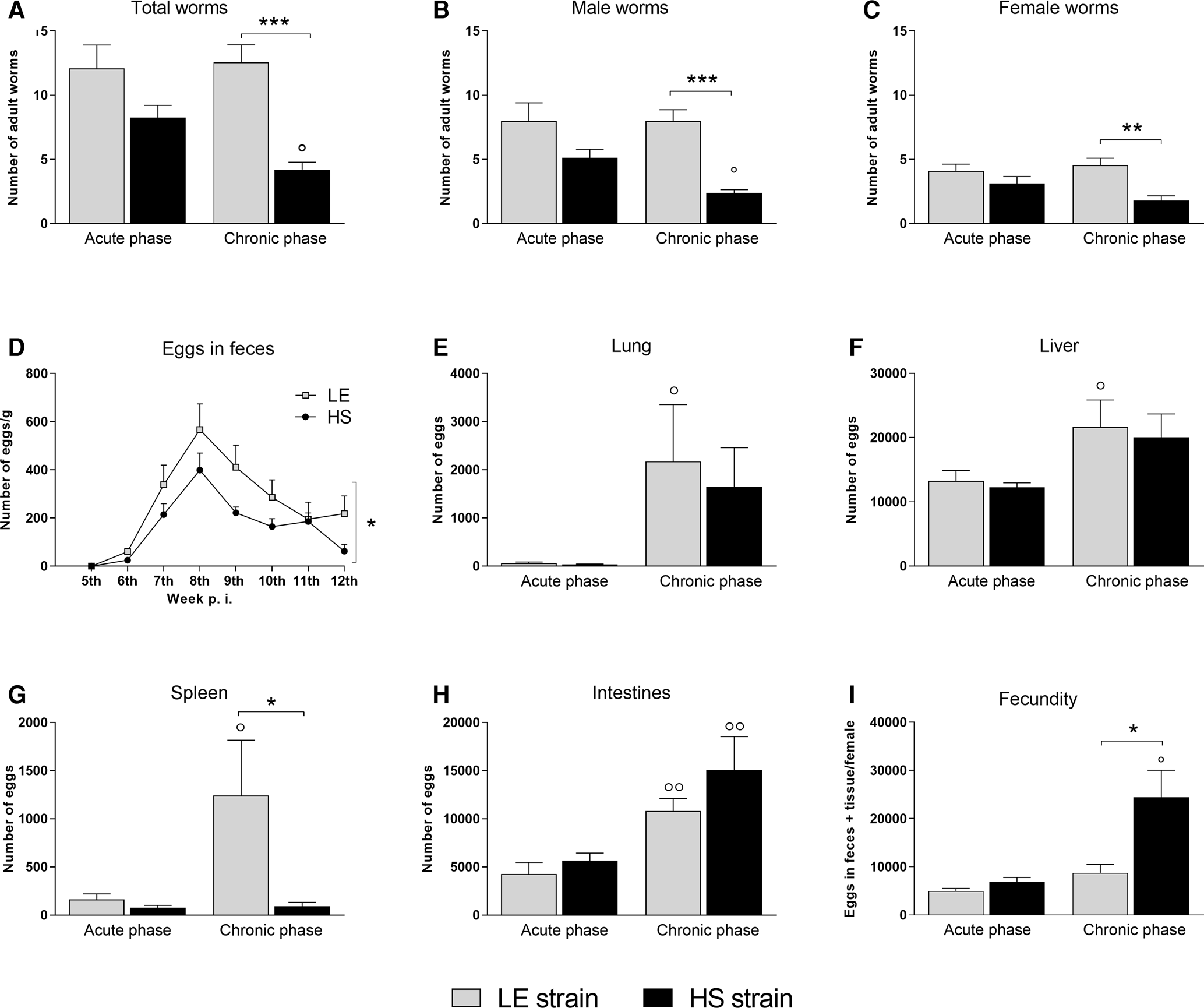
Fig. 1. Parasite burden. Number of total worms (A), males (B) and females (C) recovered from the circulatory system, number of eggs eliminated in feces (D) and retained in tissues (E–H) and fecundity of female worms (I) in BALB/c mice infected with Schistosoma mansoni from the LE (human reference) and the HS (Holochilus sciureus) strains. BALB/c mice were subcutaneously infected with 50 S. mansoni cercariae from either LE or HS parasite strain and euthanized at week 8 (acute phase) and 12 post-infection (chronic phase). Data are representative of 2 independent experiments (7–10 animals/phase of infection), and is presented as mean ± standard error. Two-way ANOVA followed by Bonferroni's post-test was performed for comparison between groups. °Statistically different data between the phases (acute vs chronic) of infection by the same strain. *Statistically different data between LE vs HS groups at the same time point (° or * P < 0.05; °° or ** P < 0.01; °°° or *** P < 0.001).
The pre-patent period of S. mansoni infection in BALB/c mice was similar (41 days of infection) for both strains (Fig. 1D). However, at the 6th week of infection, only 25% (5 egg-positive mice in a total of 20 infected mice) of the BALB/c mice infected with the HS strain eliminated parasite eggs in feces, while 75% (15/20) of mice infected with LE strain already had parasite eggs in their feces; this frequency varied significantly between the strains (P < 0.05). Although the number of schistosome eggs eliminated in the feces of mice infected with either parasite strain showed no significant differences at each time point evaluated, the number of eggs eliminated by mice infected with the HS strain was always lower than that from mice infected with the LE strain. Therefore, the sum of the number of eggs eliminated during the examination was significantly lower in mice infected with the HS strain of S. mansoni (Fig. 1D).
Even though the number of worms recovered from HS-infected mice was statistically lower than that in LE-infected mice, the number of parasite eggs retained in the different host organs of the infected mice increased progressively during infection with both strains, with no statistical differences between them in the lungs, liver and intestines (Fig. 1E, F and H). However, a statistically smaller number of eggs were observed to be retained in the spleen of mice that were chronically infected with the HS strain compared with the LE strain (Fig. 1G).
Although the number of female worms recovered from HS-infected mice was statistically lower than that from LE-infected mice, the fecundity of S. mansoni from the HS strain increased progressively. Therefore, in chronic experimental schistosomiasis, females from the HS strain showed a higher fecundity compared with female worms from the LE strain (Fig. 1I).
Liver immunopathology
Cytokine profile
In general, there was an increase in the production of most cytokines (TFN-α, IFN-γ, IL-12, IL-17, IL-4, IL-5, IL-33 and IL-10) in the liver homogenate of infected animals compared to uninfected animals for either parasite strains. TFN-α (Fig. 2A), IFN-γ (Fig. 2B), IL-12 (Fig. 2C) and IL-17 (Fig. 2D) showed a progressive increase in the liver homogenate after the infection, with no significant differences between parasite strains. The liver levels of IL-4 (Fig. 2E) and IL-33 (Fig. 2H) were also similar between the strains. In contrast, animals chronically infected with the HS strain showed significantly lower concentrations of IL-5 (Fig. 2F), IL-13 (Fig. 2G) and IL-10 (Fig. 2I) in liver homogenates than the LE strain.

Fig. 2. Liver cytokines. Quantification of cytokine levels of Th1 (TNF-α, IFN-γ and IL-12) (A–C), Th17 (IL-17) (D), Th2 (IL-4, IL-5, IL-13 and IL-33) (E-H) and regulatory (IL-10) profiles (I) in liver homogenate of uninfected BALB/c mice and mice infected with LE (human reference) and HS (Holochilus sciureus) strains of Schistosoma mansoni. BALB/c mice were subcutaneously infected with 50 S. mansoni cercariae from either LE or HS parasite strain and euthanized at week 8 (acute phase) and 12 post-infection (chronic phase). Data are representative of 2 independent experiments (7–10 animals/phase of infection) and presented as mean ± standard error. Two-way ANOVA followed by Bonferroni's post-test was performed for comparison between the groups. # Statistically significant data compared with the control group; ° Statistically different data between the phases (acute vs chronic) of infection by the same strain. * Statistically different data between the LE vs HS groups at the same time point (#, ° or * P < 0.05; ##, °° or ** P < 0.01; ###, °°° or *** P < 0.001; ####, °°°° or **** P < 0.0001).
Granuloma and hydroxyproline evaluation
Morphometric analysis showed that liver granulomas formed during the HS strain infection were larger than those of the LE strain, especially in chronic schistosomiasis (Fig. 3E). In acute experimental schistosomiasis, the histopathological evaluation revealed that both strains induced the formation of granulomas with intense extracellular matrix deposition and inflammatory infiltration (Fig. 3A and B). However, liver granulomas induced by infection with HS strain showed greater disarray of the cellular infiltration and extracellular matrix deposition, and the hepatic sinusoids showed greater dilatation and congestion (Fig. 3B). Regarding chronic schistosomiasis, the granulomas formed by LE strain infection were composed of organized collagen fibres and little cellular infiltration (Fig. 3C). In contrast, chronic infection with the HS strain induced the formation of granulomas with the presence of many inflammatory cells and a large amount of disorganized collagen fibres (Fig. 3D).
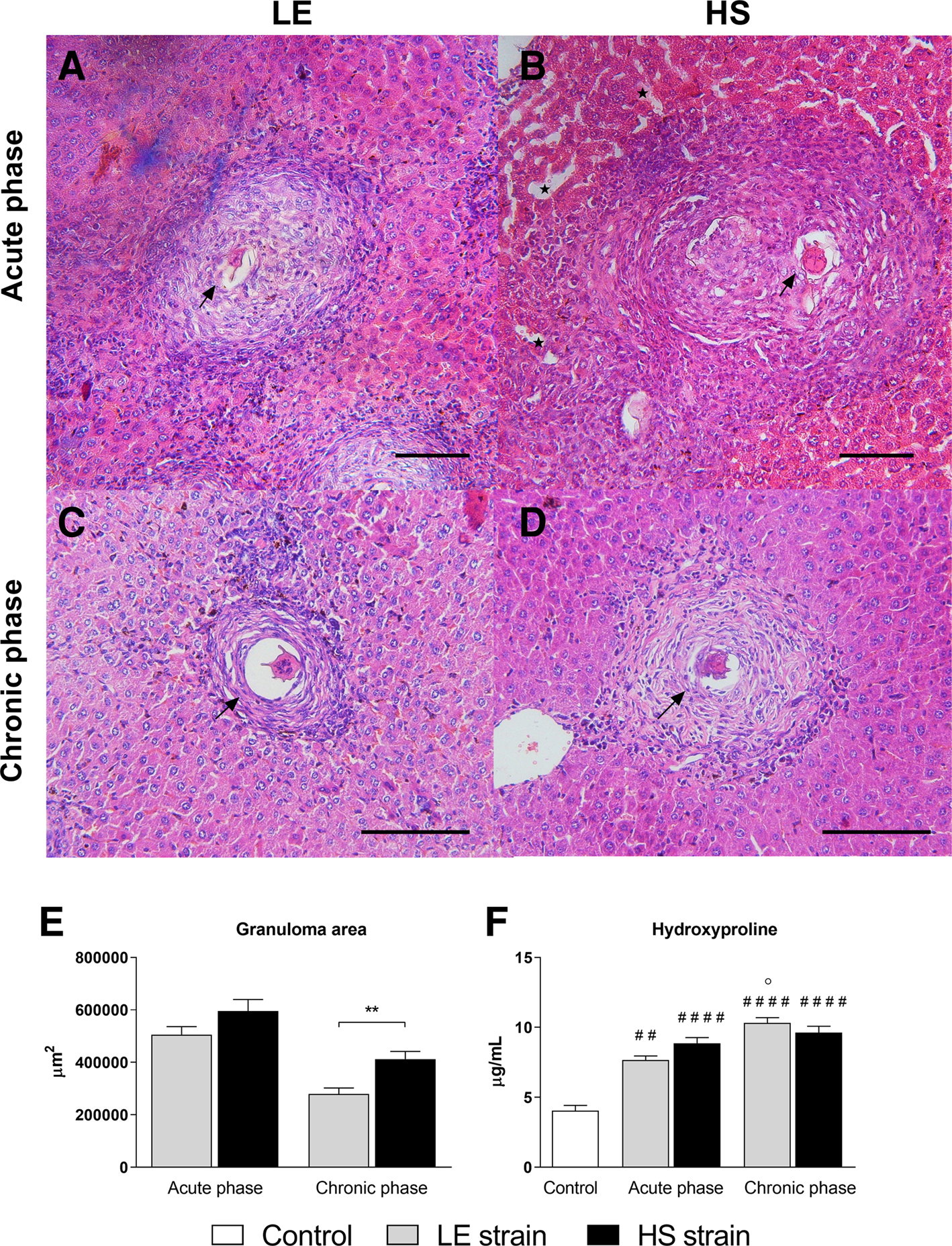
Fig. 3. Liver histopathology. Photomicrographs of HE-stained liver sections (20×; scale bar = 100 μm) from BALB/c mice infected with LE (human reference) and HS (Holochilus sciureus) strains of Schistosoma mansoni at the acute (A and B) and chronic (C and D) phases of schistosomiasis. Morphometry of hepatic granuloma area (E). Concentration of hydroxyproline in liver homogenate (F). BALB/c mice were subcutaneously infected with 50 S. mansoni cercariae from either LE or HS parasite strain and euthanized at week 8 (acute phase) or 12 post-infection (chronic phase). At least 35 isolated granulomas containing a single viable egg (black arrows) were measured in each experimental group for the granuloma area assessment. Black stars indicate hepatic sinusoids with greater dilatation and congestion. Data are representative of 2 independent experiments (7–10 animals/phase of infection) and presented as mean ± standard error. Mann–Whitney test was performed to compare granuloma area, and two-way ANOVA followed by Bonferroni's post-test was performed to compare hydroxyproline concentrations between groups. # Statistically different data compared with the control group; ° Statistically different data between phases (acute vs chronic) of infection by the same strain. * Statistically different data between LE and HS groups at the same time point (#, ° or * P < 0.05; ##, °° or ** P < 0.01; ###, °°° or *** P < 0.001; ####, °°°° or **** P < 0.0001).
Infection with either parasite strain resulted in a significant increase in liver hydroxyproline content in the experimental groups compared with the control group. However, in BALB/c mice infected with the LE strain, hydroxyproline concentrations progressively increased during the chronic phase of schistosomiasis, whereas in the group infected with the HS strain, hydroxyproline content was similar at 8 and 12 weeks of S. mansoni infection (Fig. 3F).
Tissue cellular infiltration and activation
The animals infected with the LE or HS parasite strains, regardless of the phase of schistosomiasis, showed high levels of EPO and MPO in the liver parenchyma when compared with the uninfected animals, indicating greater recruitment and activation of eosinophils and neutrophils, respectively (Fig. 4A and B). However, EPO activity in the liver homogenate of BALB/c mice acutely infected with the HS strain of the trematode was significantly lower than that in the livers of animals infected with the LE strain (Fig. 4A). In contrast, MPO activity levels in the liver of infected animals were statistically similar for both the parasite strains (Fig. 4B).

Fig. 4. Hepatic cellular infiltration and activation. Enzymatic activity of eosinophil peroxidase (A), myeloperoxidase (B), N-acetyl-β-d-glucosaminidase (C), arginase activity (D) and nitrite concentration (E) in the liver homogenates of uninfected BALB/c mice and mice infected with Schistosoma mansoni of the LE (human reference) and HS (Holochilus sciureus) strains. BALB/c mice were subcutaneously infected with 50 S. mansoni cercariae from either the LE or HS parasite strain and euthanized at week 8 (acute phase) and 12 post-infection (chronic phase). Data are representative of 2 independent experiments (7–10 animals/phase of infection) and presented as mean ± standard error. Two-way ANOVA followed by Bonferroni's post-test was performed for the comparison between the groups. # Statistically significant data compared with the control group; ° Statistically different data between the phases (acute vs chronic) of infection by the same strain. * Statistically different data between the LE vs HS groups at the same time point (#, ° or * P < 0.05; ##, °° or ** P < 0.01; ###, °°° or *** P < 0.001; ####, °°°° or **** P < 0.0001).
Regarding macrophages, there was a significant increase in NAG activity levels in the liver homogenates of animals infected with the LE and HS parasite strains in both phases of infection when compared with the control group, indicating greater recruitment and activation of these cells in the liver. Moreover, no significant differences in NAG activity were detected in the liver homogenates of mice infected with LE when compared with the HS strains of S. mansoni. Only animals that were chronically infected with the LE strain showed increased NAG levels compared to their own acute phase (Fig. 4C).
Additionally, the activation profile of infiltrated macrophages was indirectly determined by nitric oxide production (M1 mark) and arginase activity (M2 mark). Thus, arginase activity in the liver was also significantly elevated in animals infected with both S. mansoni strains compared with the control group. However, there were no differences in the levels of arginase activity between the phases of infection or among the evaluated S. mansoni strains (Fig. 4D). In contrast, the concentration of nitrite in liver homogenates, the NO end-product, was higher in animals infected with the LE and HS parasite strains in the acute phase of schistosomiasis compared to that in the control group. However, the concentration of nitrite increased progressively only in mice infected with the S. mansoni HS strain. Therefore, in chronically infected mice, the concentration of nitrite in the liver was significantly higher in HS- mice than in LE-infected mice (Fig. 4E).
Small intestine immunopathology
Cytokine profile
Schistosoma mansoni infection also resulted in a progressive increase in cytokine levels in intestinal homogenates from mice infected with either parasite strain. During acute schistosomiasis, the concentrations of TFN-α (Fig. 5A), IFN-γ (Fig. 5B), IL-12 (Fig. 5C), IL-17 (Fig. 5D), IL-5 (Fig. 5F) and IL-33 (Fig. 5H) were significantly higher in the HS-infected mice compared to the LE-infected mice, which was different from the pattern observed in the liver. IL-4 concentrations in intestinal homogenates were significantly higher in animals infected with either parasite strain than those in the control group, with no significant differences between them in acute and chronic schistosomiasis (Fig. 5E). Moreover, the HS strain induced a significant increase in TNF-α and a reduction in IL-5 compared with the LE strain after 12 weeks of S. mansoni infection (Fig. 5A and F, respectively).
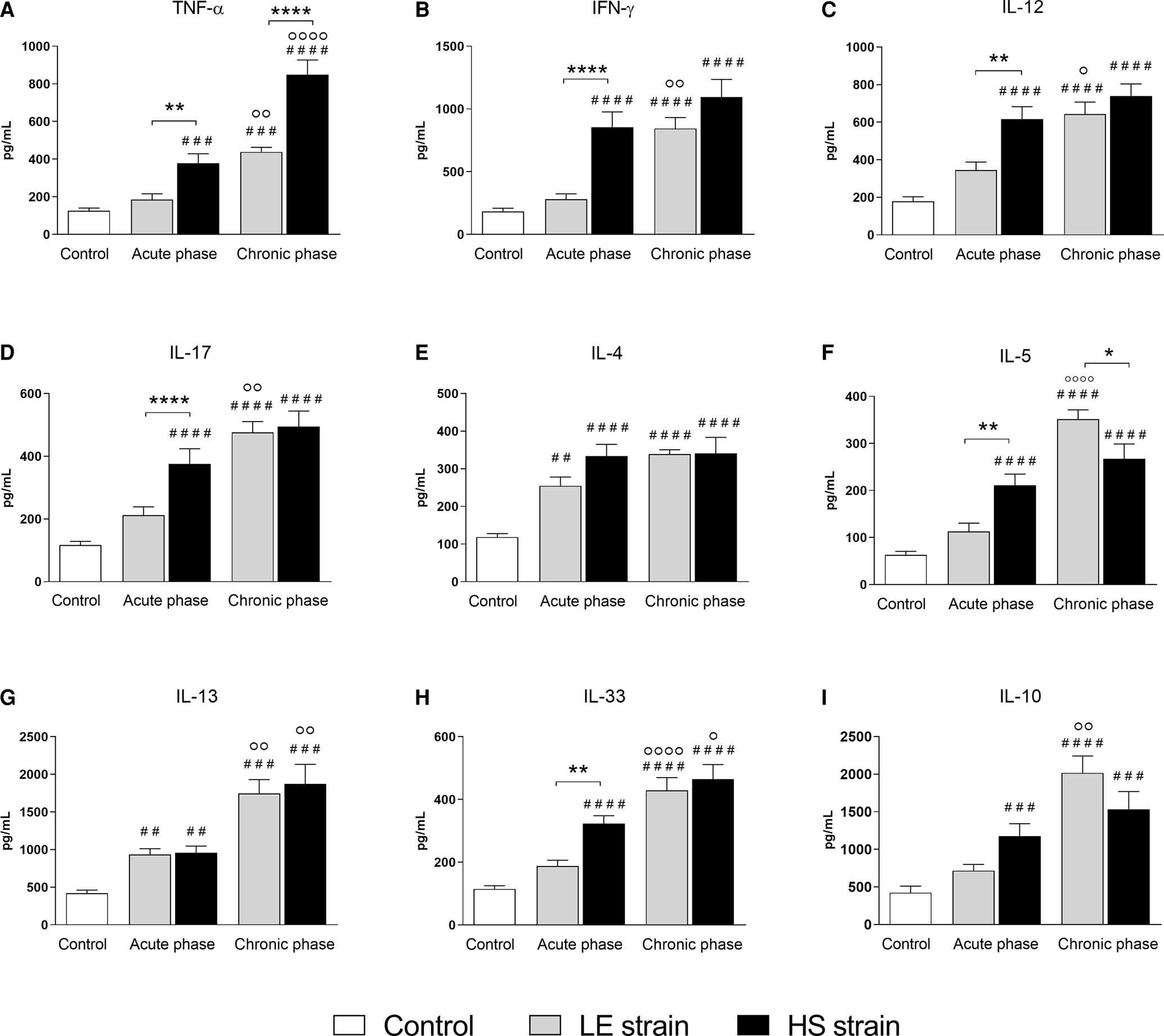
Fig. 5. Small intestine cytokines. Quantification of cytokine levels of Th1 (TNF-α, IFN-γ and IL-12) (A–C), Th17 (IL-17) (D), Th2 (IL-4, IL-5, Il-13 and IL-33) (E–H) and regulatory (IL-10) profiles (I) in ileum homogenate of uninfected BALB/c mice and mice infected with Schistosoma mansoni from the LE (human reference) and the HS (Holochilus sciureus) strains. BALB/c mice were subcutaneously infected with 50 S. mansoni cercariae from either LE or HS parasite strain and euthanized at week 8 (acute phase) and 12 post-infection (chronic phase). Data are representative of 2 independent experiments (7–10 animals/phase of infection) and presented as mean ± standard error. Two-way ANOVA followed by Bonferroni's post-test was performed for comparison between the groups. # Statistically significant data compared with the control group; ° Statistically different data between the phases (acute vs chronic) of infection by the same strain. * Statistically different data between the LE vs HS groups at the same time point (#, ° or * P < 0.05; ##, °° or ** P < 0.01; ###, °°° or *** P < 0.001; ####, °°°° or **** P < 0.0001).
Granuloma and hydroxyproline evaluation
Morphometric analysis of intestinal granulomas showed that infection with the HS strain also induced the formation of larger granulomas compared with the LE strain, mainly in acute schistosomiasis (Fig. 6A, B and E). In the chronic phase of schistosomiasis, there was a reduction in the size of intestinal granulomas in mice infected with either parasite strain, with no significant differences between them (Fig. 6C, D and E). Histopathological analysis also revealed that the ileum of mice infected with the HS strain of S. mansoni showed areas of intense cellular infiltration in the lamina propria and thickening of the muscular layer of the mucosa, which was less evident in mice infected with the LE strain. In addition, the small intestine of HS-infected mice showed areas of epithelial erosion and disruption of normal villus architecture (Fig. 6D), which was not frequently observed in mice infected with the LE strain of S. mansoni (Fig. 6C).

Fig. 6. Small intestine histopathology. Photomicrographs of HE-stained ileum sections (20×; scale bar = 100 μm) from BALB/c mice infected with LE (human reference) and HS (Holochilus sciureus) strains of Schistosoma mansoni in acute (A and B) and chronic (C and D) phases of schistosomiasis. Morphometry of intestinal granuloma area (E). Concentration of hydroxyproline in intestinal homogenate (F). BALB/c mice were subcutaneously infected with 50 cercariae from either LE or HS strain of S. mansoni and euthanized at week 8 (acute phase) or 12 post-infection (chronic phase). At least 15 isolated granulomas containing a single viable egg (black arrows) per group were measured for granuloma area assessment. The black stars indicate the muscular layer of the mucosa (the size of the star is proportional to the degree of alteration of tissue). The blank arrows show the destruction of the small intestine villi. Data are representative of 2 independent experiments (7–10 animals/phase of infection) and presented as mean ± standard error. Mann–Whitney test was performed to compare the granuloma area, and two-way ANOVA followed by Bonferroni's post-test was performed to compare hydroxyproline concentrations between the groups. # Statistically significant data compared with the control group; ° Statistically different data between the phases (acute vs chronic) of infection by the same strain. * Statistically different data between the LE vs HS groups at the same time point (#, or * P < 0.05; ##, °° or ** P < 0.01; ###, °°° or *** P < 0.001).
The hydroxyproline content in the intestinal homogenate of animals experimentally infected with either the LE or HS strain in acute schistosomiasis was similar. Moreover, there was a progressive increase in hydroxyproline concentration in animals chronically infected with either parasite strains, particularly in LE-infected mice (Fig. 6F).
Tissue cellular infiltration and activation
Regarding the tissue infiltration/activation of polymorphonuclear cells in the small intestine, animals infected with the LE parasite strain showed an increase in EPO and MPO levels compared with the uninfected animals, indicating an increase in eosinophil and neutrophil recruitment and activation, respectively (Fig. 7A and B). A statistically similar increase in EPO activity was detected in the intestinal homogenate of mice infected with either the HS or the LE strain of the parasite, with no significant differences between the two infected groups (Fig. 7A). Acute infection with S. mansoni in both the strains also induced a significant increase in MPO activity in the intestinal homogenate, but the enzymatic activity was significantly reduced in the intestinal homogenate of HS-infected mice after 12 weeks of infection; therefore, the MPO activity in animals chronically infected with HS was significantly lower than that in the LE-infected group (Fig. 7B).

Fig. 7. Small intestinal cellular infiltration and activation. Enzymatic activity of eosinophil peroxidase (A), myeloperoxidase (B), N-acetyl-β-d-glucosaminidase (C), arginase activity (D) and nitrite concentration (E) in ileum homogenate of uninfected BALB/c mice and mice infected with LE (human reference) and HS (Holochilus sciureus) strains of Schistosoma mansoni. BALB/c mice were subcutaneously infected with 50 S. mansoni cercariae from either the LE or HS parasite strain and euthanized at week 8 (acute phase) and 12 post-infection (chronic phase). Data are representative of 2 independent experiments (7–10 animals/phase of infection) and presented as mean ± standard error. Two-way ANOVA followed by Bonferroni's post-test was performed for the comparison between the groups. # Statistically significant data compared with the control group; °Statistically different data between the phases (acute vs chronic) of infection by the same strain. * Statistically different data between the LE vs HS groups (#, ° or * P < 0.05; ##, °° or ** P < 0.01; ###, °°° or *** P < 0.001; ####, °°°° or **** P < 0.0001).
Animals chronically infected with either the LE or HS strains of S. mansoni showed high levels of NAG compared with uninfected animals, indicating greater recruitment of macrophages to the small intestine. Moreover, there was an increase in NAG activity in the intestinal homogenate from the acute to the chronic phase of infection for both strains. Regarding the macrophage activation profile in the intestinal parenchyma, only mice infected with the LE strain of S. mansoni showed a progressive increase in arginase activity, resulting in a significant difference compared with the control group during chronic schistosomiasis (Fig. 7D). In contrast, the nitrite concentration in the intestine homogenate was significantly higher in all infected animals (LE or HS parasite strain) compared to that in uninfected animals. However, in the acute phase of schistosomiasis, the nitrite concentration was significantly higher in the intestinal homogenate of HS-infected mice than in LE-infected mice (Fig. 7E), indicating a predominant Th1-inflammatory environment in these infected animals.
Clinical signs of schistosomiasis morbidity
The severity of morbidity associated with schistosomiasis in mice experimentally infected with either parasite strain was comparatively evaluated by assessing the frequency of occult blood in the feces, red blood cell count and haemoglobin concentration in blood samples. Additionally, the transaminase activity, weight variation and mortality rate were evaluated.
Animals experimentally infected with the LE or HS strain of S. mansoni showed a progressive increase in occult blood in their feces during infection. During acute schistosomiasis, occult blood was detected in 40% of the stool samples of mice infected with HS strain (4 mice positive for occult blood in feces of 10 infected animals) compared to 20% (2/10) in mice infected with the LE strain of the parasite. During the chronic phase of the experimental infection, 100% (7/7) of HS-infected and 86% (6/7) of the LE-infected animals showed the presence of occult blood in feces.
To assess anaemia in infected mice, haemoglobin concentration and the number of red blood cells were evaluated. There was no difference in the number of red blood cells between any groups (Fig. 8A); however, infection with S. mansoni (LE or HS strain) resulted in a significant reduction in haemoglobin levels in both phases of schistosomiasis when compared with uninfected mice (Fig. 8B).

Fig. 8. Schistosomiasis severity. Red blood cells count (A), haemoglobin concentration (B), plasma activity of aspartate aminotransferase (AST) (C) and alanine aminotransferase (ALT) (D), bodyweight variation (E) and mortality rate (F) of uninfected BALB/c mice and infected with LE (human reference) and HS (Holochilus sciureus) strains of Schistosoma mansoni. BALB/c mice were subcutaneously infected with 50 S. mansoni cercariae from either LE or HS parasite strain and euthanized at 8- (acute phase) and 12-weeks post-infection (chronic phase). Data compile results from 2 independent experiments (14–20 animals/phase of infection) and are presented as mean ± standard error. Two-way ANOVA followed by Bonferroni's post-test was performed for the comparison of data from blood tests, transaminases activity and body weight variation between the groups. The log-rank test was performed to compare the mortality curves between the groups. # Statistically significant data compared with the control group; ° Statistically different data between phases (acute vs chronic) of infection by the same strain. * Statistically different data between LE vs HS groups at the same time point (#, ° or * P < 0.05; ##, °° or ** P < 0.01; ###, °°° or *** P < 0.001; ####, °°°° or **** P < 0.0001).
As demonstrated in previous studies using experimental models (Oliveira et al., Reference Oliveira, Rodrigues, Moreira, Rodrigues, Maggi, Resende and Negrão-Corrêa2022) and in the human population (Dessie et al., Reference Dessie, Lema and Aemero2020), S. mansoni infection induced increased transaminase activity in the circulation, indicating host liver injury. In the current study, infection with the HS strain of S. mansoni induced a significant increase in AST activity in the plasma compared to the activity level detected in uninfected animals and in mice infected with the LE-strain of the parasite in both phases of experimental schistosomiasis (Fig. 8C). There was a significant increase in ALT activity levels during acute schistosomiasis, which was reduced during the chronic phase of the disease in mice infected with either parasite strain. However, in acute schistosomiasis, HS-infected mice showed significantly higher ALT activity than LE-infected mice (Fig. 8D) suggesting greater liver damage.
Finally, to evaluate the outcome of schistosomiasis in the experimentally infected mice, weight variations and mortality rates were monitored. All experimental animals (infected or not infected) showed similar weight gain up to the 6th week of experimental infection. After this period, mice infected with either strain (LE or HS) of S. mansoni showed weight loss during post-postural acute schistosomiasis, resulting in significantly lower body weight gain kinetics compared to the control group; however, there were no differences in the kinetics of weight gain between the mice infected with either parasite strain (Fig. 8E). However, experimental infection with S. mansoni in the HS strain induced an early (in the acute phase, 50 days) and statistically high mortality rate when compared with both the control group and the LE-infected mice (Fig. 8F), suggesting a more severe schistosomiasis morbidity.
Discussion
The trematodes S. mansoni use humans as their main vertebrate hosts; however, natural infections by this parasite in wild rodents have also been reported at sites with active and persistent transmission of schistosomiasis in Africa (Duplantier and Sène, Reference Duplantier and Sène2000; Catalano et al., Reference Catalano, Sène, Diouf, Fall, Borlase, Léger, Bâ and Webster2018) and in the Americas (Théron, Reference Théron1984; Théron et al., Reference Théron, Pointier, Morand, Imbert-Establet and Borel1992; Gentile et al., Reference Gentile, Costa-Neto, Gonçalves, Bonecker, Fernandes, Garcia, Barreto, Soares, D'Andrea, Peralta and Rey2006; Do Carmo-Silva et al., Reference do Carmo-Silva, Teles-Reis, Silva-Soares, Rodrigues, Lira, Nogueira, Viegas-Melo, Cardoso, Miranda and Silva-Souza2019). Several studies (Théron, Reference Théron1984; Barral et al., Reference Barral, Morand, Pointier and Théron1996; Sire et al., Reference Sire, Durand, Pointier and Théron1999; Catalano et al., Reference Catalano, Léger, Fall, Borlase, Diop, Berger, Webster, Faye, Diouf, Rollinson, Sène, Bâ and Webster2020) have proposed that these wild rodents participate in the life cycle of S. mansoni, allowing the emergence of different strains and genotypes of the parasite, whose impact on the severity of human schistosomiasis remains unknown. In the current study, it was demonstrated that BALB/c mice were less susceptible to experimental infection with the HS strain of S. mansoni, which was isolated from naturally infected H. sciureus captured in an area that was endemic for intestinal schistosomiasis in Northeastern Brazil, compared to the experimental infection with the LE strain of S. mansoni, a parasite strain isolated from an infected human being. Moreover, HS-infected BALB/c mice showed stronger induction of the Th1/Th17 immune response in acute schistosomiasis and lower Th2/Treg response during the chronic phase of the experimental infection, which resulted in a more intense inflammatory reaction and tissue damage, accompanied by high mortality.
The parasite burden of S. mansoni is directly associated with the pathogenicity of schistosomiasis (Bina and Prata, Reference Bina and Prata2003; Euzébio et al., Reference Euzébio, Zuim, Linhares, Magalhães and Zanotti-Magalhães2012) and host susceptibility varies among S. mansoni strains (Warren, Reference Warren1967; Bastos et al., Reference Bastos, Magalhães and Pareja1979; Freire et al., Reference Freire, Rodrigues-Silva, Machado-Silva and Rey2003; Martinez et al., Reference Martinez, Neves, Oliveira, Machado-Silva and Rey2003; Euzébio et al., Reference Euzébio, Zuim, Linhares, Magalhães and Zanotti-Magalhães2012). The current data with the human LE strain and previous studies using S. mansoni obtained from infected humans of different geographic areas have shown that the number of adult worms recovered ranges from 21 to 60% of the infective dose (Warren, Reference Warren1967; Anderson and Cheever, Reference Anderson and Cheever1972; Freire et al., Reference Freire, Rodrigues-Silva, Machado-Silva and Rey2003; Martinez et al., Reference Martinez, Neves, Oliveira, Machado-Silva and Rey2003). The recovered rate is much higher when compared to the findings of the current study for the experimental infection with the HS parasite strain, which showed recovery rates of 16.5 and 8.4%, in the acute and chronic phases of schistosomiasis, respectively. Differences in parasitological parameters were also verified by Martinez et al. (Reference Martinez, Neves, Oliveira, Machado-Silva and Rey2003), who compared the experimental infection using two different strains of S. mansoni: the CMO strain, which was isolated from infected Oryzomys subflavus captured in the state of Rio Grande do Norte, Brazil, and the CM strain, which was isolated from a human from the state of Pernambuco, Brazil. Infection with the CMO strain caused fewer eggs to be eliminated in feces and retained in the large intestine when compared with the experimental infection with the CM strain. In contrast, Freire et al. (Reference Freire, Rodrigues-Silva, Machado-Silva and Rey2003) demonstrated that experimental infection of mice with the SR strain of S. mansoni isolated from N. squamipes captured in the city of Rio de Janeiro, Brazil, had a similar parasite burden as mice experimentally infected with the human SH strain isolated from the same location. However, it is important to consider that these 2 parasite isolates were obtained from the same schistosomiasis transmission zone, and the same parasite strain could possibly be responsible for the wild rodent and human infections. Contrarily, even when comparing strains from the same location, Bastos et al. (Reference Bastos, Magalhães and Pareja1979) showed that mice experimentally infected with the S. mansoni strain obtained from naturally infected wild rodents had a lower number of worms recovered when compared with the experimental infection using the human strain of the parasite. The previous data, together with the findings of the current study, suggest that S. mansoni isolated from wild rodents would have lower parasite–host compatibility in experimental models.
However, it is important to mention that the LE strain of S. mansoni, used as a reference in the current study, has been maintained in successive infections of hamsters and B. glabrata under laboratory conditions since its isolation (Pellegrino and Katz, Reference Pellegrino, Katz and Dawes1968). In addition to the host species from which the parasites were originally isolated (human vs wild rodent), the long-term maintenance of the LE parasite strain could also have contributed to the biological differences in the current study. Nevertheless, analysing data from published works that investigated the infectivity rate of the LE strain of S. mansoni in mice over the years, we observed that, just after the isolation, the adult worm recovery rate in experimental infections with the human-isolated parasite was even higher, reaching 31–34% (Katz et al., Reference Katz, Dias, Araújo and Souza1973; Araújo et al., Reference Araújo, Souza, Dias and Katz1986), dropping to 20–25% in recent publications (Maggi et al., Reference Maggi, Rocha, Camelo, Fernandes and Negrão-Corrêa2021; Oliveira et al., Reference Oliveira, Rodrigues, Moreira, Rodrigues, Maggi, Resende and Negrão-Corrêa2022), a rate similar to that observed in the current study. Therefore, published data confirm that the long-term maintenance of S. mansoni from the LE strain under laboratory conditions contributes to modifying the infectivity rate of the parasite; however, it attenuated the difference between LE and HS parasite strains, indicating that the original host has an important role in this biological aspect of the parasite strain.
Aside from the reduced number of adult worms recovered in mice infected with S. mansoni from the HS strain, the female worms of this parasite strain showed a higher fecundity rate. It is possible that the lower worm density detected in BALB/c mice infected with the HS strain of S. mansoni favoured an environment with less competition for nutrients (Webster et al., Reference Webster, Neves, Webster, Pennance, Rabone, Gouvras, Allan, Walker and Rollinson2020), which may have allowed females to reach their maximum reproductive potential, leading to higher fecundity rates. In addition, the important changes observed in the immune response profile may have affected anti-fecundity mechanisms. The differences in worm fecundity rates observed between S. mansoni from the HS and LE strains would be determinant of the disease severity and deserves further studies.
As mentioned, the infectivity and fecundity rate of S. mansoni would be influenced by the host's immune response, a determinant factor in the host–parasite interaction (McManus et al., Reference McManus, Dunne, Sacko, Utzinger, Vennervald and Zhou2018; Angeles et al., Reference Angeles, Mercado and Rivera2020; Hambrook and Hanington, Reference Hambrook and Hanington2021). Moreover, the type and intensity of the immune response produced by vertebrate hosts are also essential for understanding the development of morbidity associated with schistosomiasis (Pearce and Macdonald, Reference Pearce and MacDonald2002; Abath et al., Reference Abath, Morais, Montenegro, Wynn and Montenegro2006). Interestingly, despite the lower parasite burden, experimental infection with the HS strain of S. mansoni induced profound changes in the host immune response and pathology of experimental schistosomiasis, when compared with the reference human strain (LE) used in the current study. Specifically, HS-infected mice showed a significant reduction in type-2 immune response markers in the liver, such as EPO activity and concentrations of IL-5 and IL-13, which was accompanied by an increase in NO-end products, especially during chronic schistosomiasis. A significant reduction in IL-5 concentration was also detected in the intestinal homogenate of mice that were chronically infected with the HS strain. Previous studies have shown that schistosomiasis predominantly induces a type-2 immune response associated with high levels of IL-4, IL-5 and IL-13, which favours tissue eosinophil infiltration and M2 macrophage activation (Dunne et al., Reference Dunne, Butterworth, Fulford, Kariuki, Langley, Ouma, Capron, Pierce and Sturrock1992; Cheever et al., Reference Cheever, Hoffmann and Wynn2000; Pearce and Macdonald, Reference Pearce and MacDonald2002; Pearce et al., Reference Pearce, Kane, Sun, Taylor, McKee and Cervi2004; Masamba and Kappo, Reference Masamba and Kappo2021). Furthermore, it has been demonstrated that the absence of an adequate type-2 immune response would impair adequate granuloma formation and contribute to uncontrolled type-1 inflammation, causing a higher mortality rate in mice (Brunet et al., Reference Brunet, Finkelman, Cheever, Kopf and Pearce1997). In addition to IL-4, high levels of IL-13 are necessary for the adequate polarization of M2 macrophages, which is essential to reduce the effects of more severe schistosomiasis (Herbert et al., Reference Herbert, Hölscher, Mohrs, Arendse, Schwegmann, Radwanska, Leeto, Kirsch, Hall, Mossmann, Claussen, Förster and Brombacher2004). Furthermore, it was also demonstrated that high levels of Th1 cytokines (TNF-α and IFN-γ) and low production of IL-5 (Th2) in humans were associated with severe cases of hepatosplenic schistosomiasis (Mwatha et al., Reference Mwatha, Kimani, Kamau, Mbugua, Ouma, Mumo, Fulford, Jones, Butterworth, Roberts and Dunne1998).
Parallel to a reduction in the Th2 response in the liver, mice infected with the HS strain of S. mansoni also had lower concentrations of IL-10 during chronic schistosomiasis. The production of IL-10 in chronic schistosomiasis has been associated with an increased proliferation of a regulatory network of cells, which modulated the immune response and promoted host survival, as it controlled the intensity of tissue damage and fibrosis induced by granuloma formation (Booth et al., Reference Booth, Mwatha, Joseph, Jones, Kadzo, Ireri, Kazibwe, Kemijumbi, Kariuki, Kimani, Ouma, Kabatereine, Vennervald and Dunne2004; Hesse et al., Reference Hesse, Piccirillo, Belkaid, Prufer, Mentink-Kane, Leusink, Cheever, Shevach and Wynn2004; Martins-Leite et al., Reference Martins-Leite, Gazzinelli, Alves-Oliveira, Gazzinelli, Malaquias, Correa-Oliveira, Teixeira-Carvalho and Silveira2008). Moreover, modulation of the immune response promoted by cytokine IL-10 was found to be essential for parasite survival, as it also regulated the action of effector cells against the parasite (Hesse et al., Reference Hesse, Piccirillo, Belkaid, Prufer, Mentink-Kane, Leusink, Cheever, Shevach and Wynn2004; Angeles et al., Reference Angeles, Mercado and Rivera2020); this is in line with the lower recovery rate of HS strain worms in the chronic phase of schistosomiasis, which was verified in the current study.
In the intestine, there is a clear induction of the Th1/Th17 inflammatory profile in the acute phase of infection, with a significant increase in TNF-α, IFN-γ, IL-12 and IL-17 concentrations, accompanied by higher detection of nitrite. Previous experimental studies have shown that the predominance of a Type-1 response during schistosomiasis is associated with the destruction of schistosomula in the lungs (Smythies et al., Reference Smythies, Betts, Coulson, Dowling and Wilson1996) and intense tissue damage, such as intestinal haemorrhage, cachexia and hepatotoxicity, caused by the high production of NO induced by TNF-α (Brunet et al., Reference Brunet, Finkelman, Cheever, Kopf and Pearce1997; Hoffmann et al., Reference Hoffmann, Cheever and Wynn2000). It is well known that a predominantly type-1 inflammatory environment favours the classical activation of NO-producing macrophages (Coulson et al., Reference Coulson, Smythies, Betts, Mabbott, Sternberg, Wei, Liew and Wilson1998; Gordon, Reference Gordon2003), a molecule capable of generating extensive tissue oxidative injury and which was associated with high mortality during experimental schistosomiasis (La Flamme et al., Reference La Flamme, Patton, Bauman and Pearce2001; Barron and Wynn, Reference Barron and Wynn2011). In addition, it has been demonstrated that the Th17 response is closely associated with the severity of schistosomiasis, as it can promote exacerbated inflammation (Rutitzky and Stadecker, Reference Rutitzky and Stadecker2006; Zhang et al., Reference Zhang, Chen, Gao, Hou, Gu, Gui, Huang, Liu, Ren, Wang and Shen2012) and liver fibrosis (Wang et al., Reference Wang, Liang, Wang, Zhu, Gong, Zhang, Li and Xia2015). Therefore, infection with the HS lineage of S. mansoni induces strong Th1/Th17-associated intestinal inflammation, which probably promotes an increase in the activation of NO-producing M1 macrophages.
Interestingly, even with a predominance of type-1 immune response in the intestinal tissue in the acute phase of schistosomiasis, infection by the HS strain also promoted an increase in the cytokine levels of the type 2 profile, such as IL-33 and IL-5. The high levels of these 2 cytokines were expected, since IL-33 is considered an alarmin that is produced and released in the extracellular space under conditions of cell/tissue damage (Pastorelli et al., Reference Pastorelli, Garg, Hoang, Spina, Mattioli, Scarpa, Fiocchi, Vecchi and Pizarro2010, Reference Pastorelli, De Salvo, Cominelli, Vecchi and Pizarro2011). Furthermore, IL-33 can promote the activation of type 2 innate endothelial cells (ILC2), which produce high levels of IL-13 and IL-5 (Vannella et al., Reference Vannella, Ramalingam, Borthwick, Barron, Hart, Thompson, Kindrachuk, Cheever, White, Budelsky, Comeau, Smith and Wynn2016). Specifically, in the chronic phase of infection, the HS strain also induced a progressive increase in the levels of TNF-α in the small intestine, which demonstrated the occurrence of persistent inflammation in this tissue. LPS-stimulated splenocytes from S. mansoni-infected mice that were depleted of CD4 + cells showed a high production of TNF-α, which may be associated with possible septicaemia caused by bacterial translocation due to the intestinal barrier being compromised (Fallon et al., Reference Fallon, Richardson, Smith and Dunne2000). An important control of bacterial infections is performed by neutrophils (Alves-Filho et al., Reference Alves-Filho, Sônego, Souto, Freitas, Verri, Auxiliadora-Martins, Basile-Filho, McKenzie, Xu, Cunha and Liew2010; Kovach and Standiford, Reference Kovach and Standiford2012). In the current study, we demonstrated a reduction in MPO levels in the small intestine of animals that were chronically infected with the HS strain of S. mansoni, which could contribute to ineffective control of possible bacterial dissemination; however, further studies are required to better explore this hypothesis.
Previous studies (Lenzi et al., Reference Lenzi, Kimmel, Schechtman, Pelajo-Machado, Romanha, Pacheco, Mariano and Lenzi1998; Cheever et al., Reference Cheever, Hoffmann and Wynn2000; Hams et al., Reference Hams, Aviello and Fallon2013; Schwartz and Fallon, Reference Schwartz and Fallon2018) have demonstrated that granuloma formation has a dual role in schistosomiasis: the cellular response helps limit the tissue damage caused by lytic substances secreted by the trapped parasite eggs; however, if not well-modulated, the granulomatous response is also responsible for uncontrolled hepatic fibrosis that leads to severe hepatosplenomegaly cases of schistosomiasis. Therefore, the severity of schistosomiasis depends on the balance between granuloma formation and modulation. Additionally, granuloma formation is intrinsically related to the immune response profile induced by the vertebrate host against S. mansoni infection (Hams et al., Reference Hams, Aviello and Fallon2013; Costain et al., Reference Costain, MacDonald and Smits2018), and this response was significantly modified in mice experimentally infected with the HS strain of S. mansoni, which had an impact on schistosomiasis-associated morbidity. Histopathological analysis confirmed that HS-infected mice showed larger and more disorganized granulomas, which caused greater destruction of the hepatic and intestinal tissues. These more severe lesions induced by the experimental infection with the HS strain of S. mansoni were confirmed by the increased activity of ALT and AST in plasma, higher frequency of occult blood in the feces and higher mortality compared to LE-infected mice. ALT and AST are enzymes that participate in the cellular citric acid cycle and are mainly present in the intracellular environment of hepatocytes (Giannini et al., Reference Giannini, Testa and Savarino2005). Therefore, high levels of these enzymes in circulation, especially ALT, are indicative of intense liver damage in both humans (Da Silva et al., Reference Da Silva, Del-Rei, Fraga, Leony, Souza and Santos2018; Dessie et al., Reference Dessie, Lema and Aemero2020) and experimental models of infection with S. mansoni (Rezende et al., Reference Rezende, Moreira, Fernandes, Rodrigues and Negrão-Corrêa2020; Oliveira et al., Reference Oliveira, Rodrigues, Moreira, Rodrigues, Maggi, Resende and Negrão-Corrêa2022). Additionally, the presence of occult blood in feces is a biomarker of high intestinal damage and is widely used to assess the severity of schistosomiasis in humans (Betson et al., Reference Betson, Sousa-Figueiredo, Kabatereine and Stothard2012; Bustinduy et al., Reference Bustinduy, Sousa-Figueiredo, Adriko, Betson, Fenwick, Kabatereine and Stothard2013). Based on the immunopathological profile induced by the experimental infection with S. mansoni HS strain, it is reasonable to propose that the combination of a reduced type 2 and modulatory immune responses in the liver, and a progressive increase in type 1 intestinal inflammation may have led to higher mortality in HS-infected mice when compared with the human LE strain infection. However, it is important to consider that, even though the experimental infection of S. mansoni in mice reproduces many aspects observed in human disease, experimental models of infection have many limitations (Fallon, Reference Fallon2000; Cheever et al., Reference Cheever, Lenzi, Lenzi and Andrade2002). The results of the current study may not represent all the complexity of human infection, and further investigation in human populations from endemic areas where wild rodents participate in the transmission of schistosomiasis is necessary to confirm the impact of these parasite strains on the host's immune response and pathology.
In summary, the data from the current study showed that experimental infection with the S. mansoni strain isolated from H. sciureus in BALB/c mice resulted in low parasite recovery; however, this parasite strain induced a more intense inflammatory response and tissue damage, which led to a greater severity of schistosomiasis.
Acknowledgements
We thank José Carlos dos Reis and Elizabete De Lacorte for the technical support provided during these experiments.
Author contribution
GSM and DAN-C: Conceptualization. GSM, JGMR, MCR, SDR, GMAC, JKAOS, LM, VFR, VGO and DAN-C: Performed experiments. GSM, JGMR, GMAC, JKAOS, VFR and DAN-C: Data analysis. GSM and DAN-C: Supervision and project administration. GSM, JGMR, GMAC, JKAOS and DAN-C: Writing and editing the original draft. All the authors reviewed and approved the final version of this manuscript.
Financial support
DAN-C received research fellowship from Conselho Nacional de Desenvolvimento Cientifico e Tecnológico – CNPq- Brazil. GSM received financial support and a fellowship from Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA-BRAZIL, grant number BD-02007/19). The funder had no role in the study design, data collection and analysis or in the decision to publish the manuscript.
Conflicts of interest
The authors declare there are no competing interests.
Ethical standards
The experimental procedures were approved by the Ethics Committee on Animal Use (protocols n°46/2019 and n°368/2018) of the Federal University of Minas Gerais (UFMG, Brazil). The capture of H. sciureus was authorized by the Biodiversity Authorization and Information System (n°67253-1) and the project is registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (registration number AB9E2EC).