Introduction
The Microsporidia are a hyper-diverse phylum of spore-forming parasites infecting hosts from most animal taxa and an increasing number of protists, from all global biomes (Stentiford et al. Reference Stentiford, Becnel, Weiss, Keeling, Didier, Williams, Bjornson, Kent, Freeman, Brown, Troemel, Roesel, Sokolova, Snowden and Solter2016a,Reference Stentiford, Kerr, Bateman, Feist, Bass, Abollo Rodriguez, Ramilo and Villalbab). Of the 200 or more genera described to date, around half infect aquatic organisms including fish (20 + genera), aquatic arthropods (50 + genera) and a wide array of aquatic non-arthropod invertebrates and protists (including hyper-parasites) (Stentiford et al. Reference Stentiford, Bateman, Feist, Stone and Dunn2013a). They are found in hosts from freshwater, brackish and marine environments, and from a wide range of habitats including lakes, rivers, transitional waters, shorelines, the offshore ocean surface and the deep ocean floor. Combining this wide distribution with relatively scant detailed study of potential host taxa concludes that their diversity in water is vastly under-reported (Appeltans et al. Reference Appeltans, Ahyong, Anderson, Angel, Artois, Bailly, Bamber, Barber, Bartsch, Berta, Błażewicz-Paszkowycz, Bock, Boxshall, Boyko, Brandão, Bray, Bruce, Cairns, Chan, Cheng, Collins, Cribb, Curini-Galletti, Dahdouh-Guebas, Davie, Dawson, De Clerck, Decock, De Grave, de Voogd, Domning, Emig, Erséus, Eschmeyer, Fauchald, Fautin, Feist, Fransen, Furuya, Garcia-Alvarez, Gerken, Gibson, Gittenberger, Gofas, Gómez-Daglio, Gordon, Guiry, Hernandez, Hoeksema, Hopcroft, Jaume, Kirk, Koedam, Koenemann, Kolb, Kristensen, Kroh, Lambert, Lazarus, Lemaitre, Longshaw, Lowry, Macpherson, Madin, Mah, Mapstone, McLaughlin, Mees, Meland, Messing, Mills, Molodtsova, Mooi, Neuhaus, Ng, Nielsen, Norenburg, Opresko, Osawa, Paulay, Perrin, Pilger, Poore, Pugh, Read, Reimer, Rius, Rocha, Saiz-Salinas, Scarabino, Schierwater, Schmidt-Rhaesa, Schnabel, Schotte, Schuchert, Schwabe, Segers, Self-Sullivan, Shenkar, Siegel, Sterrer, Stöhr, Swalla, Tasker, Thuesen, Timm, Todaro, Turon, Tyler, Uetz, van der Land, Vanhoorne, van Ofwegen, van Soest, Vanaverbeke, Walker-Smith, Walter, Warren, Williams, Wilson and Costello2012; Stentiford et al. Reference Stentiford, Bateman, Feist, Stone and Dunn2013a). Recent commentaries have proposed that due to their opportunistic nature and their propensity to infect immune-suppressed organisms, that they may be classified as emerging pathogens in taxonomically diverse hosts throughout the animal–human food chain (Stentiford et al. Reference Stentiford, Becnel, Weiss, Keeling, Didier, Williams, Bjornson, Kent, Freeman, Brown, Troemel, Roesel, Sokolova, Snowden and Solter2016a,Reference Stentiford, Kerr, Bateman, Feist, Bass, Abollo Rodriguez, Ramilo and Villalbab).
Within aquatic arthropods, the skeletal musculature provides a particularly common site of infection for Microsporidia. At least 16 genera (Agmasoma, Ameson, Cucumispora, Inodosporus, Myospora, Nadelspora, Neillimelba, Nosema, Octosporea, Ormieresia, Paradoxium, Perezia, Pleistophora, Thelohania, Vairimorpha, Vavraia) replicate within the musculature, often causing widespread disruption in normal tissue structure, generation of very high parasite burdens and in many cases, the appearance of externally visible clinical signs (see Stentiford et al. Reference Stentiford, Bateman, Feist, Stone and Dunn2013a). The appearance of such clinical signs, coupled with the direct economic value of the musculature of many commercially exploited crustacean hosts, serves to unite the aquatic pathologist with samples of infected hosts revealed during field surveys and by fishers (Stentiford et al. Reference Stentiford, Bateman, Small, Moss, Shields, Reece and Tuck2010, Reference Stentiford, Ross and Kerr2015).
The plasticity of form, and manner of interaction between Microsporidia and the host muscle cell cytoplasm, is particularly varied. Several taxa exist in direct contact with the host cell cytoplasm (e.g. Ameson metacarcini, Small et al. Reference Small, Meyer, Stentiford, Dunham, Bateman and Shields2014) while others develop completely within an interfacial membrane (sporophorous vesicle), separated from organelles of the host cell (e.g. Inodosporus octospora Overstreet and Weidner, Reference Overstreet and Weidner1974; Azevedo et al. Reference Azevedo, Corral and Vivares2000). The musculature similarly does not appear to constrain the morphology of the microsporidian spore per se; ranging from ovoid and hirsute in genera such as Ameson (Small et al. Reference Small, Meyer, Stentiford, Dunham, Bateman and Shields2014), through rod-shaped and smooth in Myospora (Stentiford et al. Reference Stentiford, Bateman, Small, Moss, Shields, Reece and Tuck2010), to needle-like in Nadelspora (Olson et al. Reference Olson, Tiekotter and Reno1994). An increasingly consistent application of combined approaches to typing of known and novel microsporidian taxa (using pathology, morphology and molecular systematics) has revealed that morphology alone is a poor indicator of relatedness (Stentiford et al. Reference Stentiford, Bateman, Feist, Stone and Dunn2013a). In terms of the muscle-infecting microsporidians, the potential synonymy (rather than co-infection) of Nadelspora (smooth, needle-like spore) and Ameson (ovoid, hirsute spores) in individual host crabs (Carcinus maenas) provides an extreme example of this plasticity (Stentiford et al. Reference Stentiford, Bateman, Feist, Chambers and Stone2013b). Improved phylogenies containing increasing numbers of representatives from across the phylum show that the muscle-infecting microsporidians of aquatic arthropods do not necessarily reside within a single closely related clade but more likely exist across a broader grouping of parasites infecting other aquatic invertebrates and fish (Stentiford et al. Reference Stentiford, Bateman, Feist, Stone and Dunn2013a). The potential that microsporidian taxa infecting the musculature of aquatic invertebrates (particularly crustaceans) complete their lifecycles within fish has been proposed (Lom and Nilsen, Reference Lom and Nilsen2003). However, to date, no definitive studies have conclusively demonstrated this phenomenon.
Here, we report the discovery of a novel microsporidian parasite infecting the musculature of the common prawn (Palaemon serratus) from UK waters. The parasite infection displays similar external clinical signs (opaque musculature, lethargy) to previously described I. octospora infections in P. serratus but is otherwise distinct in terms of pathogenesis, morphology and phylogeny. In our study, while both I. octospora and the novel taxon were found infecting individual prawns sampled from the same population, co-infections were not observed. Based upon sequencing of a fragment of the ssrDNA gene from both parasites, we provide the first phylogenetic analysis of I. octospora, and the novel microsporidian described herein. Based upon these analyses, we reveal a potential synonymy between the genus Inodosporus and the fish-infecting microsporidian genus Kabatana and further, a very close relationship between the novel microsporidian described herein and members of the fish-infecting genus Ovipleistohora. Our discovery provides strong evidence for trophic transfer of microsporidians within clade 5 (Vossbrinck et al. Reference Vossbrinck, Debrunner-Vossbrinck, Weiss, Weiss and Becnel2014) of the phylum, between crustacean and fish hosts. Furthermore, it demonstrates that morphologically and phylogenetically distinct microsporidians can infect the same tissues of the same host species to impart clinical signs which mimic infection with the other.
Materials and methods
Field sampling
Prawns (P. serratus) were captured by a commercial fisherman in October 2016 using baited traps, set at a depth of 20–35 m in Carrick Roads (Fal Estuary), Cornwall (between the entrance of the Fal at 50 08 35N and 05 01 43W and west of St. Just in Roseland at 50 11 03N and 05 02 54W). Ten prawns displaying typical clinical signs previously associated with microsporidiosis in other crustacean hosts (lethargy, opaque tissues underlying carapace; Stentiford et al. Reference Stentiford, Ross and Kerr2015) were separated from the remaining catch (of several hundred animals) following visual inspection and transported live to shore. Specimens displaying these clinical signs (Fig. 1) were placed on ice and subsequently sampled at the quayside for histology, transmission electron microscopy (TEM) and molecular diagnostics.
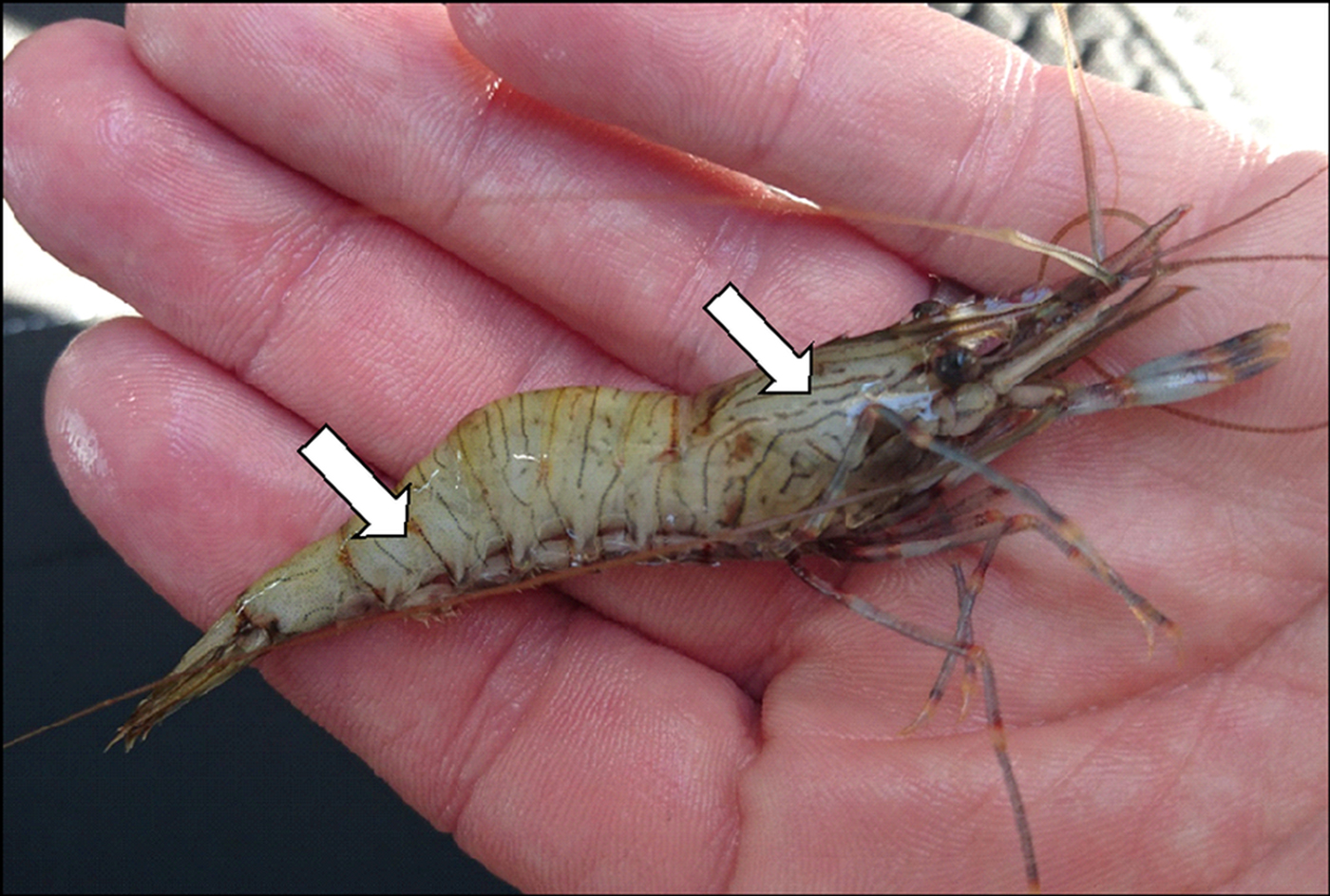
Fig. 1. Clinical signs of microsporidiosis in Palaemon serratus. Opacity and whitening of the skeletal muscles in the abdomen, thorax and limbs was observed in prawns infected with either Inodosporus octospora or Ovipleistophora arlo n.sp. Co-infections were not observed.
Histology and TEM
For histological assessment, the hepatopancreas, gills, heart, midgut, gonad and skeletal muscles were dissected from each prawn displaying clinical signs of microsporidiosis. Excised samples were placed immediately into Davidson's seawater fixative and fixation allowed to proceed for 24 h before transfer to 70% industrial methylated spirit prior to processing. Fixed samples were processed to wax in a vacuum infiltration processor using standard protocols and 3–5 µ m sections were cut using a rotary microtome prior to mounting on glass slides and staining with haematoxylin and eosin (HE). Stained sections were analysed by light microscopy (Nikon Eclipse E800) and digital images were taken using the Lucia™ Screen Measurement System (Nikon, UK). For electron microscopy, 2 mm3 blocks of skeletal muscle, obtained both from the abdomen and thorax, were fixed in 2·5% glutaraldehyde in 0·1 m sodium cacodylate buffer (pH 7·4), for 12 h at room temperature prior to rinsing in 0·1 m sodium cacodylate buffer (pH 7·4) and post-fixation in 1% osmium tetroxide in 0·1 m sodium cacodylate buffer for 1 h. Specimens were washed in two changes of 0·1 m sodium cacodylate buffer and dehydrated through a graded acetone series prior to embedding in Agar 100 epoxy resin (Agar Scientific, Agar 100 pre-mix kit medium) and polymerising overnight at 60 °C in an oven. Semi-thin (0·5–1 µm) sections were stained with Toluidine blue for viewing with a light microscope to identify suitable target areas. Ultrathin sections (70–90 nm) of target areas were mounted on uncoated copper grids and stained with 2% aqueous uranyl acetate and Reynolds’ lead citrate. Grids were examined using a JEOL JEM 1400 transmission electron microscope and digital images captured using an AMT XR80 camera and AMT V602 software.
DNA extraction, PCR, sequencing and phylogenetic analysis
Samples of opaque skeletal muscle from the abdomen and thorax, corresponding to those sampled for histology and electron microscopy, were excised for the sequencing of part of the small subunit ribosomal RNA (SSU rRNA) gene. Total DNA was extracted from muscle homogenates of eight individual P. serratus. Initial homogenization was conducted using Lysing Matrix A FastPrep® tubes with 1 : 10 (w/v) tissue to G2 buffer/Proteinase K (Qiagen, UK) and a Fast Prep cell disrupter (1 min at 5 m s−1). Homogenized samples were incubated for 3 h at 56 °C. Total DNA was extracted using the EZ1 DNA tissue kit and BioRobot® EZ1 (Qiagen, UK) following manufacturer instructions. The partial SSU rRNA gene fragment was amplified using the new microsporidian-specific primer combination CTMicrosp (5′-CACCAGGTTGATTCTGCCTGACG-3′) and Microsp1342r (5′-ACGGGCGGTGTGTACAAAGAACAG-3′), which amplify c. 1100–1300 bp of the microsporidian SSU rRNA gene (depending on the lineage), and in our experience, are the most frequently successful of various microsporidian-specific primer combinations. All PCR reactions were performed in a 25 µL reaction mix consisting of 1 X Green Go Taq buffer, 1 mm MgCl2, 0·25 mm dNTPs, 400 pmol each of the forward and reverse primer, 0·625 units Go Taq Flexi (Promega, UK), 1 µL extracted nucleic acid, and nuclease-free water up to 25 µL. A negative control without template was run alongside the samples. All amplifications were performed using the following thermal cycler programme on a DNA Engine Tetrad® 2 Peltier Thermal Cycler: 95 °C×5 min followed by 35 cycles of 95 °C for 30 s, 63 °C for 30 s and 72 °C for 1 min and 30 s, followed by 72 °C for 10 min, and held at 4 °C. Amplification products were checked on 1% agarose gels stained with ethidium bromide and visualized using a UV illuminator. Products within the expected size range were excised from the gels, purified using the GeneJET Gel Extraction Kit (Thermo Fisher Scientific, UK) and sequenced by Eurofins MWG Operon commercial sequencing facility with the Sanger sequencing method. Analysis of the sequences was completed using BioEdit Sequence Alignment Editor software, version 7.2.5 (Hall, Reference Hall1999).
The two sequences generated by this study [RA16082-1C (‘type 1’; 1210 bp) and RA16082-4C (‘type 2’; 1224 bp)] were aligned using the E-ins-I algorithm within mafft version 7 (Katoh and Standley, Reference Katoh and Standley2013) with 59 other sequences, including their closest named relatives determined by blastn-searches against GenBank (January 2017), representatives of the five proposed microsporidian clades of Vossbrinck et al. (Reference Vossbrinck, Debrunner-Vossbrinck, Weiss, Weiss and Becnel2014), deeply branching microsporidians (including metchnikovellids, Mitosporidium, Nucleophaga and Paramicrosporidium), and a rozellid and aphelid, with a holozoan outgroup. The resulting alignment was analysed using maximum likelihood in RAxML BlackBox version 8 (Stamatakis, Reference Stamatakis2014) [generalized time-reversible (GTR) model with CAT approximation (all parameters estimated from the data); bootstrap values mapped onto the tree with the highest likelihood value]. A Bayesian consensus tree was constructed using MrBayes v 3.2.5 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Hohna, Larget, Liu, Suchard and Huelsenbeck2012). Two separate MC3 runs with randomly generated starting trees were carried out for five million generations each with one cold and three heated chains. The evolutionary model applied included a GTR substitution matrix, a four-category autocorrelated γ correction and the covarion model. All parameters were estimated from the data. Trees were sampled every 1000 generations. The first 1·25 m generations were discarded as burn-in (trees sampled before the likelihood plots reached stationarity) and a consensus tree was constructed from the remaining sample. The two 18S rDNA sequences generated by this study are available from GenBank (accession numbers to be confirmed). All phylogenetic analyses were performed via the Cipres Science Gateway (Miller et al. Reference Miller, Pfeiffer and Schwartz2010).
Results
Histopathology
Histology of prawns displaying externally visible clinical signs of microsporidiosis revealed widespread colonization of the skeletal musculature with two apparently different pathogens (hereafter referred to as type 1 and type 2). Two prawns were infected with type 1, while the remaining eight were infected with type 2. In all cases, muscle fibres and constituent myofibrils of the major abdominal flexor muscles of the abdomen were replaced by masses of parasite life stages. Despite the relatively low power of our analysis (n = 10 animals), prawns only appeared to be infected with either the type 1 or type 2 microsporidian only; histology did not reveal co-infections.
In type 1, parasite-filled cysts extended longitudinally throughout muscle fibres (Fig. 2A). Merogonal and sporogonal life stages were observed (Fig. 2B), with apparent culmination in sets of eight mature spores contained within an interfacial membrane resembling a sporophorous vesicle (Fig. 2C). On numerous occasions, ruptured muscle fibres were associated with haemocyte infiltration and the formation of melanized aggregates (Fig. 2D). Smears produced from fixed material and viewed under phase-contrast microscopy confirmed the presence of eight spores within an interfacial membrane, and the occurrence of three distinctive tail-like appendages protruding from the surface of the mature spore within (Fig. 2E and F). Type 1 histology was consistent with infection by I. octospora, reported previously in the same host by Azevedo et al. (Reference Azevedo, Corral and Vivares2000).
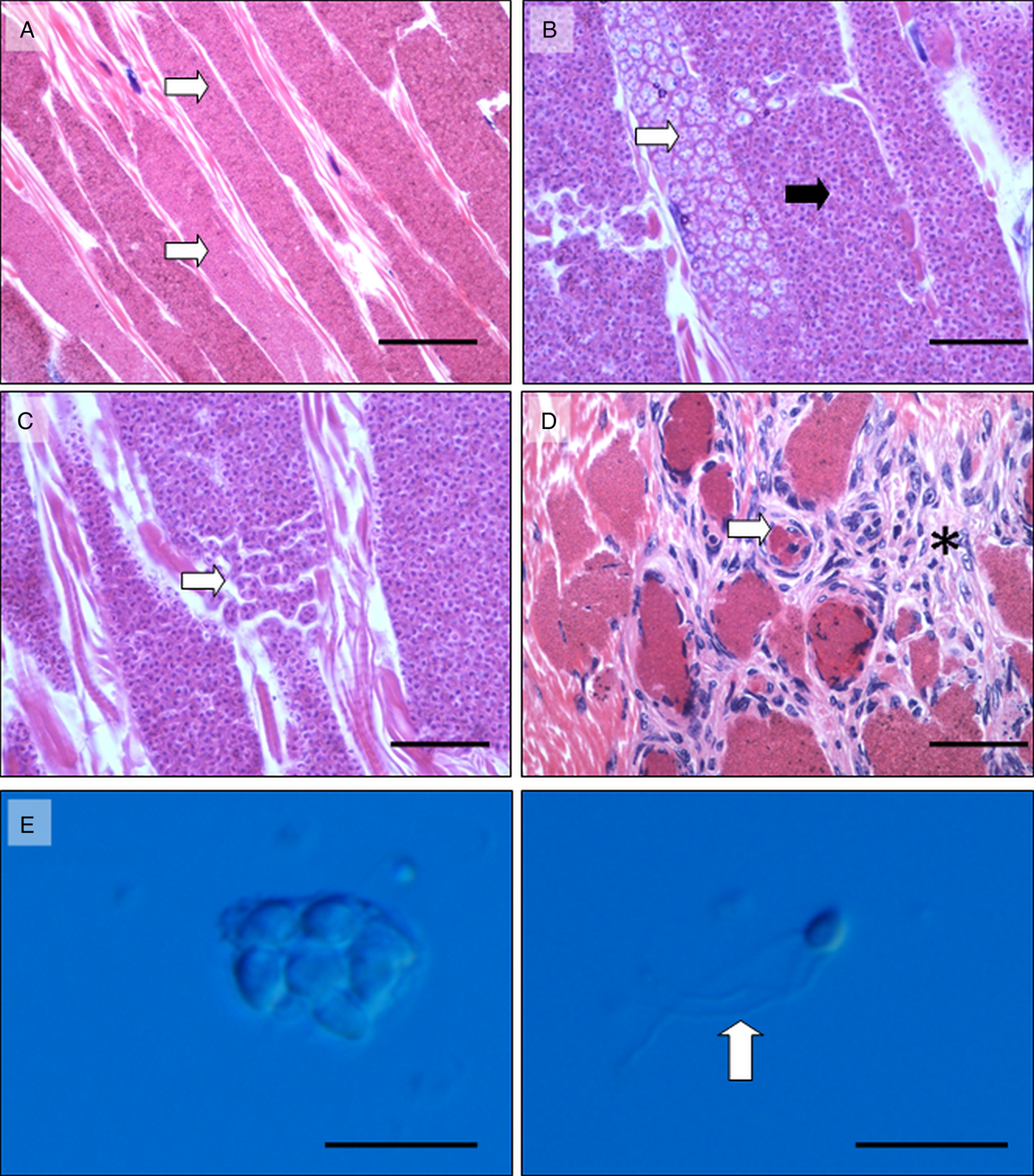
Fig. 2. Histopathology of Inodosporus octospora infection in musculature of Palaemon serratus. (A) Large parasite-filled cysts (white arrows) replace normal muscle fibres. Scale 100 µ m. (B) Mature spores (black arrow) and presumptive earlier life stages (white arrows) appeared to develop synchronously within individual muscle fibres. Scale 50 µ m. (C). Mature spores were contained within an interfacial membrane in sets of eight (white arrow). Scale 25 µ m. (D) Rupture of infected muscle fibres (white arrow) and infiltration of musculature with host haemocytes (asterisk). Scale 100 µ m. (E) Phase-contrast image of sporophorous vesicle containing eight (six fully visible) mature spores. Scale 10 µ m. (F) Phase-contrast image of single spore, liberate from the sporophorous vesicle and possessing three prominent hair-like protrusions (arrow). Scale 10 µ m. Images A–D, HE histology. Images E and F phase-contrast microscopy.
In type 2, parasite-filled cysts also extended longitudinally along muscle fibres, effectively replacing myofibrils and the muscle cell cytoplasm (Fig. 3A–C). In a similar manner to type 1 infections, rupture of infected muscle fibres and presumed exposure of microsporidian parasite life stages to circulating haemocytes elicited haemocyte infiltration and formation of melanized aggregates in type 2-infected prawns (Fig. 3D). Sporophorus vesicles contained up to 16 mature pyriform spores (Fig. 3E, F). Despite these gross similarities between infection types, merogony and early sporogony in the type 2 microsporidian was particularly distinctive. Very early meronts, with dividing nuclei appeared as isolated, deep-blue stained cells amongst eosinophilic myofibres (Fig. 4A). Nuclear division led to the development of multi-nucleate meront plasmodia (Fig. 4B, C), which became increasingly surrounded by an eosinophilic granular substance bound within the sporophorous vesicle which separated parasite life stages from the host cell cytoplasm. When infected muscle fibres were viewed in longitudinal section, a progressive development of microsporidian life stages along the fibre was observed. Early basophilic meronts proceeded to form multi-nucleate meront plasmodia, and distinctive rosette-like plasmodia (containing at least of 16 nuclei). Separation of rosette ‘buds’ to form presumed early sporonts and sporoblasts was also observed. In all cases, the distinctive granular eosinophilic substance between the parasite and the sporophorous vesicle wall was clearly visible (Fig. 4D). Type 2 histology was not consistent with infection by I. octospora as reported in this host by Azevedo et al. (Reference Azevedo, Corral and Vivares2000).

Fig. 3. Histopathology of Ovipleistophora arlo n.sp. infection in musculature of Palaemon serratus. (A) Large parasite-filled cysts (white arrows) replace normal muscle fibres. Scale 100 µ m. (B) Parasite cysts (white arrow) progressively replace myofibrils (black arrow). Scale 25 µ m. (C). Mature spores were contained within an interfacial membrane in sets of at least 16 (white arrow). Scale 25 µ m. (D) Rupture of infected muscle fibres (white arrow) and infiltration of musculature with host haemocytes (asterisk). Scale 100 µ m. (E) Phase-contrast image of sporophorous vesicle containing >16 mature spores. Scale 10 µ m. (F) Phase-contrast image of single spores, liberate from the sporophorous vesicle and not possessing the prominent hair-like protrusions seen in Inodosporus octospora. Scale 10 µ m. Images A–D, HE histology. Images E and F phase-contrast microscopy.

Fig. 4. Merogony and early sporogony of Ovipleistophora arlo n.sp. in the musculature of Palaemon serratus. (A) Early meronts undergoing nuclear division (arrow). The fine granular substance between the meront wall and the interfacial membrane which contains developing parasites (see 4B) is not yet apparent. (B) Further nuclear divisions lead to merogonal plasmodia (arrow), now separated from the interfacial membrane (black arrow) by an eosinophilic granular substance (asterisk) which remains at all other stages of development. (C) Merogony of multiple parasite sets contained within interfacial membranes (black arrows) and forming a discrete cyst within the musculature. (D) Discrete parasite sets within interfacial membranes and forming discrete cysts within the musculature. In many cases, development of parasite sets appeared to occur in linear sequence (e.g. in the direction of the white arrow), culminating in distinctive ‘rosette-like’ clusters which preceded separation of sporoblasts (black arrows). Adjacent cysts (Cy) contain mature spores, still enclosed within the interfacial membrane developed during merogony. All scale bars 10 µ m. All images HE histology.
Ultrastructure
TEM applied to samples of prawns infected with either type 1 or type 2 microsporidiosis revealed multiple life stages of two morphologically distinct microsporidian parasites within the cytoplasm of host muscle cells. As observed in histological analyses, co-infections were not observed in samples observed using TEM.
As reflected in histology, ultrastructural characteristics of the type 1 microsporidian were consistent with previous observations of I. octospora infecting this host (Azevedo et al. Reference Azevedo, Corral and Vivares2000). Although we did not commonly observe meronts and could not confirm the presence of a diplokaryon state in early development, the formation of eight uninucleate sporonts within a defined sporophorous vesicle, the size and structure of sporoblasts and mature spores and particularly, the presence of several tail-like appendages protruding from the spore surface (and folded within the confines of the sporophorous vesicle) were highly consistent with this description (see Fig. 5A–H). We conclude from these observations that type 1 microsporidiosis as observed here is due to I. octospora.

Fig. 5. Sporogonal development of Inodosporus octospora in the musculature of Palaemon serratus. All life stages occurred within an interfacial membrane separating the parasites/s from the host cell cytoplasm and were consistent with the description by Azevedo et al. (Reference Azevedo, Corral and Vivares2000). Diplokaryotic merogonal stages were infrequently observed in the two infected P. serratus examined. (A–D) Differential representation of unikaryotic sporont cell numbers within the interfacial membrane (arrows) with 1- (A), 2- (B), 4- (C) and 6- (D) visible cells (the latter representing the mature 8-cell set within an individual packet). In all stages, a fine granular substance (and occasional membranous elements) filled the space between the sporont wall and the interfacial membrane (asterisks). Membranes were increasingly frequent within the space as sporonts matured to sporoblasts (E,F) and mature spores (G) (black arrows). Mature spores possessed numerous tail-like appendages (white arrow) which apparently emanated from the exospore and extended in to the granular space between mature spores and the interfacial membrane (asterisk). Scale A–D 500 nm. Scale E 1 µ m. Scale F, G, H 500 nm. All transmission electron microscopy
Ultrastructural characteristics of the type 2 microsporidian were not consistent with previous observations of I. octospora infecting this host (Azevedo et al. Reference Azevedo, Corral and Vivares2000). In line with the development cycle observed in histology, apparently uninucleate meronts, bound by a simple membrane (Fig. 6A) and contained within an interfacial membrane, underwent nuclear division (Fig. 6B) to form large multi-nucleate meronts (Fig. 6C). A fine granular substance occupied the space between the meront wall and the interfacial membrane (Fig. 6D). This granular substance remained during thickening of the meront wall to form the sporont plasmodium (Fig. 6E), and separation of apparently uninucleate sporonts from this plasmodium (Fig. 6F). Uninucleate sporonts often possessed a crenelated cell wall (Fig. 7A) and were arranged in ‘rosettes’ (Fig. 7B), a feature observed in histological preparations (see Fig. 4E). Development of precursors of the spore-extrusion apparatus (polar filament, anchoring disk) only occurred after separation of uninucleate sporonts from plasmodia, marking their progression to sporoblasts (Fig. 7C). Maturing sporoblasts displayed an increasingly organized internal arrangement of 7–8 turns of a tapering polar filament (Fig. 7D), a feature retained in the mature spore (Fig. 7E, F). During latter stages of spore maturation, the granular substance contained within the space between parasite cells and the interfacial membrane organized in to circular crystalline stacks (Fig. 7D, E, F). Crystalline stacks were not apparently attached to the surface of parasite cells. Mature spores measured 4·237 µ m (s.e. ± 0·03 µ m) by 2·258 µ m (s.e. ± 0·02 µ m) (n = 30), were uninucleate, contained eight coils of a tapering polar filament, arranged in a single rank along the spore wall. Spores possessed a broad, umbrella-shaped anchoring disc covering the anterior region of the bi-laminar polaroplast (inset, Fig. 7F). The spore wall was trilaminar with a thin electron-lucent exospore and a thickened, electron-lucent endospore overlaying the plasmalemma (Fig. 7E, F). Although not possible to enumerate accurately by TEM, sporophorous vesicles appeared to contain at least 16 spores (Fig. 3).

Fig. 6. Merogony and early sporogony of Ovipleistophora arlo n. sp. in the musculature of Palaemon serratus. (A) Apparently uninucleate meronts (nuclei depicted as 1n) developed in close contact with the muscle cell cytoplasm although separation of the two closely opposed membranes of the cell wall (white arrow) may be the precursor to formation of the interfacial membrane that contains all developing life stages thereafter. (B, C) Nuclear division (1n) served to increase the nuclear contingent of the meront as it formed large, granular and vacuolated cells within an interfacial membrane (black arrow). A fine granular substance (asterisk) filled the space between the meront/sporont wall and the interfacial membrane. (D) Transition from the meront to sporont was cryptic but involved thickening of the parasite cell wall and gradual compartmentalization (white arrow) of the sporont plasmodium. (E) Multiple parasite sets, contained within interfacial membranes (black arrow) were often closely opposed in ‘cysts’ but themselves were not apparently contained within a further bounding membrane. Instead, they were in direct contact with surrounding host musculature (Mu). (F). Separation of uninucleate sporonts (1n) from the sporont plasmodium precedes development of spore-extrusion precursors in the early sporoblast (polar filament precursors labelled with white arrow). Scale A, B, E, F 500 nm; C, D 1 µ m. All images transmission electron microscopy.

Fig. 7. Sporogony of Ovipleistophora arlo n. sp. in the musculature of Palaemon serratus. (A, B) Separation of uninucleate sporonts appeared to occur randomly, or in a more orderly manner leading to creation of sporont ‘rosettes’ which corresponded to development observed via histology. In case of the latter, sporonts often possessed a crenelated cell wall (black arrow) and vacuolated cytoplasm (white arrow). In all cases, sporonts resided within the fine, granular matrix within the interfacial membrane (asterisks). (C) Sporoblasts were uninucleate (1n) and in early stages contained a disorganized polar filament precursor (white arrow) and an electron-lucent vacuole (black arrow). (D) Later stage sporoblasts possessed a tapering polar filament, coiled 8–9 times in a single rank along the inner edge of the endospore (black arrow), an electron-dense polar vacuole (white arrow) and a labyrinthic structure resembling Golgi (white arrow head). The granular matrix filling the space between the spore wall and interfacial membrane started to arrange in to disk-like crystalline stacks which remained through later stages of development to the mature spore (black arrow head). (E, F) Mature spore with trilaminar wall composed of thin exospore, thick electron-lucent endospore (white arrow) and thin endospore. An anchoring disk (inset in F) terminated the tapering polar filament (black arrow) at the slightly constricted anterior of the spore (white arrowhead), after passing through a laminar polaroplast (white asterisk in Inset). The disk-like crystalline stacks in the space between the spore wall and interfacial membrane became increasingly densely packed (black asterisk). Mature spores existed in packets of at least 16 within the interfacial membrane, corresponding with histological observations. Scale A, D, E 500 nm; Scale B, C 2 µ m; Scale F 100 nm. All transmission electron microscopy.
Molecular systematics
Type 1. The ultrastructure of the type 1 microsporidian parasite described herein is consistent with I. octospora Overstreet and Weidner, Reference Overstreet and Weidner1974, infecting P. serratus in Europe. The parasite had previously been reported as Thelohania octospora (Pixell-Goodrich, Reference Pixell-Goodrich1920; Codreanu, Reference Codreanu and Corradetti1966) and later, Orthohelohania octospora (Codreanu et al. Reference Codreanu, Codreanu-Balcescu and Porchet-Hennerb1974) before being transferred to a novel genus Inodosporus (Overstreet and Weidner, Reference Overstreet and Weidner1974) based upon the presence of distinctive spore tails. Azevedo et al. (Reference Azevedo, Corral and Vivares2000) provided the first comprehensive ultrastructural analysis of I. octospora though no phylogenetic information was presented. In the current study, based on sequencing of 1210 bp of the SSU rRNA gene, we demonstrate that I. octospora branches within clade 5 of the Microsporidia (Vossbrinck et al. Reference Vossbrinck, Debrunner-Vossbrinck, Weiss, Weiss and Becnel2014) and specifically, with members of the fish-infecting genus Kabatana (Dyková and Tonguthai, 2000). Our 1210 bp SSU rDNA sequence of I. octospora is identical to EU682928 (Kabatana sp. JI 2008) from the two-spot goby (Gobiusculus flavescens) and has 98·4% sequence similarity with JQ062989 (Kabatana sp. JS 2012), from the Arrow goby (Clevelandia ios). Therefore, our data provide strong evidence for synonymy between the genera Inodosporus (Overstreet and Weidner, Reference Overstreet and Weidner1974), and Kabatana (Lom et al. Reference Lom, Dyková and Tonguthai2000). Further, there is potential for synonymy between EU682928 (Kabatana sp. JI 2008 Barber et al. Reference Barber, Davies, Ironside, Forsgren and Amundsen2009, infecting G. flavescens) and I. octospora, which may be clarified by further experimental and phylogenetic studies.
Type 2. Based upon both morphological and phylogenetic data, Pekkarinen et al. (Reference Pekkarinen, Lom and Nilsen2002) established the genus Ovipleistophora to discriminate several oocyte-infecting pathogens in fish from other members of the genus Pleistophora. At present, the genus contains two species, Ovipleistophora mirandellae (Vaney and Conte, Reference Vaney and Conte1901) and Ovipleistophora ovariae (Summerfelt, Reference Summerfelt1964), both infecting the oocytes of freshwater fish. Although the morphology of the type 2 microsporidian described here infecting P. serratus is not at all consistent with that of existing members of the genus Ovipleistophora, phylogenetic data propose a very close relationship; the 1224 bp type 2 sequence having 99·5% similarity to AJ252954 [(O.) Pleistophora mirandellae from roach Rutilus rutilus, and 98·9% similarity to AF356223 O. mirandellae from common ruffe Gymnocephalus cernuus]. A third freshwater fish-infecting microsporidian, Pleistophora ovariae, infecting the oocytes of the golden shiner (Notemigonus crysoleucas) also groups with these parasites and is thus likely a member of the genus Ovipleistophora based upon the taxon description of Pekkarinen et al. (Reference Pekkarinen, Lom and Nilsen2002). Two other microsporidians Pleistophora hyphessobryconis KM458272 (Sanders et al. Reference Sanders, Lawrence, Nichols, Brubaker, Peterson, Murray and Kent2010) and Pleistophora beebei KX099692 (Casal et al. Reference Casal, Matos, Rocha, Sindeaux-Neto, Al-Quraishy and Azevedo2016), infecting the muscles of tropical freshwater fish, form a subclade to Ovipleistophora in our analysis. We conclude that based upon similarity in SSU rDNA, the type 2 microsporidian parasite detected in P. serratus is a member of the genus Ovipleistophora. However, the lack of identity in SSU rDNA to known taxa within the genus when coupled with the different ecological context in which the type 2 parasite was detected (marine) provides support for erection of a new species within the genus: Ovipleistophora arlo n. sp.
Taxonomic summary
Type 1 microsporidian; I. octosporaOverstreet and Weidner, Reference Overstreet and Weidner1974
Based upon histological and ultrastructural evidence, the type 1 microsporidian described herein is a neotype of I. octospora (Overstreet and Weidner, Reference Overstreet and Weidner1974). Phylogenetic analysis, using partial sequence of the SSU rDNA gene for I. octospora is provided here for the first time, and gives evidence both for its placement within clade 5 of the Microsporidia (Vossbrinck et al. Reference Vossbrinck, Debrunner-Vossbrinck, Weiss, Weiss and Becnel2014), and for potential synonymy between the genera Inodosporus and Kabatana. Although morphologically distinct, the identity in SSU rDNA sequence between I. octospora and the Kabatana sp. (NCBI accession #EU682928) infecting the muscles of two-spot goby (G. flavescens) from Sweden (Barber et al. Reference Barber, Davies, Ironside, Forsgren and Amundsen2009) raises the prospect that these two parasites are the same. If proven by further experimental or phylogenetic analysis, by the principle of priority, Kabatana sp. would be become a junior synonym of Inodosporus, and specific taxa such as #EU682928 (Barber et al. Reference Barber, Davies, Ironside, Forsgren and Amundsen2009) may be synonymized with I. octospora. We also acknowledge that as currently presented, our data cannot yet support this synonymization.
Type 2 microsporidian; O. arlo n.sp.
Phylum Microsporidia (Balbiani, Reference Balbiani1882)
Clade 5 (Vossbrinck et al. Reference Vossbrinck, Debrunner-Vossbrinck, Weiss, Weiss and Becnel2014)
Genus Ovipleistophora (Perkarinnen et al. 2002)
Parasites described to date infect the oocytes of freshwater fish and their digenean hyper-parasites (Lovy and Friend, Reference Lovy and Friend2017). The type species O. mirandellae colonizes the cytoplasm of the eggs of common ruffe (G. cernuus) and roach (R. rutilus) in Europe. It possesses unikaryotic nuclei throughout development and meronts are covered with an elaborate surface membrane and a thick surface coat consisting of masses of vesicles in a finely granular substance. Sporogony is polysporoblastic, proceeding by segmentation of the sporogonial plasmodium, leading to large number of spores within sporophorous vesicles. Spores possess a large posterior vacuole and are dimorphic (macro- and microspores), differing in overall size, size of polar vacuole and number of turns of the polar filament. Based upon SSU rDNA phylogeny, the type species groups closely with another fish egg-infecting taxon, O. ovariae (Summerfelt, Reference Summerfelt1964) in the golden shiner (N. crysoleucas) (Pekkarinen et al. Reference Pekkarinen, Lom and Nilsen2002). Morphological description of the type 2 microsporidian described herein is not consistent with that of existing members of the genus. Our placement of the type 2 microsporidian in Ovipleistophora is based upon high similarity of SSU rDNA sequence between the prawn parasite and O. mirandellae AJ252954 infecting roach (99·5%) and AF356223 infecting common ruffe (98·9%). Based upon this similarity, while noting limitation of utilizing morphology as a reliable indicator of relatedness in the Microsporidia (Stentiford et al. Reference Stentiford, Bateman, Feist, Stone and Dunn2013a), we extend the generic description of the genus to include specific features observed in infected P. serratus: all stages occur within skeletal muscles of infected prawns. Uninucleate meronts bound by a simple membrane contained within an interfacial membrane undergo nuclear division to form multi-nucleate meronts. A fine granular substance occupies the space between the meront wall and the interfacial membrane, remaining throughout sporogony. Sporonts which separate from the sporont plasmodium are initially bound by a crenelated cell wall and form ‘rosettes’. Precursors of spore-extrusion elements (polar filament, anchoring disk) occur after separation of sporonts from plasmodia, marking their progression to sporoblasts. Maturing sporoblasts display 7–8 turns of a tapering polar filament. During latter stages of spore maturation, the granular substance contained within the space between parasite cells and the interfacial membrane organizes in to circular crystalline stacks; though these do not apparently associate with the spore wall. Mature spores measure 4·237 µ m (s.e. ± 0·03μm) by 2·258 µm (s.e. ± 0·02 µ m) (n = 30), are uninucleate, contain eight turns of a tapering polar filament, arranged in a single rank along the spore wall. Spores possess a broad, umbrella-shaped anchoring disc covering the anterior region of the bi-laminar polaroplast and a trilaminar wall with thin electron-lucent exospore and thickened, electron-lucent endospore overlaying the plasmalemma. All life stages are contained within a sporophorous vesicle, in maturity containing at least 16 spores.
Ovipleistophora arlo. n. sp Stentiford, Ross, Minardi, Feist, Bateman, Gainey, Troman and Bass
Description as for prawn-specific life stages of the genus Ovipleistophora. Distinctive partial SSU rDNA gene sequence from closest branching neighbour, O. mirandellae per the methods outlined in this study.
Type host. Common prawn (P. serratus) (Decapoda: Palaemonidae).
Site of infection. Skeletal musculature of abdomen and thorax.
Type locality. Carrick Roads (Fal Estuary), Cornwall (between 50 08 35N and 05 01 43W, and 50 11 03N and 05 02 54W).
Etymology. The generic epithet follows that described for Ovipleistophora by Perkarinen et al. (2002) and indicates a propensity for infection of the oocytes of fish. The specific epithet is derived from the first name (Arlo) of the son of the first author who ably assisted during the fieldwork components of this study.
Type material. Histological sections, TEM resin and digital pathology preparations are deposited in the Registry of Aquatic Pathology (RAP) at the Cefas Weymouth Laboratory, UK. Ovipleistophora arlo SSU rDNA gene sequences from samples collected in the United Kingdom (UK) have been deposited in Gen-Bank (accession numbers to be assigned).
Discussion
In this paper, we report the discovery of a novel microsporidian parasite infecting the musculature of the common prawn (P. serratus) from UK waters. Infection with the parasite (herein O. arlo n. sp.) elicits similar clinical signs to the previously described microsporidian I. octospora, also known to infect the musculature of P. serratus from Europe (Azevedo et al. Reference Azevedo, Corral and Vivares2000). However, despite similar clinical manifestation and the presence of both parasites in the same population of prawns, morphology, development cycle, and phylogeny of O. arlo and I. octospora is otherwise entirely distinct.
Evidence of a crustacean fish lifecycle for I. octospora
We provide the first phylogenetic sequence data for I. octospora and demonstrate potential synonymy between the genera Inodosporus and Kabatana. Furthermore, we show that at least based upon SSU rDNA sequence, the I. octospora in prawns may be the same parasite as Kabatana sp. JI 2008 (Genbank #EU682928) infecting two-spot goby G. flavescens sampled from Swedish waters (Barber et al. Reference Barber, Davies, Ironside, Forsgren and Amundsen2009). In G. flavescens, Kabatana sp. JI 2008 is known to infect the skeletal musculature (Barber et al. Reference Barber, Davies, Ironside, Forsgren and Amundsen2009). Morphologically, the description of Kabatana sp. JI 2008 differs from that of I. octospora infecting P. serratus (this study). However, the well-documented capacity for significant between-host (e.g. Nylund et al. Reference Nylund, Nylund, Watanabe, Arnesen and Karlsbank2009) and even within-host (Stentiford et al. Reference Stentiford, Bateman, Feist, Chambers and Stone2013b) morphological plasticity of the same microsporidian taxon in different part of its life cycle do not preclude a hypothesis that I. octospora and Kabatana sp. JI 2008 may be the same pathogen. Given that P. serratus and G. flavescens have considerable niche overlap in European marine habitats (Collins, Reference Collins1981; Potts et al. Reference Potts, Edwards and Costello1990; http://www.marlin.ac.uk/), there is potential for this parasite to cycle between these hosts. Although not supported fully by data presented in the current study, by principles of taxonomic priority, the genus Kabatana would therefore be considered a junior synonym of Inodosporus, and specific taxa therein may also be re-assigned as appropriate. Given that Kabatana spp. have been described infecting fish hosts from numerous locations around the globe (McGourty et al. Reference McGourty, Kinziger, Hendrickson, Goldsmith, Casal and Azevedo2007; Barber et al. Reference Barber, Davies, Ironside, Forsgren and Amundsen2009), the potential for observations presented in this paper to represent a generic lifecycle strategy in this taxon requires consideration. Further, our discovery of potential synonymy between a crustacean- and fish-infecting microsporidian occupying an overlapping marine niche provides strong support for fish-invertebrate life cycling within at least those microsporidians within clade 5 (Vossbrinck et al. Reference Vossbrinck, Debrunner-Vossbrinck, Weiss, Weiss and Becnel2014), a phenomenon previously proposed, but not demonstrated by Lom and Nilsen (Reference Lom and Nilsen2003).

Fig. 8. Bayesian SSU rRNA phylogeny including type 1 (Inodosporus octospora) and type 2 (Ovipleistophora arlo n. sp.) microsporidians described in this study, and 59 other sequences representing microsporidians in clades 1–5, rozellids and aphelids, rooted on Holozoa. A total of 1340 alignment positions were analysed. Bayesian posterior probabilities/maximum likelihood bootstrap values are indicated on nodes; black filled circles indicate values of ⩾0·95 and 95%, respectively.
Close relations between O. arlo and other fish-infecting microsporidians in clade 5
The finding of a taxonomically distinct microsporidian to I. octospora, also infecting the skeletal musculature of P. serratus, collected from the same location at the same time of year, appears remarkable. Ovipleistophora arlo n.sp. elicited identical clinical signs to I. octospora but was otherwise distinctive in terms of morphology, life cycle and phylogeny. Given that infection with O. arlo mimicked the external clinical signs of infection with the previously known pathogen I. octospora, it is pertinent to note how consistent application of sensitive and specific diagnostics able to define candidate agents is a critical element of disease surveillance (Stentiford et al. Reference Stentiford, Feist, Stone, Peeler and Bass2014). Challenge to the parasite competitive exclusion principle (in which occupation of a host or specific tissue therein occurs at the exclusion of other parasites; Hardin, Reference Hardin1960) is increasingly occurring in other host–pathogen systems, principally driven by more consistent application of discriminatory molecular diagnostics which expose infection by different taxa (Fitt et al. Reference Fitt, Huang, van den Bosch and West2006). Temporal niche partitioning (where parasites may occupy a host or tissue therein at different times in its life history) is more commonly accepted (Hamelin et al. Reference Hamelin, Bisson, Desprez-Loustau, Fabre and Mailleret2016). Given that cases of co-infection of I. octospora and O. arlo were not recorded in P. serratus during this study (via histology, TEM or molecular diagnostics, albeit on a relatively small number of animals), competitive exclusion (or even temporal niche partitioning) cannot be excluded at this point. However, given the likely fatal nature of the disease associated with infection by both parasites, temporal niche partitioning at least, appears unlikely.
Despite distinction in host type, tissue tropism and even habitat (marine), O. arlo is a very close phylogenetic relation to other known members of the genus Ovipleistophora. Based upon SSU rRNA gene sequence O. arlo is most similar to AJ252954 (O.)P. mirandellae infecting roach (R. rutilus) and AF356223 O. mirandellae infecting common ruffe (G. cernuus), both freshwater fish inhabiting European waterways (99·5% and 98·9% similarity, respectively). O vipleistophora arlo also clades with a third freshwater fish-infecting microsporidian, (O.)P. ovariae infecting the tropical freshwater golden shiner (N. crysoleucas). All of these fish-infecting microsporidians colonize the oocytes of their host and are likely vertically transmitted (Pekkarinen et al. Reference Pekkarinen, Lom and Nilsen2002). Furthermore, each of these fish species are batch spawners, laying their sticky eggs on stones and vegetation (Mills, Reference Mills1981; Brown et al. Reference Brown, Selgeby and Collins1998) or even in the nests of other fish species (Shao, Reference Shao1997). The presence of a member of the genus Ovipleistophora in an invertebrate host from a marine/estuarine environment (this study) initially appears surprising. However, given that most of the studies on microsporidiosis in fish have focussed on commercially exploited, or model taxa, under-reporting of both host range, and parasitic strategy within the osteichthyds is thought highly likely (Stentiford et al. Reference Stentiford, Bateman, Feist, Stone and Dunn2013a). Fish species exhibiting oviparity (internal fertilization, shedding of zygotes to the water) or ovuliparity (external fertilization following shedding of eggs and sperm to the water) (Lodé, Reference Lodé2012) appear to be the most likely candidate hosts for Ovipleistophora or other oocyte-infecting microsporidians. In habitats such as the Fal estuary, those fish taxa which deposit their eggs in overlapping niches with P. serratus provide a potential means for re-infection of naïve prawns via egg predation. Members of the Gobiidae, one of the largest families of teleost fish are widespread in global estuarine, brackish and even freshwater environments (Patzner et al. Reference Patzner, Van Tassell, Kovačiĉ and Kapoor2011). The female attaches her eggs to benthic surfaces prior to fertilization and guarding by the male (Hoese, Reference Hoese, Paxton and Eschmeyer1998). Gobies are relatively omnivorous consumers with a predilection for benthic crustaceans (Collins, Reference Collins1981). In some cases, gobies form symbiotic associations (burrow sharing for egg laying) with benthic shrimp (Helfman et al. Reference Helfman, Colette and Facey1997). There are at least 10 species of goby known to inhabit waters of the UK, the distribution of most of which includes the English Channel (http://www.marlin.ac.uk/). Given the wide distribution of P. serratus in UK (and European) waters, and their preponderance for inhabiting sub-tidal habitats down to 40 m depth, all of these goby species have potentially overlapping habitat niches with the prawn (http://www.marlin.ac.uk/). Highly abundant species such as the common goby (Pomatoschistus microps), sand goby (Pomatoschistus minutus), two-spotted goby (G. flavescens) and painted goby (Pomatoschistus pictus) appear therefore to be excellent candidate hosts for O. arlo and are worthy of further investigation. The potential synonymy between Kabatana and Inodosporus (section 4·1) supports the principle that prawn-gobiid microsporidian lifecycles are likely. Further, these discoveries expose a wider potential for trophic transfer of microsporidians across clade 5 of the phylum.
Recent studies have provided evidence of a novel Ovipleistophora (O. diplostomuri) causing infection in a marine fish (Lepomis macrochirus) and its digenean parasite Posthodiplostomum minimum (Lovy and Friend, Reference Lovy and Friend2017); the authors proposing infection of the digenean follows prior infection of fish tissues. Infection leads to degeneration of the encysted worm suggesting that either P. mimumum is a dead-end host for O. diplostomuri, or that it forms an additional medium for the transmission of the parasite via predation on tissues of the fish host. The phylogenetic distinction of O. diplostomuri from other known digenean-infecting microsporidia (e.g. Unikaryon legeri in cockles; Stentiford et al. Reference Stentiford, Becnel, Weiss, Keeling, Didier, Williams, Bjornson, Kent, Freeman, Brown, Troemel, Roesel, Sokolova, Snowden and Solter2016a,Reference Stentiford, Kerr, Bateman, Feist, Bass, Abollo Rodriguez, Ramilo and Villalbab) reveals a potential for diverse microsporidians to capitalize on the existing parasitic life cycle strategies of other parasites, or an ability to opportunistically utilize a wide array of cellular substrates present within host tissues (in this case digenean cysts) for their own propagation.
The muscle as a ‘seat of infection’ for the Microsporidia
The Phylum Arthropoda (including the subphyla Insecta, Arachnida, Myriapoda and the Crustacea) are the most species-rich ecological animal guild on the planet. With over one million described taxa they comprise over 80% of known animal species (Zhang, Reference Zhang2011), with a potential for several million more currently unknown types (Ødegaard, Reference Ødegaard2000). The Insecta is certainly the most species rich (Thompson, Reference Thompson1994), while the Crustacea contains at least 67 000 described species, occupying the full spectrum of terrestrial and aquatic habitats (Zhang, Reference Zhang2011). Specific groups (such as krill, copepods) are hyper-abundant in the global oceans and likely represent the greatest biomass of any animal group on the planet (Atkinson et al. Reference Atkinson, Siegel, Pakhomov, Jessopp and Loeb2009).
It is pertinent to consider also that the Arthropoda are the animal group in which the Microsporidia appear to have colonized most successfully, with numerous known microsporidian genera infecting insects and aquatic crustacean hosts; their presence in other invertebrate taxa (e.g. Mollusca) is rare in comparison (Stentiford et al. Reference Stentiford, Bateman, Feist, Stone and Dunn2013a). Particularly within the crustaceans, the muscle provides the most common site of infection; invasion of the mitochondria-rich zone beneath the sarcolemma preceding colonization of the muscle fibre by masses of merogonal and sporogonal life stages (and accompanied by complete degeneration of normal muscle structure and function) (e.g. Stentiford et al. Reference Stentiford, Ross and Kerr2015). In many crustaceans, the musculature comprises the most significant proportion of the body mass, particularly in the Decapoda where it is exploited as a human food resource in fished and farmed species. In large-clawed crabs (such as the European edible crab, Cancer pagurus), musculature comprises around 50% of the wet tissue mass (Barrento et al. Reference Barrento, Marques, Teixeira, Carvalho, Vaz-Pires and Nunes2009), a similar proportion to that observed in pandalid and penaeid prawns (Crawford, Reference Crawford1980). The combination of hyper-diversity and abundance, the high proportion of musculature in the crustacean body plan, and its biochemical (carbohydrate-rich) and physiological (mitochondria-rich) composition has apparently provided an ideal medium for radiation of the phylum Microsporidia within the arthropods. The intense colonization of the musculature of microsporidian-infected arthropod hosts effectively creates a ‘parasite factory’ within terrestrial and aquatic ecosystems, facilitating generation of infectious propagules (spores), and a means for their transmission to other hosts. In the case of microsporidians within clade 5, we propose that this scenario underpins multi-trophic transmission of parasites at least from crustacean to fish hosts. The importance of crustaceans in marine food webs and particularly, their role in the diet of fish (Turner, Reference Turner2004) supports this principle (Turner, Reference Turner2004).
The crustacean musculature has not restricted the morphological spectrum of those microsporidians which reside there. Development can occur in direct contact with the host sarcoplasm (e.g. Myospora, Stentiford et al. Reference Stentiford, Bateman, Small, Moss, Shields, Reece and Tuck2010), or within interfacial membranes which separate developing life stages from contact with host organelles (e.g. Thelohania) (Henneguy and Thelohan, Reference Henneguy and Thelohan1892). Spore stages range in shape from ovoid (e.g. Paradoxium, Stentiford et al. Reference Stentiford, Ross and Kerr2015), to bacilliform (Myospora, Stentiford et al. Reference Stentiford, Bateman, Small, Moss, Shields, Reece and Tuck2010) to needle-like (e.g. Nadelspora, Olson et al. Reference Olson, Tiekotter and Reno1994); and from smooth (e.g. Myospora) to highly ornamented (e.g. Ameson, Small et al. Reference Small, Meyer, Stentiford, Dunham, Bateman and Shields2014), a trait which has been speculated to facilitate transmission in aquatic systems (Vávra and Lukeš, Reference Vávra and Lukeš2013). The demonstration of infection potential across an extremely wide array of host taxa (from protists to humans), with emphasis on taxonomic radiation in hyper-abundant prey items within terrestrial and aquatic food chains (arthropods) proposes that trophic transfer of microsporidians will be increasingly defined as a core transmission strategy in the phylum. The consistent application of tools which allow for accurate diagnosis and systematics of specific taxa, wherever they may reside in these food chains, will provide clarity on this complexity, and will provide a means by which those microsporidians with the greatest burden on animal and human health have the potential to be controlled.
Acknowledgements
The authors acknowledge the assistance of fisherman Mr Cameron Henry for bringing infected prawns to our attention, and for obtaining the animals used in this study.
Financial support
This work was supported by funding from the UK Department of Environment, Food and Rural Affairs (Defra) under contract #FC1212 (to GDS) and FB002 (to SWF), and to the European Commission under contract C6928 (to GDS and KB). DB was additionally supported by a NERC Standard Research Grant (NE/H000887/1) and the Natural History Museum, London.











