Introduction
Toxoplasma gondii infections are prevalent in humans and animals worldwide. The ingestion of undercooked infected meat or consumption of food and water contaminated with oocysts excreted in cat feces are the main sources of infection. Cats are everywhere and a single cat can excrete millions of oocysts that can remain viable in the environment for months under natural conditions (Dubey, Reference Dubey2010). Estimation of oocyst contamination of the environment is difficult because of low numbers present in soil or water (Lélu et al., Reference Lélu, Villena, Dardé, Aubert, Geers, Dupuis, Marnef, Poulle, Gotteland, Dumètre and Gilot-Fromont2012). Wild and domestic birds are excellent sentinels of environmental contamination with T. gondii oocysts because herbivorous birds feed on the ground, and birds of prey consume hundreds of rodents and other small mammals yearly that are important intermediate hosts of T. gondii (Dubey et al., Reference Dubey, Pena, Cerqueira-Cézar, Murata, Kwok, Yang, Gennari and Su2020; Iemmi et al., Reference Iemmi, Vismarra, Mangia, Zanin, Genchi, Lanfranchi, Kramer, Formenti and Ferrari2020). Migratory birds (penguins, geese and others) can transport the parasite across seas (Sandström et al., Reference Sandström, Buma, Hoye, Prop, van der Jeugd, Voslamber, Madsen and Loonen2013). Some species (turkeys, geese, ducks and ostriches) are part of food supply for humans. A study estimated that billions of birds are consumed by cats yearly (Loss et al., Reference Loss, Will and Marra2013). Thus, there is great potential for the spread of T. gondii oocysts in the environment.
We recently reviewed the biology of T. gondii infections in chickens (Gallus domesticus) (Dubey et al., Reference Dubey, Pena, Cerqueira-Cézar, Murata, Kwok, Yang, Gennari and Su2020). Here, T. gondii infections in other avian species, including domestic turkeys, ducks, geese, ratites and other avian species, are reviewed.
Turkeys (Meleagris gallopavo)
Antibodies to T. gondii were detected from 11.0 to 89.8% of turkeys surveyed (Table 1). Using a kinetic recombinant antigen (GRA7 and GRA8) ELISA, T. gondii antibodies were detected in 387 (20.2%) of 1913 sera from 14 turkey farms in different areas of Germany (Koethe et al., Reference Koethe, Pott, Ludewig, Bangoura, Zöller, Daugschies, Tenter, Spekker, Bittame, Mercier, Fehlhaber and Straubinger2011). Seroprevalence varied greatly among farms and within the individual farms, depending on fattening cycles and season which turkeys were slaughtered. Seroprevalences were higher in turkeys slaughtered in summer vs in fall or winter (Koethe et al., Reference Koethe, Pott, Ludewig, Bangoura, Zöller, Daugschies, Tenter, Spekker, Bittame, Mercier, Fehlhaber and Straubinger2011).
Table 1. Seroprevalence of Toxoplasma gondii in turkeys (Meleagris gallopavo)

ELISA, enzyme-linked immunosorbent assay; FR, free-range; IHA, indirect haemagglutination assay; LAT, latex agglutination test; MAT, modified agglutination test (Dubey and Desmonts, Reference Dubey and Desmonts1987).
a IHA (Toxo-IHA Fumouze, Diagnostics, France).
b LAT (Toxo latex kit from Bio-kit-SA, Barcelona, Spain).
A very high rate of infection was reported in a study from Iran (Sarkari et al., Reference Sarkari, Asgari, Bagherian, Ashkani Esfahani, Kalantari, Mohammadpour, Ashrafmansori, Amerinia and Sabet Sarvestani2014). Antibodies to T. gondii were found in 89.8% of turkeys (Table 1) and T. gondii DNA was detected in 61.6% of turkey tissues (Table 2). Viable T. gondii was isolated from the muscle of 5 wild hunted turkeys in the USA (Table 3).
Table 2. Isolation of viable Toxoplasma gondii from wild bird by bioassay in mice and/or cats
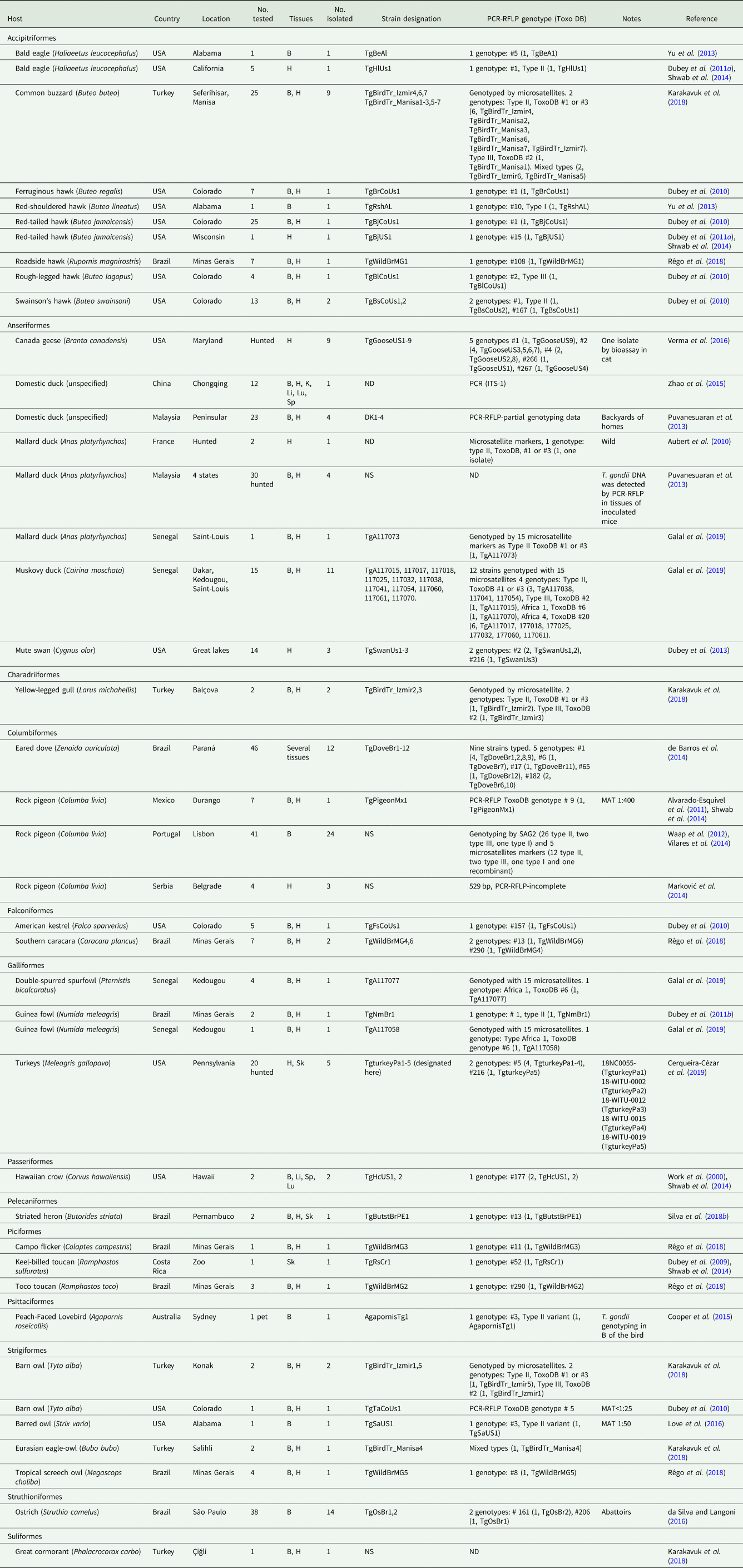
B, brain; H, heart; K, kidney; Li, liver; Lu, lung; Sk, skeletal muscle; Sp, spleen; NS, not stated; ND, no data; PCR, polymerase chain reaction; PCR-RFLP, Restriction fragment length polymorphism.
Table 3. Toxoplasma gondii DNA from tissues of wild birds

B, brain; Bl, blood; H, heart; G, gizzard; Li, liver; Lu, lung; Sk, muscle; Sp, spleen; Tg, Toxoplasma gondii; ND, not done; NS, not stated; PCR, polymerase chain reaction; N-PCR, nested PCR; RT-PCR, real-time PCR; PCR, polymerase chain reaction; PCR-RFLP, Restriction fragment length polymorphism.
Turkeys are considered resistant to clinical toxoplasmosis and there were no reports of clinical toxoplasmosis since 2009. Experimentally, turkeys inoculated intravenously with T. gondii tachyzoites or oocysts orally remained healthy, irrespective of the dose (Bangoura et al., Reference Bangoura, Zöller, Koethe, Ludewig, Pott, Fehlhaber, Straubinger and Daugschies2013; Zöller et al., Reference Zöller, Koethe, Ludewig, Pott, Daugschies, Straubinger, Fehlhaber and Bangoura2013; Hotop et al., Reference Hotop, Buschtöns, Bangoura, Zöller, Koethe, Spekker-Bosker, Hotop, Tenter, Däubener, Straubinger and Groß2014; Maksimov et al., Reference Maksimov, Basso, Zerweck, Schutkowski, Reimer, Maksimov, Conraths and Schares2018). A kinetic ELISA was developed based on a mixture of recombinant dense granule antigens GRA7 and GRA8 using sera from turkeys inoculated intravenously with Me49 tachyzoites; SAG1 antigen was not suitable in this ELISA using recombinant SAG1 (Koethe et al., Reference Koethe, Pott, Ludewig, Bangoura, Zöller, Daugschies, Tenter, Spekker, Bittame, Mercier, Fehlhaber and Straubinger2011). In a subsequent study, information on a large panel of 101 synthetic peptides was obtained on sera from 18 turkeys intravenously inoculated with tachyzoites of 3 strains of T. gondii (RH-Type I, Me49-Type II and NED-Type III). The authors concluded that by using selected peptides, it was possible to serotype strains up to 9 weeks post-inoculation (p.i.) (Maksimov et al., Reference Maksimov, Basso, Zerweck, Schutkowski, Reimer, Maksimov, Conraths and Schares2018). Antibodies peaked at 5–7 weeks p.i., using the SAG1-ELISA, and the results varied with the T. gondii isolate. Similar results were obtained by the indirect fluorescent antibody assay (IFA) (Maksimov et al., Reference Maksimov, Basso, Zerweck, Schutkowski, Reimer, Maksimov, Conraths and Schares2018).
In turkeys orally inoculated with oocysts, the parasite was widely disseminated in turkey tissues (Bangoura et al., Reference Bangoura, Zöller, Koethe, Ludewig, Pott, Fehlhaber, Straubinger and Daugschies2013). Inoculated turkeys were euthanized 6 or 12 weeks p.i. and parasite distribution was assessed by polymerase chain reaction (PCR); no difference was found with respect to 6 or 12 weeks p.i. Brain, heart and drumstick were most frequently infected tissues. The route of inoculation could affect the distribution of parasite DNA. In turkeys inoculated intravenously with tachyzoites, liver, pectoral muscle, heart and brain were affected in decreasing order (Zöller et al., Reference Zöller, Koethe, Ludewig, Pott, Daugschies, Straubinger, Fehlhaber and Bangoura2013). Judging from the histopathological results, the number of T. gondii in turkey tissues was low. Tissue cysts were found in imprints of 1 liver, and 2 pectoral muscles of turkeys parenterally inoculated with tachyzoites (Zöller et al., Reference Zöller, Koethe, Ludewig, Pott, Daugschies, Straubinger, Fehlhaber and Bangoura2013). By using magnetic-capture PCR and 100 g samples, most T. gondii were found in the brain and heart (Koethe et al., Reference Koethe, Straubinger, Pott, Bangoura, Geuthner, Daugschies and Ludewig2015). Parasite burden was higher in the drumstick vs pectoral muscles.
Ducks (Anas spp.) and geese
Ducks are important for the economy of some countries, especially China. Ducks are a good source of meat and eggs for human consumption. To our knowledge, there are no reports of clinical toxoplasmosis in domestic ducks or geese, but antibodies are common (Tables 4 and 5).
Table 4. Seroprevalence of Toxoplasma gondii in domestic ducks

NS, not stated; ELISA, enzyme-linked immunosorbent assay; IFA, indirect fluorescent antibody test; IHAT, indirect haemagglutination test; LAT, latex agglutination test; MAT, modified agglutination test (Dubey and Desmonts, Reference Dubey and Desmonts1987); FR, free range; Tg, Toxoplasma gondii; AS, association; IHC, immunohistochemical.
a IHA (Toxo-IHA Fumouze Diagnostics, France); LAT (PLASMATECH Co., UK); MAT (Toxo-Screen DA®, Biomerieux, Lyon, France). This is the same test as MAT.
Table 5. Seroprevalence of Toxoplasma gondii in domestic goose

AS, association; FR, free-range; Tg, Toxoplasma gondii; ELISA, enzyme-linked immunosorbent assay; IFA, indirect fluorescent antibody test; IHA, indirect haemagglutination assay; MAT, modified agglutination test (Dubey and Desmonts, Reference Dubey and Desmonts1987).
a IHA kit (Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, China).
Toxoplasma gondii DNA was found in 9 (7.8%) of 115 muscle samples from 115 ducks and 2 of 42 geese (4.7%) (Zou et al., Reference Zou, Nie, Zhang, Zou, Zhu and Cong2017). Viable T. gondii was isolated from tissues of ducks in China, France and Malaysia (Table 3).
Ostriches (Struthio camelus) and other ratites
Ratite meat is lean and its human consumption is increasing. Serologic data are summarized in Table 6. Antibodies to T. gondii were detected up to 80.0% of sera (Table 6).
Table 6. Seroprevalence of Toxoplasma gondii in ratites

MAT, modified agglutination test (Dubey and Desmonts, Reference Dubey and Desmonts1987); AS, association; Tg, Toxoplasma gondii.
Viable T. gondii was demonstrated in ostrich tissues. In a Brazilian report, T. gondii was detected in brains of ostriches and in soil samples from paddocks (da Silva and Langoni, Reference da Silva and Langoni2016). Brain (25 g) samples of 38 seropositive and 20 seronegative ostriches were bioassayed in mice. Toxoplasma gondii was isolated from 14 seropositive but not from seronegative ostriches. All strains were apparently pathogenic for mice. Toxoplasma gondii DNA was found in peritoneal exudates of mice inoculated from tissues of 8 ostriches, and in brains of mice inoculated with 6 ostrich samples. Nothing was said concerning finding viable T. gondii. Of interest is the report of finding T. gondii-like oocysts microscopically in soil samples from 5 of 20 paddocks; all were confirmed by PCR. In repeat sampling, T. gondii-like oocysts were found microscopically in soil samples from 9 of 20 paddocks and results were confirmed by PCR. It should be noted that only a few oocysts are normally present in soil and their detection is a challenge (J.P.D. own observation).
In marked contrast to the Brazilian report, in a Chinese study, T. gondii DNA was not detected in any of the 293 hearts and 77 brains of ostriches (Feng et al., Reference Feng, Lu, Wang, Zhang and Yang2017).
A study in Egypt found T. gondii DNA in the blood of 9 of 120 ostriches (El-Madawy and Metawea, Reference El-Madawy and Metawea2013). These authors also tested tissues of 5 ostriches that had died of toxoplasmosis-like illness; T. gondii DNA was found in the brains of 5, hearts of 3 and leg muscle of 1 (El-Madawy and Metawea, Reference El-Madawy and Metawea2013) (Comment J.P.D. – tissues of these ostriches should be examined histologically for verification of molecular results).
Other wild avian species
Data on T. gondii seroprevalence, viable parasite and DNA characterization are arranged by scientific order of birds, by region and chronologically in Tables 2, 3 and 7.
Carnivorous birds
Prevalence of T. gondii in carnivorous birds reflects the prevalence of the parasite in their prey. For example, owls consume hundreds of rodents yearly. In a recent survey of kestrels at an airport site in Italy, T. gondii antibodies were detected in 33.3% of kestrel trapped during 2016 and 14.3% of 91 kestrel during 2017; seroprevalence was lower in juveniles than in adults (Iemmi et al., Reference Iemmi, Vismarra, Mangia, Zanin, Genchi, Lanfranchi, Kramer, Formenti and Ferrari2020). Based on the remnants of animals in the feces of kestrels, rodents were a major component of the kestrel diet (Iemmi et al., Reference Iemmi, Vismarra, Mangia, Zanin, Genchi, Lanfranchi, Kramer, Formenti and Ferrari2020). In a large sample size of raptors in Spain, T. gondii antibodies were found in 51.0% of 96 common buzzard, 17.7% of 175 Griffon vulture and 17.0% of Spanish Imperial eagle (Cabezón et al., Reference Cabezón, García-Bocanegra, Molina-López, Marco, Blanco, Höfle, Margalida, Bach-Raich, Darwich, Echeverría, Obón, Hernández, Lavín, Dubey and Almería2011). Among the raptors, vultures are considered resistant to clinical toxoplasmosis and other microbial infections in general. In 2 large surveys, T. gondii antibodies were found in 17.7% of 175 Gyps fulvus in Spain (Cabezón et al., Reference Cabezón, García-Bocanegra, Molina-López, Marco, Blanco, Höfle, Margalida, Bach-Raich, Darwich, Echeverría, Obón, Hernández, Lavín, Dubey and Almería2011) but twice (39.6% of 101) more were infected in Israel (Salant et al., Reference Salant, Hamburger, King and Baneth2013); the results are comparable because modified agglutination test (MAT) at the serum dilution of 1:25 was used in both studies. Finding of T. gondii DNA in tissues of 43 of 48 (89.6%) wild birds (mostly carnivorous) in Turkey suggests a very high prevalence of the parasite in local rodents (Karakavuk et al., Reference Karakavuk, Aldemir, Mercier, Atalay Sahar, Can, Murat, Döndüren, Can, Özdemir, Degirmenci Döskaya, Pektas, Dardé, Gürüz and Döskaya2018). Of 281 raptors from a rehabilitation centre in the USA, 34.5% were seropositive with highest (46.0%) prevalence in Barred owl (Love et al., Reference Love, Kwok, Verma, Dubey and Bellah2016)
Viable T. gondii was isolated (Table 2) and DNA demonstrated (Table 3) from several species of carnivorous birds. The highest prevalence of DNA was in Buteo buteo from Turkey; 23 (92.0%) of 25 were infected (Table 3).
Experimental infection of crested caracara with T. gondii
An experiment on caracara in Brazil provided useful information. Caracaras are raptors/scavengers with wide distribution. Eight caracaras that could not be released from a rehabilitation centre were used in this experiment. They were serologically negative to T. gondii by IFA (cut-off 1:40). Five caracaras were fed rodent (Calomys callosus) infected with Me49 strain of T. gondii. The birds were euthanized 68 days p.i. Blood was collected for serological examination weekly or more often. Seroconversion occurred between 5 and 14 days p.i. The serological response was erratic. In 1 bird (No.6), transient antibody was observed on day 14 and then at 45 days p.i. The highest antibody titer was 1:650. Antibodies became undetectable at 68 days p.i. in 2 birds. By immunohistochemistry, T. gondii was detected in the hearts and muscles of all 5 caracaras and demonstrated by bioassay in the hearts of 2 birds. Three control caracaras not inoculated with T. gondii remained serologically negative to T. gondii (Vitaliano et al., Reference Vitaliano, Mineo, André, Machado, Mineo and Werther2010).
Herbivorous/insectivorous birds
Seroprevalence varied depending on the geography, host and the habitat (Table 7). For example, in the ground feeding pigeon (Columba livia), seroprevalences varied from 1.6 to 35.7% (Table 7). In a large sample size of pigeons, a low prevalence (1.9% of 521) was reported; the pigeons were from Durango City, Mexico that has a dry climate (Alvarado-Esquivel et al., Reference Alvarado-Esquivel, Rajendran, Ferreira, Kwok, Choudhary, Alvarado-Esquivel, Rodríguez-Peña, Villena and Dubey2011). This low prevalence is likely related to the effect of climate on oocyst survival. In another survey from cold climate, only 3.9% of pigeons from Colorado, USA were seropositive (Dubey et al., Reference Dubey, Felix and Kwok2010). Toxoplasma gondii antibodies were detected in 11.8% of 35 pigeons and 9.5% of 620 quails that were destined for human consumption in China (Cong et al., Reference Cong, Huang, Zhou, Xu, Wu, Yan, Zhao, Song and Zhu2012, Reference Cong, Chi, Sun, Shan, Kang, Meng and Qian2017a). Finding of T. gondii DNA in the muscles of 6.4% of 390 quails indicates that the parasite was present in these birds destined for human consumption (Cong et al., Reference Cong, Ju, Zhang, Meng, Ma, Qian and Zhu2017b). Similarly, T. gondii DNA was detected in 5.4% of 280 wild ducks and 3.4% of 350 common pheasants hunted for human consumption in the Czech Republic (Skorpikova et al., Reference Skorpikova, Reslova, Lorencova, Plhal, Drimaj, Kamler and Slany2018).
Table 7. Serologic prevalence of antibodies to Toxoplasma gondii in wild birds

ELISA, enzyme-linked immunosorbent assay. Unless stated otherwise, ELISA=ELISA in-house; IFA, indirect fluorescent antibody test; IHA, indirect haemagglutination assay; LAT, latex agglutination test; MAT, modified agglutination test (Dubey and Desmonts, Reference Dubey and Desmonts1987); Tg, Toxoplasma gondii; AS, positive association; IHC, immunohistochemical.
a ELISA Vekto-Toxo antibodies kits (Vektor-Best, Russian Federation); IHA (ImmunoHAI-Toxoplasmose, Wama Diagnostic, São Carlos, SP, Brazil); WAMA Diagnosis. Imuno-HAI; LAT Pastorex toxo, Bio-Rad Laboratories s.r.o., Prague, Czech Republic; MAT (New Life Diagnostic LLC, Carlsbad, CA, USA).
b ELISA ID Screen Avian Toxoplasmosis Indirect (ID VET, France); IHA (Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, China); LAT Latex agglutination test (Antec Diagnostic Products, Bridport, Dorset, UK); MAT (Toxo-Screen DA®, Biomerieux, Lyon, France). This is the same test as MAT.
Toxoplasma gondii infection in sea gulls and other scavenging birds indicates contamination of marine/lake waters with oocysts. In one study, 22.8% of 479 yellow-legged gulls (Larus michahellis) were seropositive to T. gondii (Cabezón et al., Reference Cabezón, Cerdbà-Cuéllar, Morera, García-Bocanegra, González-Solís, Napp, Ribas, Blanch-Lázaro, Fernández-Aguilar, Antilles, López-Soria, Lorca-Oró, Dubey and Almería2016). In another investigation, T. gondii antibodies (assessed by ELISA) were detected in 233 of 1122 freshly laid L. michahellis eggs (Gamble et al., Reference Gamble, Ramos, Parra-Torres, Mercier, Galal, Pearce-Duvet, Villena, Montalvo, González-Solís, Hammouda, Oro, Selmi and Boulinier2019). The freshness of eggs was verified by immersion in water; such eggs do not float in water (Gamble et al., Reference Gamble, Ramos, Parra-Torres, Mercier, Galal, Pearce-Duvet, Villena, Montalvo, González-Solís, Hammouda, Oro, Selmi and Boulinier2019). The occurrence of T. gondii antibodies in 4 other species of sea birds (Sula spp. and Phaeton spp.) from Brazil (Gennari et al., Reference Gennari, Niemeyer, Soares, Musso, Siqueira, Catão-Dias, Dias and Dubey2016b) and in 19.9% of 659 black-headed gulls (Chroicocephalus ridibundus) in China (Miao et al., Reference Miao, Han, Xiang, Yuan, Liu, Duan, Zhu and Zou2014) indicates that T. gondii infection is common in sea birds. Toxoplasma gondii oocysts from feline feces can be washed into sewage and freshwater run-off and contaminate marine waters. Antibodies to T. gondii were detected in 8.5% of 632 mute swans (Cygnus olor) from the USA and viable T. gondii was isolated from hearts of 3 (Dubey et al., Reference Dubey, Choudhary, Kwok, Ferreira, Oliveira, Verma, Marks, Pedersen, Mickley, Randall, Arsnoe and Su2013). Mute swan is an invasive species present in US waters; infection in these hosts is indicative of oocyst contamination.
Reports of viable T. gondii and parasite DNA from tissues of wild birds are summarized in Tables 2 and 3, respectively.
Experimental infection of pigeons with T. gondii
Little information is available concerning the efficacy of different diagnostic methods for the detection of T. gondii infection in wild birds. A diagnostically useful experimental study was conducted in pigeons in Brazil (de Godoi et al., Reference de Godoi, Nishi, Pena and Gennari2010). Sixteen seronegative pigeons (C. livia) were inoculated orally with 50 oocysts of the T. gondii VEG strain and divided into 4 groups of 4 pigeons each and euthanized at 15, 30, 45 and 60 days p.i. One pigeon died of toxoplasmosis on day 23 p.i. and tissue cysts were found in its brain; other pigeons remained healthy. All pigeons were seronegative by the MAT (cut-off 1:5) and IFA (cut-off 1:4) before feeding oocysts and developed antibody titers of more than 1:4000 by both MAT and IFA. Tissues (brain, heart, muscle) were tested by bioassay in mice and by PCR. Viable T. gondii was isolated from 5 of 12 pigeons and DNA was detected by nested PCR in tissues of 7 of 12 pigeons. By serology and bioassay, none of the 160 naturally exposed pigeons were positive for T. gondii providing further evidence of the validity of serology (de Godoi et al., Reference de Godoi, Nishi, Pena and Gennari2010).
Migratory birds
Toxoplasma gondii infections in migratory birds are of epidemiological significance because the parasite can be transported with the host and the introduction of T. gondii in new geographic locations can disturb the equilibrium (Gennari et al., Reference Gennari, Ogrzewalska, Soares, Saraiva, Pinter, Labruna and Dubey2014). For example, ToxoDB genotype #9 (Chinese 1) occurs mainly in China but has been occasionally found in other countries, including the USA and Mexico. Whether migratory birds could have transported the parasite is a possibility. Antibodies to T. gondii were detected in Magellanic penguins in Chile and these birds migrate throughout South American coastline (Acosta et al., Reference Acosta, Soares, Mayorga, Alves, Soares and Gennari2018, Reference Acosta, Souza-Filho, Muñoz-Leal, Soares, Heinemann, Moreno, González-Acuña and Gennari2019).
A study of migratory and non-migratory geese revealed interesting results (Sandström et al., Reference Sandström, Buma, Hoye, Prop, van der Jeugd, Voslamber, Madsen and Loonen2013). A total of 2675 birds, both adults and juveniles, of 4 goose species (Anser anser, n = 266; A. brachyrhynchus, n = 787; Branta canadensis, n = 79; B. leucopsis, n = 1543) at Arctic brood-rearing areas in Russia and on Svalbard, and temperate wintering grounds in the Netherlands and Denmark (migratory populations) as well as temperate brood-rearing grounds (the Netherlands, non-migratory populations) were tested for T. gondii antibodies (MAT, 1:40). Only adult B. leucopsis were seropositive: T. gondii antibodies were found in 14.8% of 811 adults from Svalbard and 17.7% of 157 from Russia sampled during summer but not in any of the 456 juveniles sampled in summer (Arctic) and summer and winter in the Netherlands. Similar results were obtained with 3 other species of goose (Sandström et al., Reference Sandström, Buma, Hoye, Prop, van der Jeugd, Voslamber, Madsen and Loonen2013). The authors concluded that geese become infected postnatally at wintering groups.
In a study of migratory birds and resident Nearctic brown lemmings (Lemmus trimucronatus) from Arctic Canada, T. gondii infections were detected only in migratory geese (Table 1) and not in resident lemmings (Elmore et al., Reference Elmore, Samelius, Fernando, Alisauskas and Jenkins2015).
Clinical toxoplasmosis in wild birds
Little is known of clinical toxoplasmosis among wild birds in nature. Among all avian species, most severe toxoplasmosis has been reported in canaries (Serinus canaria), Hawaiian geese (Branta sandvicensis) and Hawaiian crows (Corvus hawaiiensis); reports between 1988 and 2009 were summarized previously (Dubey, Reference Dubey2010). In the past decade, few cases of fatal toxoplasmosis were documented in captive birds or those from National Parks (Table 8).
Table 8. Clinical toxoplasmosis in wild birds

IHC, immunohistochemistry; PCR, polymerase chain reaction; TEM, transmission electron microscopy; PCR-RFLP, Restriction fragment length polymorphism.
Hawaiian crows (C. hawaiiensis) and Nene goose (B. sandvicensis) are endangered native species in Hawaii. Nene is the largest extant terrestrial bird in Hawaiian Island and the official state bird (Work et al., Reference Work, Dagenais, Rameyer and Breeden2015). Of 300 Nene examined at necropsy, inflammatory conditions were found in 69 and 16.0% of these were thought to be toxoplasmosis (Work et al., Reference Work, Dagenais, Rameyer and Breeden2015). Thus, 11 geese died of toxoplasmosis. The presence of cats in colonies near the native bird sites is thought to be a source of T. gondii oocysts for the birds (Lepczyk et al., Reference Lepczyk, Haman, Sizemore and Farmer2020).
Two episodes of clinical toxoplasmosis were reported in guinea fowl in the USA and Brazil. An owner in Mississippi, USA lost 7 of 20 backyard guinea fowls. Birds were lethargic before death. Two dead birds were necropsied. Severe lesions of multifocal necrosis, fibrin exudation and inflammation of spleen, lung, heart and bone marrow were seen microscopically in 1 and mild lesions in the other guinea fowl. Toxoplasma gondii was identified histologically in tissues of both birds and the diagnosis was confirmed by PCR (Jones et al., Reference Jones, Wilson, Fitzgerald and Kiupel2012).
The Brazilian outbreak of clinical toxoplasmosis was reported in guinea fowl on a chicken farm; the farm had 47 chickens (G. domesticus) and 29 guinea fowl (Vielmo et al., Reference Vielmo, Pena, Panziera, Bianchi, De Lorenzo, Oliveira, Alves, Gennari, Pavarini, de Barros and Driemeier2019). Of these 76 birds, 22 (13 chickens and 9 guinea fowl) had clinical signs and 15 (9 chickens, 6 guinea fowl) died. Two guinea fowl were examined at necropsy and both had toxoplasmosis (Table 8).
Genetic diversity of T. gondii isolates
PCR-RFLP genetic data based on extraction of DNA from host tissue are summarized in Table 9 and from the live tachyzoites in Table 10. A total of 102 samples from birds were genotyped in this summary (Table 10), including 75 from viable T. gondii isolates (Table 2) and 27 from DNA extracted from tissues of birds (Table 10). Overall, genotype distribution follows the global patterns recognized previously (Shwab et al., Reference Shwab, Zhu, Majumdar, Pena, Gennari, Dubey and Su2014; Su and Dubey, Reference Su and Dubey2020), with ToxoDB genotypes #1 and #3 (collectively known as Type II), and genotype #2 (known as Type III) being dominant in Africa and Europe. Most genotypes identified in the Americas were diverse and different from those in the Old World. Of interest is the predominance of ToxoDB genotype #9 (Chinese 1) in China and its rare occurrence in Mexico (Alvarado-Esquivel et al., Reference Alvarado-Esquivel, Rajendran, Ferreira, Kwok, Choudhary, Alvarado-Esquivel, Rodríguez-Peña, Villena and Dubey2011; Shwab et al., Reference Shwab, Zhu, Majumdar, Pena, Gennari, Dubey and Su2014). Type I isolates (ToxoDB genotype #10) are considered rare worldwide. It was detected in a hunted turkey in 4 tree sparrows in China (Table 3). As this genotype is highly virulent to mice and relatively easy to isolate by bioassay, future study to obtain isolates for genotyping is needed to confirm the findings. Also, of interest is the finding of ToxoDB genotypes #4 and #5, together known as Type 12, in North America but their rare frequency from the rest of the world.
Table 10. Distribution of PCR-RFLP (ToxoDB) Toxoplasma gondii genotypes from wild birds from different continents/countries

a DNA from tissues. Twenty-seven of the 102 samples were DNA from bird tissues.
Table 9. Toxoplasma gondii genotypes based on DNA directly from host tissue

Conclusions
Here, we summarized seroprevalence, clinical disease, epidemiology and genetic diversity of T. gondii strains isolated from wild birds worldwide for the past decade. It is obvious that T. gondii infection in raptors is common and they are excellent sentinels to monitor T. gondii in rodents and small other animals. Detection of T. gondii antibodies in eggs offers a non-invasive sampling method. In one investigation, T. gondii antibodies (assessed by ELISA) were detected in 233 of 1122 freshly laid yellow-legged gulls (L. michahellis) eggs (Gamble et al., Reference Gamble, Ramos, Parra-Torres, Mercier, Galal, Pearce-Duvet, Villena, Montalvo, González-Solís, Hammouda, Oro, Selmi and Boulinier2019). Finding antibodies in sea gulls indicates contamination of fresh and marine waters with T. gondii oocysts. In general, T. gondii infection in herbivorous birds is a good measure of oocyst contamination in the environment. Genetic studies revealed low genetic diversity in Europe, Asia, Africa and the USA, but higher diversity of T. gondii in South America. A study of migratory and non-migratory geese at Arctic brood-rearing areas in Russia and on Svalbard, and temperate wintering grounds in the Netherlands and Denmark (migratory populations) revealed that geese become infected postnatally at wintering groups (Sandström et al., Reference Sandström, Buma, Hoye, Prop, van der Jeugd, Voslamber, Madsen and Loonen2013).
Acknowledgements
This research was supported in part by an appointment of Camila K. Cerqueira-Cézar and Fernando H. A. Murata to the Agricultural Research Service (ARS) Research Participation administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the US Department of Energy (DOE) and the US Department of Agriculture (USDA). ORISE was managed by ORAU under DOE contract number DE-SC 0014664. All opinions expressed in this paper were the authors' and did not necessarily reflect the policies and views of USDA, ARS, DOE or ORAU/ORISE. We thank Dr Yurong Yang for help with Chinese references.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Not applicable.













