Introduction
Digenean flatworms are major parasites of ruminants and are the aetiological agents for pancreatic eurytrematosis (PE). PE is an under diagnosed or possibly neglected parasitosis in goats and sheep in the Americas. The disease is well-documented in cattle in Brazil and Asia (Tang and Tang, Reference Tang and Tang1977; Tang and Lin, Reference Tang and Lin1980; Bassani et al., Reference Bassani, Sangioni, Saut, Headley and Yamamura2006; Schwertz et al., Reference Schwertz, Lucca, Silva, Baska, Bonetto, Gabriel, Centofanti and Mendes2015; Okajima et al., Reference Okajima, Shibata, Takahashi, Nagafuchi, Okajima and Nonaka2016). There are a few reports of PE in small ruminants and existent reports are primarily from Asia (Tang and Lin, Reference Tang and Lin1980; Graydon et al., Reference Graydon, Carmichael, Sanchez, Weidosari and Widjayanti1992; Dorny et al., Reference Dorny, Batubara, Iskander and Pandey1996; Ma et al., Reference Ma, He, Li, Guo, Pan, Wang, Zhang and Liu2014).
Eurytrema sp. are flukes from the Dicrocoeliidae family that infect pancreatic ducts of ruminants (Bassani et al., Reference Bassani, Sangioni, Saut, Headley and Yamamura2006; Sakamoto and Oikawa, Reference Sakamoto and Oikawa2007; Leite et al., Reference Leite, Lopes-Torres, Souza, Neves, Gomes and Machado-Silva2020). The life cycle of Eurytrema sp. is complex and includes invertebrate hosts such as land snails and grasshoppers, with ruminants as definitive hosts (Tang and Tang, Reference Tang and Tang1977; Schwertz et al., Reference Schwertz, Lucca, Silva, Baska, Bonetto, Gabriel, Centofanti and Mendes2015). Eurytrema cladorchis, Eurytrema pancreaticum, and Eurytrema coelomaticum infections have been reported in ruminants in Asia (Tang and Lin, Reference Tang and Lin1980; Graydon et al., Reference Graydon, Carmichael, Sanchez, Weidosari and Widjayanti1992; Ma et al., Reference Ma, He, Li, Guo, Pan, Wang, Zhang and Liu2014; Okajima et al., Reference Okajima, Shibata, Takahashi, Nagafuchi, Okajima and Nonaka2016), whereas E. coelomaticum causes PE in cattle in Brazil (Ilha et al., Reference Ilha, Loretti and Reis2005; Bassani et al., Reference Bassani, Sangioni, Saut, Headley and Yamamura2006; Schwertz et al., Reference Schwertz, Gabriel, Henker, Bottari, Carmo, Guarda, Morescoc, Machado, Morsch, Schetinger, Stedillea, Baska, Matteif, Silva and Mendes2016a, Reference Schwertz, Carmo, Bottari, Silva, Gabriel, Lucca, Guarda, Moresco, Machado, Morsch, Schetinger, Stefani, Mendes and Silva2016b). Most Eurytrema sp. infections are asymptomatic, although severe clinical signs and death have been rarely detected in sheep naturally infected by E. pancreaticum and also in cattle parasitized by E. coelomaticum (Kono et al., Reference Kono, Sakamoto, Yasuda, Kitano, Togoe and Yamamoto1980; Tang and Lin, Reference Tang and Lin1980; Graydon et al., Reference Graydon, Carmichael, Sanchez, Weidosari and Widjayanti1992; Dorny et al., Reference Dorny, Batubara, Iskander and Pandey1996; Ilha et al., Reference Ilha, Loretti and Reis2005; Ma et al., Reference Ma, He, Li, Guo, Pan, Wang, Zhang and Liu2014; Schwertz et al., Reference Schwertz, Gabriel, Henker, Bottari, Carmo, Guarda, Morescoc, Machado, Morsch, Schetinger, Stedillea, Baska, Matteif, Silva and Mendes2016a). The severity of pancreatic injury seems to be related to the parasite load based on E. coelomaticum infections in cattle (Schwertz et al., Reference Schwertz, Gabriel, Henker, Bottari, Carmo, Guarda, Morescoc, Machado, Morsch, Schetinger, Stedillea, Baska, Matteif, Silva and Mendes2016a, Reference Schwertz, Carmo, Bottari, Silva, Gabriel, Lucca, Guarda, Moresco, Machado, Morsch, Schetinger, Stefani, Mendes and Silva2016b).
Since knowledge of clinical and pathological features of PE in goats and sheep is minimal worldwide, this disease remains obscure in the Americas. Herein, we investigated cases of PE in small ruminants in the Federal District, Midwestern Brazil. This study aims to determine the epidemiological, clinical, pathological and aetiological features of PE in goats and sheep.
Materials and methods
A survey of pancreatic trematodiasis in small ruminants (2009–2018) was conducted in the archive of necropsies at the Veterinary Pathology Laboratory, Veterinary Teaching Hospital, University of Brasilia, Federal District, Brazil. All animals included in the study exhibited pancreatic flukes regardless of the cause of death. We compiled case records, including species, age, breed, gender, date of death, season of the year and geographical location. Other findings noted during local inspection of the flock, such as contact between small ruminants and cattle in paddocks, body condition and gross findings were also compiled. Additionally, we requested information on the annual average population of small ruminants in the period covered in this study from the Secretariat of Agriculture, Federal District, Brazil.
All necropsies and viscera inspections were conducted within 4 h of death. Tissue samples of organs were fixed in 10% buffered formalin (pH 7.0), embedded in paraffin, and histological sections were stained with haematoxylin and eosin (H&E). Fresh pancreatic parasites were collected and frozen at −20°C in sterile 0.9% saline solution for molecular assays and fixed in an alcohol–formalin–acetic acid solution (70°C; ethanol 95%, formaldehyde 3% and glacial acetic acid 2%) for morphological identification of parasites.
Histological sections of the pancreas and surrounding abdominal adipose tissue were re-evaluated. A semi-quantitative analysis of injuries related to pancreatic infection by trematodes was conducted and classified as: absent (−), mild (+), moderate (++) and severe (+++). Additionally, histological slides were also stained with Masson's trichrome, reticulin and Sudan Red stains to evidence pancreatic fibrosis and adipose tissue necrosis, respectively.
Fixed trematodes from goats and sheep were clarified in 80% glacial acetic acid, evaluated by brightfield-microscopy, and morphometry conducted with ImagePro Plus 4.0 software.
Frozen samples of flukes were grouped into four pools according to location and year of cases for DNA extraction, polymerase chain reaction (PCR) and sequencing. Additionally, fresh flukes from two autochthonous asymptomatic PE cases in cattle (pool 5) were also collected for molecular identification and comparison with the parasites from goats and sheep.
Trematode DNA was extracted by using the Illustra Tissue & Cells GenomicPrep Mini Spin Kit (GE Healthcare Life Sciences do Brasil Ltda, Brazil). Using a PCR protocol with an initial denaturation step of 5 min at 94°C, followed by 40 cycles of 45 s at 94°C, 45 s at 55°C and 2 min at 72°C, the partial 18S rRNAs were amplified with previously described primers E-18S-F and E-18S-R (Zheng et al., Reference Zheng, Luo, Jing, Hu and Cai2007). PCR products were separated by electrophoresis in 2% agarose gels, stained with ethidium bromide and examined under ultraviolet light. The PCR products were purified from gel using the QIAquick Gel Extraction Kit (QiAgen, Hilden, Germany) and subjected to direct sequencing using the forward and reverse primers.
Chromatograms and quality scores for the five sequences from the pools of parasites obtained in this study were evaluated using Geneious v. 9.0.5. Trimmed sequences were submitted to the BLASTn tool (http://www.ncbi.nlm.nih.gov/BLAST). For phylogenetic analysis, 14 18S rRNA sequences of the Dicrocoeliidae family were retrieved from the GenBank database. Nucleotide alignment with partial sequences (429 bp) was carried out using the ClustalW algorithm. The Kimura 2 parameter with invariant sites was estimated as the best model according to the jModelTest v2.1.10 tool. The tree was constructed by the neighbour-joining method in MEGA v. X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018), and bootstrapping was performed with 1000 replicates. Encyclometra colubrimurorum was chosen as an outgroup in accordance with other studies (Tkach et al., Reference Tkach, Achatz, Hildebrand and Greiman2018; Hildebrand et al., Reference Hildebrand, Pyrka, Sitko, Jeżewski, Zaleśny, Tkach and Laskowski2019). The nucleotide sequences were deposited in the GenBank database and are available under the accession numbers MT973984, MT973985, MT97398, MT973987 and MT973988.
Results
The Federal District is the smallest federative unit of Brazil and comprises 5802 km2 in the Central Western region. Flocks of small ruminants in the region included an average population of 3418 goats and 21 291 sheep in the analysed period. In 10 years of necropsy records, we detected autochthonous cases of pancreatic trematodiasis in eight goats (8/62 = 12.9%) and in two sheep (2/251 = 0.8%) from three different flocks. Seven cases (70%) occurred in the Brazilian spring, while summer, autumn and winter each had one case. The data retrieved from the necropsy records are presented in Table 1.
Table 1. Data on cases of PE in small ruminants in the Federal District, Brazil, 2009–2014

Goats 01 and 02 were from a flock of 20 dairy goats, raised on a pasture of Tifton grass (Cynodon spp.), and supplemented with a commercial concentrate mixture. Both animals showed a clinical evolution over 1 to 2 months of anorexia, lethargy, weakness, marked weight loss and died within a 15-day interval of each other. Goats 03, 04, 05, 06 and 07 were part of a commercial dairy goat flock of 50 breeding stock, supplemented with chopped sugar cane and commercial concentrate mixture, and kept on Tifton grass pastures bordered by a creek with swampy margins. The goats were slaughtered for local meat consumption and trematode parasites were detected in the pancreas during routine viscera inspection. In a dairy goat flock of 90 animals (5 goats and 85 sheep), goat 08, sheep 09 and sheep 10 were kept in Tifton grass paddocks. Goat 08 showed anorexia, weight loss, cachexia and died 1 month after the onset of clinical signs. Sheep 09 was slaughtered for meat consumption, and pancreatic parasites were observed during viscera inspection. Animal 10 died of fetal dystocia and uterine rupture, and flukes were found within the pancreas at necropsy.
Goats 01, 02 and 08 (Table 2) presented gross findings and death associated with severe pancreatic fluke infection. The main pathological changes were a diffusely pale pancreas that was firm and atrophic with an irregular surface (Fig. 1A). Major pancreatic ducts were significantly dilated with numerous flattened and dark red parasites. A marked feature detected in all three goats was multifocal to coalescent firm masses representing areas of fat necrosis and haemorrhagic foci of various sizes throughout the peritoneal cavity (Fig. 2A), including mesentery (Fig. 2B), omentum (Fig. 2C) and retroperitoneal fat (Fig. 2D). Steatonecrotic foci were chalky-white or yellow, sometimes oily, and surrounded by hyperaemic halos. Goats 03–07 and sheep 09 were all asymptomatic, and no other gross changes were detected except for mildly dilated pancreatic ducts containing flukes at pancreas inspection. Sheep 10, with fetal dystocia, presented pancreatic parasitism (Fig. 1B) and fibrinous peritonitis related to uterine rupture. The local inspection of all flocks did not demonstrate contact between sheep and goats with cattle.
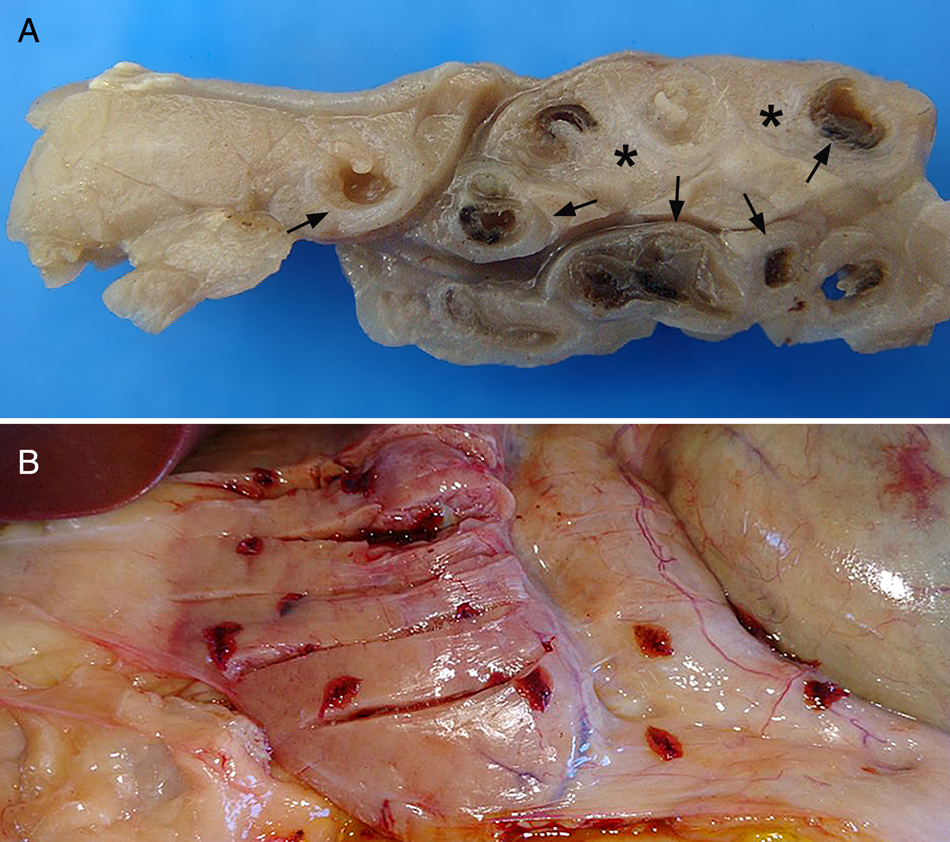
Fig. 1. (A) Goat, PE. Pancreas diffusely pale, firm, atrophic, with pancreatic ducts significantly dilated. (B) Sheep, PE. Numerous flattened and red parasites in an asymptomatic case.
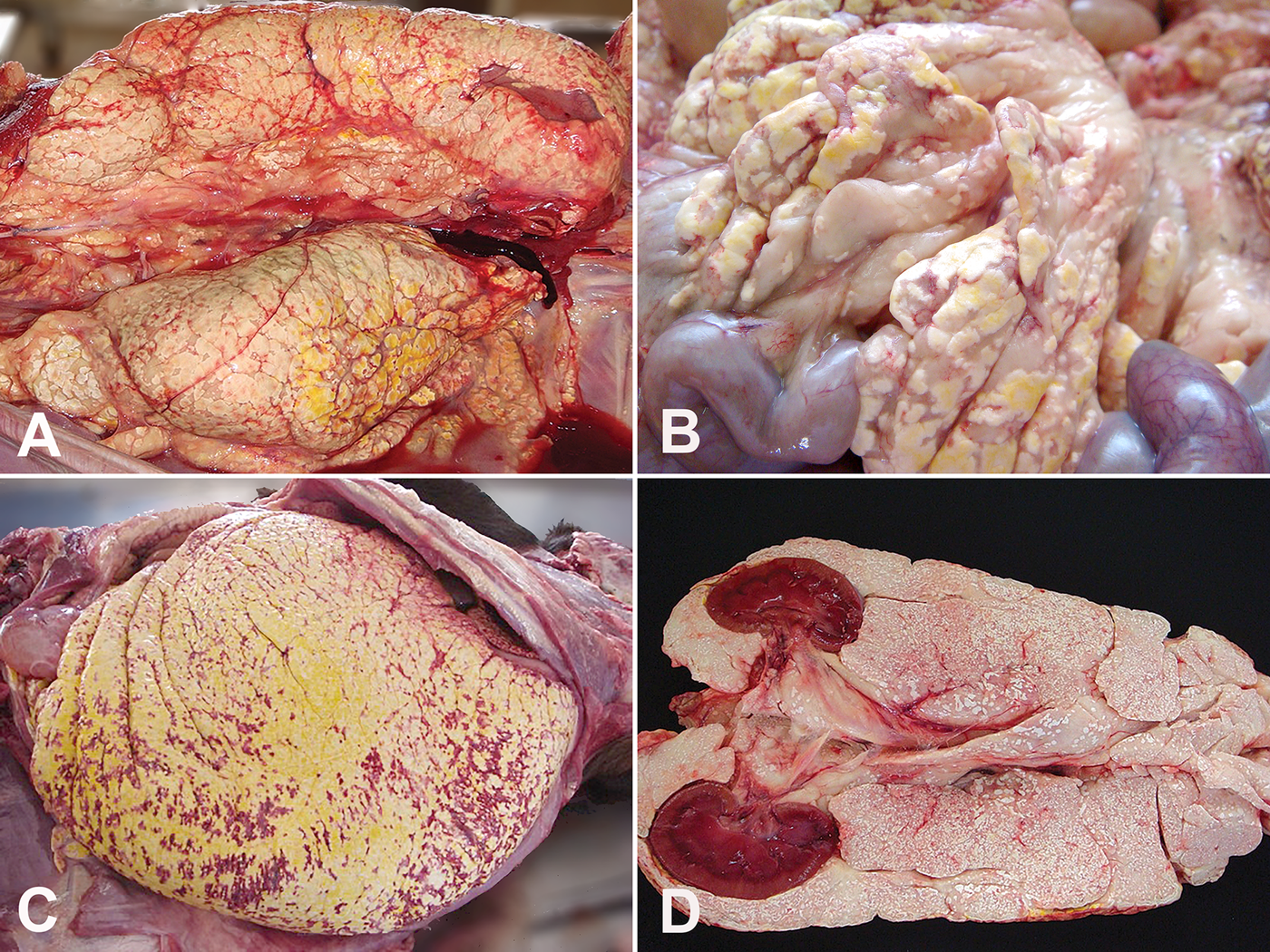
Fig. 2. Goat, PE, AFN. (A) Areas of fat tissue necrosis and haemorrhagic foci of various sizes throughout the peritoneal cavity. (B) Steatonecrosis at the mesentery. (C) Necrosis of adipose tissues of omentum. (D) Necrosis of retroperitoneal fat surrounding kidneys.
Table 2. Gross findings related to the Eurytrema coelomaticum infection in small ruminants in the Federal District, Brazil, 2009–2014

Interpretation: absence (−); mild (+); moderate (++); severe (+++).
Histologically, there was marked pancreatic interstitial fibrosis in goats 01, 02 and 08 (Table 3) surrounding atrophic acini and ducts (Fig. 3A), occasional small foci of necrosis and moderate lymphohistiocytic inflammation (Fig. 3B) with occasional eosinophils and plasma cells. The pancreatic ducts were markedly dilated, lined by hyperplastic epithelium with papillary projections towards the lumen, and contained transverse and longitudinal sections of acoelomate parasites (Fig. 3C). Flukes were filled with parenchyma, presented an external eosinophilic integument, a muscular oral sucker and uterus with thick and brown-shelled operculated eggs (Fig. 3D).
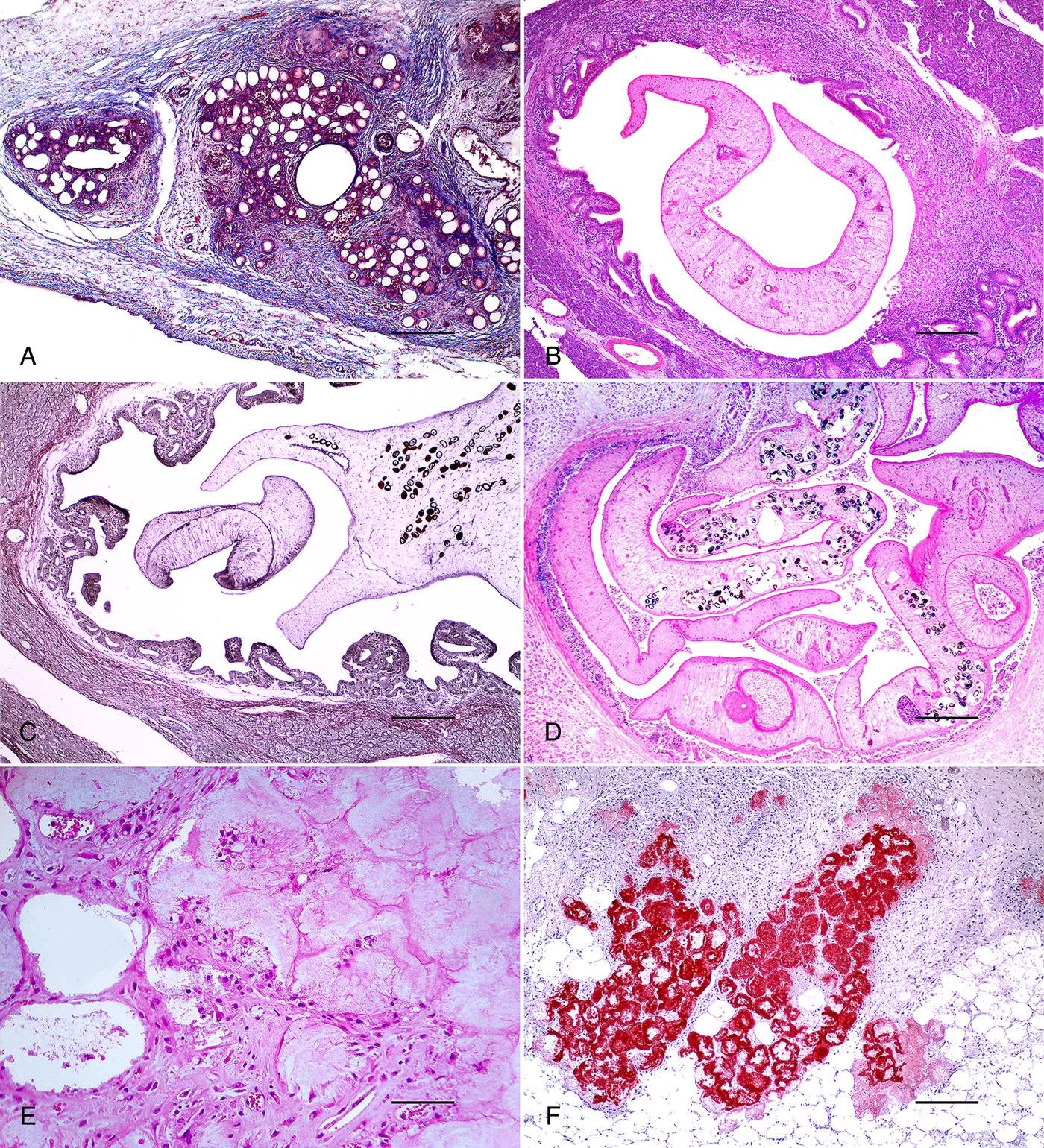
Fig. 3. Goat, PE, pancreas. (A) Interstitial fibrosis surrounding atrophic acini and ducts. Masson's trichrome stain. Bar = 250 μm. (B) Lymphohistiocytic inflammation surrounding a parasitized pancreatic duct. H&E. Bar = 250 μm. (C) Dilated pancreatic duct lined by hyperplastic epithelium and containing a section of trematode. Reticulin stain. Bar = 250 μm. (D) Transverse and longitudinal sections of acoelomate parasites filled with parenchyma, with an external eosinophilic integument, a muscular oral sucker and uterus with thick and brown-shelled operculated eggs. H&E. Bar = 250 μm. (E) Shrunken adipocytes with pycnotic nuclei and eosinophilic cytoplasm containing cholesterol crystals. H&E. Bar = 100 μm. (F) Fat necrosis areas strongly red stained. Sudan Red staining. Bar = 250 μm.
Table 3. Histopathological findings in the pancreas and abdominal fat tissues of small ruminants with PE in the Federal District, Brazil, 2009–2014

Interpretation: absence (−); mild (+); moderate (++); severe (+++).
Shrunken adipocytes with pycnotic nuclei and eosinophilic cytoplasm containing cholesterol crystals characterized the severe necrosis of abdominal fat tissues in goats 01, 02 and 08. These areas were surrounded by a lymphohistiocytic inflammatory infiltrate (Fig. 3E) with foamy macrophages and occasional multinucleated giant cells. Multifocal deposits of mineralized, amorphous, basophilic and granular material were also apparent. Areas of fat necrosis strongly stained with Sudan Red staining (Fig. 3F). Three asymptomatic goats and one sheep showed mild pancreatic duct ectasia filled with trematodes and surrounded by a mild lymphocytic inflammatory infiltrate (Table 3).
Morphological examination (Fig. 4) demonstrated dorsoventrally flat and leaf-shaped parasites with a total length of 8.79 ± 0.39 mm (9.16–7.98 mm) and a width of 5.06 ± 1.04 mm (6.49–3.37 mm). The oral sucker was located at the anterior end of the body, measuring 0.96 ± 0.06 mm (1.03–0.85 mm) × 0.91 ± 0.08 mm (1.05–0.81 mm). A small, rounded pharynx [0.33 ± 0.05 mm (0.43–0.27 mm) × 0.29 ± 0.041 mm (0.35–0.25 mm)] was followed by a short oesophagus and long caecum that branched and reached the posterior end. A ventral sucker (acetabulum) was located in the middle third of the body with the following dimensions: 0.92 ± 0.07 mm (1.03–0.85 mm) × 0.93 ± 0.06 mm (1.02–0.81 mm). Symmetrical and rounded post-acetabular testicles [left: 0.83 ± 0.17 mm (1.06–0.60 mm) × 0.76 ± 0.18 mm (0.96–0.38 mm); right: 0.80 ± 0.08 mm (0.91–0.68 mm) × 0.75 ± 0.13 mm (0.93–0.52 mm)] were present. A post-testicular ovary measuring 0.38 ± 0.12 mm (0.57–0.23 mm) × 0.36 ± 0.08 mm (0.50–0.27 mm) was identified. Vitelline glands were arranged in a lateral position to the ovary, the left measuring 1.60 ± 0.20 mm (1.78–1.29 mm) × 0.88 ± 0.16 mm (1.11–0.65 mm) and the right 1.64 ± 0.26 mm (2.07–1.23 mm) × 0.84 ± 0.20 mm (1.20–0.63 mm). Uteri extended almost the entire length of the parasite's body, and the terminal excretory pore was located at the posterior end. Flukes were identified as Eurytrema coelomaticum (Giard and Billet, 1892) based on body length and sucker size (Sakamoto and Oikawa, Reference Sakamoto and Oikawa2007; Leite et al., Reference Leite, Lopes-Torres, Souza, Neves, Gomes and Machado-Silva2020).

Fig. 4. Morphological features of Eurytrema coelomaticum adult collected from goat in Federal District, mid-western Brazil. Internal and external structures observed in a whole-mounted specimen, brightfield stereomicroscope. Ce, caeca; Ep, excretory pore; Es, oesophagus; Os, oral sucker; Ov, ovary; Ph, pharynx; Te, testis; Ut, uterus; Vs, ventral sucker; VG, vitelline gland.
Molecular identification was conducted using five pooled fluke samples: GP1DF: goats 1 and 2; GP2DF: goats 3 to 7; GP3DF: goat 8; SP4DF: sheep 9 and 10 and CP5DF: 2 autochthonous cases of PE in cattle. The five sequences from goats, sheep and cattle collected in this study showed 100% nucleotide identity with E. coelomaticum isolates (GenBank accession numbers KJ010810.1, DQ401035.1 and KJ010810.1) in BLASTn with 100% coverage. The percent sequence identity with E. pancreaticum (KY490001, KY490002, KY490004 and DQ401034) and E cladorchis (LC005981, LC005982 and LC005983) was 99.5 and 99.1%, respectively, using the Needleman–Wunsch algorithm. Phylogenetically, the five isolates were more closely related to E. coelomaticum (KJ010808, KJ010809 and KJ0108010) sequences forming a cluster supported by a high bootstrap value (Fig. 5).

Fig. 5. Neighbour-joining tree based on 18S rRNA sequence (429 bp) of 19 Dicrocoeliidae isolates. The tree is rooted with Encyclometra colubrimurorum as an outgroup and was built in MEGA v. X. Eurytrema coelomaticum isolate sequences identified in this study are shown with arrows. Asterisks represent bootstrap values equal to 75 or more. GenBank accession numbers are given in parentheses.
Discussion
PE has an undefined state in small ruminants in the Americas. In this study, a higher incidence of E. coelomaticum infection was observed in goats (12.9%) compared to that in sheep (0.8%) in the Federal District, Midwestern Brazil. Although this study is the first report of E. coelomaticum as the causal agent of PE in small ruminants in the Americas, there are several reports from Asia of Eurytrema sp. infecting goats, sheep and cattle. The prevalence of eurytrematosis in small ruminants varied from 0.84 to 63.0% in China and other regions from Asia (Tang and Tang, Reference Tang and Tang1977; Dorny et al., Reference Dorny, Batubara, Iskander and Pandey1996; Sangvaranond et al., Reference Sangvaranond, Lampa, Wongdachkajorn and Sritong2010; Ma et al., Reference Ma, He, Li, Guo, Pan, Wang, Zhang and Liu2014). Eurytrema coelomaticum infections varied from 0.1 to 72.9% in slaughtered beef cattle in Southern Brazil and Japan (Bassani et al., Reference Bassani, Sangioni, Saut, Headley and Yamamura2006; Okajima et al., Reference Okajima, Shibata, Takahashi, Nagafuchi, Okajima and Nonaka2016). Our findings, despite the limited representativeness of an archival study and a small population of goats in the region, suggest a relevant prevalence of PE in goats and a low frequency in sheep in the Federal District, Brazil.
The swampy margins of a creek in the paddock (15°38′16″S, 47°47′44″W) of one goat flock was a unique epidemiological finding of this study that could be associated with land snail development and increased risk of E. coelomaticum infection in small ruminants. Additionally, most E. coelomaticum infections in goats and sheep were detected in the rainy season, starting in spring and lasting all summer in Midwestern Brazil. Endemic areas for E. coelomaticum in some southern states of Brazil have wet weather and a well-marked rainy season, providing suitable conditions for developing land snails and grasshoppers (Ilha et al., Reference Ilha, Loretti and Reis2005; Bassani et al., Reference Bassani, Sangioni, Saut, Headley and Yamamura2006; Rachid et al., Reference Rachid, Aquino Neto, Facury-Filho, Carvalho, Valle and Vasconcelos2011). Environmental factors such as pastures grown in natural wetlands or swampy areas favourable for intermediate host development and a high density of livestock in paddocks are possible contributing factors to E. coelomaticum infection rates in the Americas.
Eurytrema sp. infections in livestock, land snails and grasshoppers were higher during rainy seasons and in regions with higher precipitation totals in China. A marked reduction in infectiousness during the cold and dry seasons was detected in intermediate hosts (Tang and Tang, Reference Tang and Tang1977; Tang et al., Reference Tang, Cui, Dong, Wang, Nulimajabu, Hongchang, Chiping, Mei and Qian1979, Reference Tang, Mei, Tang, Cui, Lu and Quian1983; Ma et al., Reference Ma, He, Li, Guo, Pan, Wang, Zhang and Liu2014). Even though there are some reports of PE in sheep and goats in Asia, many epidemiological aspects and risk factors related to PE remain uncertain. Although most cases of PE in small ruminants occurred in the wet season in the Federal District, limited information on prevalence, intermediate hosts and environmental factors prevents a definitive conclusion on disease seasonality and other risk factors.
Three goats with PE in Midwestern Brazil showed clinical signs including anorexia, lethargy, weakness, marked weight loss and a fatal outcome. A similar syndrome characterized by progressive wasting, weakness, inappetence, emaciation and death was detected in sheep with E. pancreaticum infection in North Sumatra (Graydon et al., Reference Graydon, Carmichael, Sanchez, Weidosari and Widjayanti1992). A weaned goat experimentally infected with E. cladorchis presented with digestive disorders such as lost appetite, diarrhoea and sudden death in China (Tang and Tang, Reference Tang and Tang1977). Cachexia and sudden death were also recorded in an adult goat naturally infected by E. cladorchis in Nepal (Mahato, Reference Mahato1987). Despite clinical signs detected in some animals, most cases in our study and small ruminants with PE in Asia (Tang and Tang, Reference Tang and Tang1977; Dorny et al., Reference Dorny, Batubara, Iskander and Pandey1996; Sangvaranond et al., Reference Sangvaranond, Lampa, Wongdachkajorn and Sritong2010; Ma et al., Reference Ma, He, Li, Guo, Pan, Wang, Zhang and Liu2014) were asymptomatic. Although similar clinical signs were observed in more severe infections, most PE cases in cattle were equally subclinical (Ilha et al., Reference Ilha, Loretti and Reis2005; Bassani et al., Reference Bassani, Sangioni, Saut, Headley and Yamamura2006; Quevedo et al., Reference Quevedo, Mendes, Pappen, Soares, Muller and Farias2013).
The time frame between the onset of clinical signs and a fatal outcome observed in three goats with E. coelomaticum natural infection was similar to that reported in goats in Asia (Kono et al., Reference Kono, Sakamoto, Yasuda, Kitano, Togoe and Yamamoto1980; Tang and Lin, Reference Tang and Lin1980). In general, clinical signs in PE cases with a fatal outcome in goats seem to have a shorter clinical evolution compared to the 2 to 10 months in cattle naturally infected with E. coelomaticum in Southern Brazil (Ilha et al., Reference Ilha, Loretti and Reis2005). Sheep with eurytrematosis and wasting syndrome also showed a clinical course of several months (Graydon et al., Reference Graydon, Carmichael, Sanchez, Weidosari and Widjayanti1992). Eurytrema coelomaticum chronic infections in cattle have led to considerable annual economic losses in Brazil (Ilha et al., Reference Ilha, Loretti and Reis2005; Schwertz et al., Reference Schwertz, Lucca, Silva, Baska, Bonetto, Gabriel, Centofanti and Mendes2015). PE in small ruminants is still an indeterminate disease in the Americas, therefore clinical evolution and economic losses are not yet established. Additionally, severe clinical signs detected in three goats were presumably aggravated by abdominal fat necrosis (AFN), contributing to a fatal outcome.
Overall, PE in small ruminants in the Federal District ranged from minor lesions (70.0%) to severe pancreatic parasitism by E. coelomaticum, marked pancreatitis, fibrosis, atrophy, duct ectasia, AFN and death in 37.5% of infected goats. Eurytrematosis has been reported as an incidental necropsy finding without significant pancreatic gross lesions in small ruminants in China (Ma et al., Reference Ma, He, Li, Guo, Pan, Wang, Zhang and Liu2014) and Indonesia (Dorny et al., Reference Dorny, Batubara, Iskander and Pandey1996). In contrast to the most frequent asymptomatic infections, severe chronic parasitic pancreatitis and death related to Eurytrema infection, as observed herein, was reported in the natural and experimental PE in goats and sheep (Kono et al., Reference Kono, Sakamoto, Yasuda, Kitano, Togoe and Yamamoto1980; Tang and Lin, Reference Tang and Lin1980; Mahato, Reference Mahato1987; Graydon et al., Reference Graydon, Carmichael, Sanchez, Weidosari and Widjayanti1992). Marked pancreatic fibrosis, pallor, atrophy and intraductal trematodes were pathological changes detected in cattle in Brazil with severe E. coelomaticum infections (Ilha et al., Reference Ilha, Loretti and Reis2005; Rachid et al., Reference Rachid, Aquino Neto, Facury-Filho, Carvalho, Valle and Vasconcelos2011; Quevedo et al., Reference Quevedo, Mendes, Pappen, Soares, Muller and Farias2013; Schwertz et al., Reference Schwertz, Carmo, Bottari, Silva, Gabriel, Lucca, Guarda, Moresco, Machado, Morsch, Schetinger, Stefani, Mendes and Silva2016b). Gross findings varied from a fibrotic, shrunken and smaller pancreas to an enlarged, dark and heavy parasitized organ, even without morphological changes in cattle infected with E. coelomaticum (Ilha et al., Reference Ilha, Loretti and Reis2005). Until now, Eurytrema spp. infections have not been associated with severe, fatal chronic pancreatitis in goats.
AFN was the most distinct pathological finding, observed in three goats with a fatal outcome. AFN has not been reported in small ruminants and cattle with PE and is rarely reported in domestic ruminants with other conditions (Smith et al., Reference Smith, Rotstein and Brownie2004; Santos et al., Reference Santos, Bandarra, Sonne, Pavarini and Driemeier2008; Tharwat and Buczinski, Reference Tharwat and Buczinski2012; Tani et al., Reference Tani, Pratakpiriya, Tani, Yamauchi, Hirai, Yamaguchi, Ano and Katamoto2017). The aetiology of AFN is complex and not well established (Smith et al., Reference Smith, Rotstein and Brownie2004; Tani et al., Reference Tani, Pratakpiriya, Tani, Yamauchi, Hirai, Yamaguchi, Ano and Katamoto2017) and is possibly associated with the leakage of pancreatic enzymes in PE cases. The inspection of the paddocks of the flocks in this study did not identify fescue grass, abdominal trauma was not recorded in goats with AFN and any genetic predisposition of goats or sheep to AFN remains unrecognized. Severe pancreatitis in the three goats with eurytrematosis was the only known factor that could be related to AFN. Extensive pancreatic damage caused by the E. coelomaticum infection possibly enabled the leakage of enzymatic juice from the pancreas, triggering AFN.
Microscopically, severe chronic pancreatitis, fibrosis, acinar atrophy, ductal ectasia containing cross-sections of trematode parasites and epithelial proliferation were the main pancreatic lesions observed in the three goats with fatal PE. Findings in goats and sheep with asymptomatic PE ranged from minor lesions including mild lymphocytic inflammation and pancreatic duct ectasia to the absence of pancreatic injury entirely. Experimental and natural Eurytrema sp. infection of small ruminants in Asia showed similar pathological findings (Kono et al., Reference Kono, Sakamoto, Yasuda, Kitano, Togoe and Yamamoto1980; Graydon et al., Reference Graydon, Carmichael, Sanchez, Weidosari and Widjayanti1992) and also in varying degrees in cattle infected with E. coelomaticum in Brazil (Ilha et al., Reference Ilha, Loretti and Reis2005; Bassani et al., Reference Bassani, Sangioni, Saut, Headley and Yamamura2006; Rachid et al., Reference Rachid, Aquino Neto, Facury-Filho, Carvalho, Valle and Vasconcelos2011; Quevedo et al., Reference Quevedo, Mendes, Pappen, Soares, Muller and Farias2013; Schwertz et al., Reference Schwertz, Gabriel, Henker, Bottari, Carmo, Guarda, Morescoc, Machado, Morsch, Schetinger, Stedillea, Baska, Matteif, Silva and Mendes2016a).
In our study, severe lesions in the pancreas and abdominal fat tissue damage suggest intense parasitic pancreatic inflammation in three goats. Studies on the pathogenesis of pancreatic damage related to E. coelomaticum infection in cattle have shown an increase in activity of NTPDase and 5′-nucleotidase, known as purinergic signalling enzymes with a modulating action on the inflammatory response. The serum activity of these enzymes appears to be directly associated with the grade of parasite load and inflammation of pancreatic tissues (Fávero et al., Reference Fávero, Schwertz, Doleski, Leal, Machado, Manzoni, Silva, Gabriel, Stedille, Christ, Stefani, Mendes and Silva2016). Other evidence suggests that zinc and adenosine deaminase (ADA) levels are lower in cattle infected with E. coelomaticum. Low levels of ADA, an enzyme with a significant role in modulating the immune system and limiting inflammation, may increase the pancreas' inflammatory tissue destruction in parasitized cattle (Grosskopf et al., Reference Grosskopf, Schwertz, Machado, Bottari, Silva, Gabriel, Lucca, Alves, Schetinger, Morsch, Mendes and Silva2017).
Cattle infected with E. coelomaticum showed modifications in the activity of nitric oxide and cholinesterases (inflammatory mediators), suggesting a relationship between parasitism and pancreatic inflammation (Schwertz et al., Reference Schwertz, Carmo, Bottari, Silva, Gabriel, Lucca, Guarda, Moresco, Machado, Morsch, Schetinger, Stefani, Mendes and Silva2016b). The activation of the antioxidant system, protein oxidation and lipid peroxidation were related to the degree of pathological pancreatic changes and parasite load in cattle naturally infected by E. coelomaticum (Schwertz et al., Reference Schwertz, Gabriel, Henker, Bottari, Carmo, Guarda, Morescoc, Machado, Morsch, Schetinger, Stedillea, Baska, Matteif, Silva and Mendes2016a). Regarding the still undetermined pathogenesis of PE in goats, the severity of lesions in fatal cases suggests an exacerbated pancreatic inflammatory reaction. Further investigations of inflammatory modulation and antioxidant systems are necessary to understand the extreme variability of pancreatic and surrounding tissue damage in goats with E. coelomaticum infection.
Histological findings of AFN detected in the three goats with PE were similar to steatonecrosis everywhere (Smith et al., Reference Smith, Rotstein and Brownie2004; Santos et al., Reference Santos, Bandarra, Sonne, Pavarini and Driemeier2008; Tharwat and Buczinski, Reference Tharwat and Buczinski2012; Tani et al., Reference Tani, Pratakpiriya, Tani, Yamauchi, Hirai, Yamaguchi, Ano and Katamoto2017). The seriousness of the parasitic pancreatic damage in the goats presumably resulted in severe abdominal fat tissue necrosis. Pathogenesis of steatonecrosis resulting from pancreatitis includes enzymatic necrosis of fat tissues due to the leakage of pancreatic lipolytic and proteolytic enzymes, triggering an inflammatory reaction followed by saponification (Pereda et al., Reference Pereda, Pérez, Escobar, Arduini, Asensi, Serviddio, Sabater, Aparise and Sastre2012). Leakage of lipolytic pancreatic enzymes in pancreatitis enables lipolysis, the release of unsaturated fatty acids (UFAs), and pro-inflammatory cytokines (Patel et al., Reference Patel, Trivedi, Durgampudi, Noel, Cline, DeLany, Navina and Singh2015). Besides the local generation of inflammatory mediators, UFA promotes a systemic response, amplifying the severity of pancreatic inflammation, worsening the clinical condition and outcome (Franco-Pons et al., Reference Franco-Pons, Gea-Sorlí and Closa2010; Patel et al., Reference Patel, Trivedi, Durgampudi, Noel, Cline, DeLany, Navina and Singh2015). Additionally, in experimental models, pancreatic duct obstruction furthered the development of severe pancreatic haemorrhagic necrosis (Steer, Reference Steer1992). Altogether, complex pancreatitis mechanisms may explain the severe pancreatic damage, AFN and death observed in goats with eurytrematosis in the Federal District. It is possible to hypothesize that flukes could irritate and obstruct pancreatic ducts in severe cases, causing marked inflammation, pancreatic enzyme leakage and activation and necrosis. Furthermore, the expansion of pancreatic necrosis may reach abdominal fat tissues causing the release of high levels of mediators, which enable severe systemic and local inflammatory reactions, marked AFN, and a fatal outcome.
Morphological identification of pancreatic trematodes in goats and sheep showed primary body length, oral sucker size, pharynges and a reproductive system compatible with E. coelomaticum (Sakamoto and Oikawa, Reference Sakamoto and Oikawa2007; Leite et al., Reference Leite, Lopes-Torres, Souza, Neves, Gomes and Machado-Silva2020). Differences in the morphology and size of E. coelomaticum are considered to be reliable parameters to differentiate it from E. pancreaticum (Sakamoto and Oikawa, Reference Sakamoto and Oikawa2007). Moreover, molecular assay and sequencing results also identified E. coelomaticum as the causative agent of PE in goats and sheep in the Federal District. Natural infections by E. pancreaticum and E. cladorchis have been reported in goats and sheep in Asia (Tang and Tang, Reference Tang and Tang1977; Tang and Lin, Reference Tang and Lin1980; Mahato, Reference Mahato1987; Graydon et al., Reference Graydon, Carmichael, Sanchez, Weidosari and Widjayanti1992; Dorny et al., Reference Dorny, Batubara, Iskander and Pandey1996; Ma et al., Reference Ma, He, Li, Guo, Pan, Wang, Zhang and Liu2014), and goats have been shown to be experimentally susceptible to E. coelomaticum infection (Kono et al., Reference Kono, Sakamoto, Yasuda, Kitano, Togoe and Yamamoto1980; Tang et al., Reference Tang, Mei, Tang, Cui, Lu and Quian1983).
Although E. coelomaticum has been identified as the agent responsible for PE in cattle in Southern Brazil (Ilha et al., Reference Ilha, Loretti and Reis2005; Bassani et al., Reference Bassani, Sangioni, Saut, Headley and Yamamura2006; Rachid et al., Reference Rachid, Aquino Neto, Facury-Filho, Carvalho, Valle and Vasconcelos2011; Quevedo et al., Reference Quevedo, Mendes, Pappen, Soares, Muller and Farias2013; Schwertz et al., Reference Schwertz, Gabriel, Henker, Bottari, Carmo, Guarda, Morescoc, Machado, Morsch, Schetinger, Stedillea, Baska, Matteif, Silva and Mendes2016a), the situation of natural infections in small ruminants is still undefined or under-reported in the Americas. In our necropsy records, some cases of E. coelomaticum infection in cattle were also observed in the Federal District. Unfortunately, an epidemiological investigation of these cases was not conducted, and the incidence of PE in cattle in the Federal District and all Midwestern Brazil is unknown. Therefore, it is not possible to speculate on the animal species responsible for introducing and maintaining E. coelomaticum in the region or the economic impact of the trematode on livestock production.
PE is an under diagnosed and possibly neglected parasitosis in goats and sheep in relevant regions of small ruminant production in the Americas. Our findings on PE in small ruminants highlight the relevance of E. coelomaticum infections in the Federal District, Midwestern Brazil. Unusual AFN detected in some goats with PE in Brazil suggests a high pathogenicity of E. coelomaticum infections in comparison with most cases of PE in small ruminants reported in Asia. Further epidemiological, clinical and pathological investigations must be conducted in small ruminants in the Americas to determine PE incidence in other regions and gauge economic impact on livestock production.
Acknowledgements
Special thanks to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) for partial financing (Finance Code 001) and the support of the National Council for Scientific and Technological Development (CNPQ). We thank Hannah A. Bullock for the English language editing, thoughtful comments and efforts to review and improve the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.












