INTRODUCTION
The tapeworm Ligula intestinalis is a common pseudophyllidean cestode that successively infests 3 different hosts during its parasite cycle. Teleost fish and particularly members of the Cyprinidae are the second intermediate host and are usually infested by the plerocercoid larvae of this pseudophyllidean cestode after eating parasitized zooplankton. During the infection of the fish, the tapeworm invades the abdominal cavity where it remains for the life of the host. The parasitized fish can be eaten by a piscivorous bird to complete the parasite cycle. Effects of this parasite on fish health have been studied in several species including bream, Abramis brama and white bream, Blicca bjoerkna (Barus and Prokes, Reference Barus and Prokes2002), gudgeon, Gobio gobio, rudd, Scardirius erythrophthalmus, fathead minnow, Pimephales promelas and dace, Leusiscus leusiscus (Arme and Owen, Reference Arme and Owen1968), tench, Tinca tinca (Yavuzcan et al. Reference Yavuzcan, Korkmaz and Zencir2003), and roach Rutilus rutilus (Arme and Owen, Reference Arme and Owen1968; Kennedy et al. Reference Kennedy, Shears and Shears2001). Nevertheless, the roach appears to be the specific host of L. intestinalis according to the preponderance of infections recorded (Arme and Owen, Reference Arme and Owen1968; Loot et al. Reference Loot, Poulin, Lek and Guegan2002; Jobling and Tyler, Reference Jobling and Tyler2003).
It has been well established that, from a pathogenic point of view, the second host is the most affected because L. intestinalis occupies the body cavity of the fish for several years and is responsible for harmful effects (Van Dobben, Reference Van Dobben1952; Dence, Reference Dence1958; Wilson, Reference Wilson1971). The first morphological effect appears after the rapid growth of the parasite in the fish's body cavity, characteristically distending the abdominal region. Effects include reduction of fish growth, particularly for young fish, and an apparent reduction in the ability of ligulosed fish to escape predation. For example, Van Dobben (Reference Van Dobben1952) reported that 7% of the roach caught in the river were parasitized while infected roach represented 30% of the prey found in stomachs of cormorants. In a large variety of species, infection by L. intestinalis induces an inflammatory response (Taylor and Hoole, Reference Taylor and Hoole1995) and, most importantly and specifically, impaired reproduction. This latter effect is related to the inhibition of gonadal maturation resulting in completely immature reproductive tissues. In bream, the parasite appears to be able to inhibit sex steroid production (Hecker and Karbe, Reference Hecker and Karbe2005) and aromatase activity (Hecker et al. Reference Hecker, Sanderson and Karbe2007) while cytological changes of the pituitary gland and associated reduction of gonadotrophin II (LH) synthesis have been reported in roach (Arme and Owen, Reference Arme and Owen1968; Carter et al. Reference Carter, Pierce, Dufour, Arme and Hoole2005).
The effects of L. intestinalis on fish reproduction are of importance for environmental studies specifically looking at the effects of pollution on wildlife. Parasites may contribute to the observed effects reported in contaminated sites and might greatly increase the impact and thus the risk a given species is facing in those polluted areas. But if parasitization cannot be ignored in the prognosis, it should also be taken into consideration for the diagnosis. Parasites can be important factors of impaired fish health and may generate false positive results of biomarkers of pollution. L. intestinalis appears to be able to interfere with parameters that are usually monitored in pollutant-related studies dealing with endocrine disruption. Thus, the aim of this study was to document the effects of L. intestinalis on several end-points including plasma steroid levels, induction of the yolk protein precursor vitellogenin, aromatase activity and gonad maturation in order to increase our knowledge of the specific actions of the parasite in roach.
MATERIALS AND METHODS
Sampling
From September 2005 to June 2007, 504 wild mature roach (>14 cm long) were sampled monthly from a reference site, a former sand quarry, in Normandy, France. In total, 41 parasitized fish were observed between October and April and were grouped according to season. This was defined as either the maturation period (October–December) or the spawning season (March–April). A previous study revealed that the sampling site was free from xenoestrogens according to the low plasma vitellogenin levels recorded in male roach (below 30 ng.ml−1, unpublished data). Furthermore, the mutagenicity and the oestrogenicity of the sediment extracts measured by the SOS chromotest (Couteau et al. Reference Couteau, Flaman, Minier and Cachot2008) and the YES assay (Peck et al. Reference Peck, Labadie, Minier and Hill2007), respectively, were under the detection limit (Couteau and Minier, personal communication). Fish were caught using nets. After being anaesthetized with tricaine-s (MS 222; 100 mg.l−1), blood was collected from the caudal vein using a heparinized syringe. The fish were then dissected, the gonads removed and weighed to determine the gonadosomatic index as: GSI (%)=(gonad weight)/(body weight)×100. Three pieces of 5 mm in diameter from the anterior, the middle and the posterior region of each gonad were fixed in 4% formaldehyde. The remaining gonad was immediately frozen in carbonic ice and stored at −80°C until further processing. The condition factor was calculated as (Fulton, Reference Fulton1904): CF=(body weight)/(total length3). The parasitization index (PI) was calculated as described by Hoole (Reference Hoole, Pike and Lewis1994): PI (%)=(weight of parasite)/(total fish weight)×100.
Gonad histology
Gonads were dehydrated through a series of graded ethanol (50–99·9%), cleared with xylene, and embedded in paraffin. Transverse sections of 5 μm thickness were cut and stained with haematoxylin eosin, and saffron for further microscopic observations. The categorization of cell types was based on a previous study on the gametogenesis of roach (Jafri, Reference Jafri1990; Geraudie et al. Reference Geraudie, Gerbron, Hill and Minier2009).
Vitellogenin
Plasma concentrations of vitellogenin (VTG) were measured by sandwich enzyme-linked immunosorbent assay (ELISA), using carp (Cyprinus carpio) monoclonal antibodies, according to the manufacturer's instructions (Biosense Laboratories, Bergen, Norway). Three different dilutions of each sample were assessed, i.e: 1:5000, 1:50 000, 1:500 000 and 1:50, 1:500 and 1:5000 for female and male roach respectively.
Sex steroids
Progesterone (P), 11-ketotestosterone (11-KT), and 17-β-estradiol (E2) plasma concentrations were determined by enzyme-linked immunosorbent assay (ELISA), following the manufacturer's protocol (Cayman Chemical Company, Ann Arbor, Michigan, USA). Plasma samples were diluted 10-fold for 11-KT and P assay, while a 1:5 dilution was used for E2.
Aromatase activity
Brains and gonads were homogenized with a Precellys 24 (Bertin Technologies, Montigny-le-Bretonneux, France) in 50 mM potassium phosphate buffer, pH 7·4, containing 1 mM PMSF, 1 mM EDTA and 20% glycerol (v/v) in a ratio of ½ (w/v). After centrifugation (1000 g, 20 min, 4°C), supernatants were collected and the total amount of proteins determined by Bradford assay (Bradford, Reference Bradford1976). Samples were then stored at −80°C.
Aromatase activity was determined using the tritiated water assay which quantified the release of tritiated water during the conversion of the labelled substrate [1β-3H (N)] androst-4-ene-3,17-dione to estrone (Thompson and Siiteri, Reference Thompson and Siiteri1974). Optimal conditions were determined as well as concentrations of brain proteins and labelled substrate. The duration and the temperature of the incubation were optimized.
For the aromatase assay, 500 μg of brain protein were added to a potassium phosphate buffer (50 mM) containing 1 mM NADPH. The reaction was started by the addition of 150 nM of [1β-3H (N)] androst-4-ene-3, 17-dione. After 1 h incubation at 30°C, the reaction was stopped by addition of 1 ml of chloroform. After vigorous vortexing for 30 s, the glass tubes were centrifuged (3000 g, 10 min, 4°C). The aqueous fraction was removed, vigorously vortexed with 1 ml of chloroform and then centrifuged (3000 g, 10 min, 4°C). Activated charcoal (5%, w/v) was added to the aqueous fraction to eliminate remaining organic compounds, vortexed for 30 s and centrifuged (4000 g, 20 min, 4°C). Two aliquots (150 μl) of each supernatant were distributed in a 24-well plate (Flexibles plates 24-w, Perkin Elmer) containing 750 μl of scintillation liquid (OptiPhase ‘Hi safe’ 3, Pekin Elmer). Scintillation was counted using a Liquid Scintillation Counter (Microbeta, Perkin Elmer).
Statistics
All results are expressed as means±95% confidence interval (CI). Normality was controlled using the Shapiro-Wilk's W test. Statistical comparisons were made using the Student's t-test to determine statistical significance of data from ligulosed and uninfected roach.
Results
Biological parameters
A total of 41 roach were found to be parasitized by the cestode Ligula intestinalis. This represented 8·1% of the sampled population during the late autumn (October–December) and early spring (March–April) 2005–2007. Between 1 and 10 L. intestinalis were observed in the abdominal cavity of infected fish. Calculation of the parasitization index resulted in values from 3 to 13·5% showing a low to medium impact of the developed parasite on the total weight of the sampled fish. Accordingly, a slight but significant effect in condition index was measured when comparing parasitized and non-parasitized fish using the Fulton's index (Table 1). Furthermore, a marked effect was seen in gonadal growth, with a 50% decrease in the gonado-somatic index (GSI) for both sexes (Fig. 1). The effect was more pronounced during the breeding season as the gonads were hardly developed in infected fish and the ligulosed fish GSI values were then only 25–30% of the non-infected fish (Fig. 1). No correlation could be found between the occurrence of the parasite and the length of the roach. In total, 20 male and 21 female roach were parasitized suggesting that gender had no influence on L. intestinalis development. Indeed this sex-ratio among parasitized fish was similar to that of the whole population (male: female, 1: 1·07).

Fig. 1. Gonadosomatic index (GSI) of male (♂) and female (♀) roach uninfected (black bars) or infected (grey bars) with the cestode Ligula intestinalis. Results are given as mean±C.I. Significant differences are indicated (**P<0·01; ***P<0·001).
Table 1. Morphological parameters in male and female roach uninfected or infected with the cestode Ligula intestinalis
(Significant differences between groups of fish (P<0·05) are indicated using different letters.)

Histological observations
Gonad maturation of parasitized roach was inhibited. The gonads subsequently remained immature throughout the reproductive cycle for a high proportion of ligulosed fish (Fig. 2). It was found that 80% of the male roach infested with L. intestinalis did not demonstrate any gametogenesis and only spermatogonias could be visualized. On the contrary, no immature male fish were observed among the ‘healthy’ population in spring. A similar picture was obtained with females, whereby 13·6% and 80% of the non-parasitized or ligulosed fish, respectively, showed only primary oocytes under histological examination in spring. Nevertheless, 1 parasitized male and 1 parasitized female reached complete maturity during the breeding season and displayed mature spermatozoa or secondary oocytes. No histological pathologies such as necrosis, fibrosis or inflammatory foci were observed among the ligulosed-fish, which was in line with the very low occurrence of these pathologies in the whole sampled population.

Fig. 2. Percentage of early stages of sexual maturity (measured as spermatogonia or primary oocytes in the gonad) in male (♂) and female (♀) roach uninfected (black bars) or infected (grey bars) with the cestode Ligula intestinalis.
Vitellogenin and sex steroids
Low concentrations of plasma vitellogenin (VTG <100 ng.ml.−1) were recorded in male roach whatever the sampling period, suggesting that no environmental xenoestrogens were interfering with the endocrine system in the sampling area. On the contrary, female roach had higher plasma content of this phospholipoprotein (Fig. 3). No significant differences were found in male plasma VTG between parasitized roach and unaffected fish. High VTG concentrations were recorded in unaffected females with a mean concentration reaching 895·103 ng.ml−1 in spring. However, infected female roach showed a significant reduction of their plasma VTG concentrations (5-fold in autumn and 9-fold in spring).

Fig. 3. Plasma vitellogenin (VTG) concentrations in male (♂) and female (♀) roach uninfected (black bars) or infected (grey bars) with the cestode Ligula intestinalis. Results are given as mean±C.I. Significant differences are indicated (*P<0·05).
The plasma progesterone (P) levels were similar in both male and female roach (Fig. 4). In healthy fish, the highest levels were recorded in autumn whereas no seasonal variation was observed in their parasitized counterparts. These later showed a significant decrease (of more than 3-fold) in their plasma P concentrations for both sexes in autumn. Furthermore, a significant negative correlation was observed between female P levels and the parasitization index (R2=−0·90; P<0·05).

Fig. 4. Mean concentrations of progesterone (P) in male (♂) and female (♀) roach uninfected (black bars) or infected (grey bars) with the cestode Ligula intestinalis. Results are given as mean±C.I. Significant differences are indicated (*P<0·05).
Results of measurements of 11-KT plasmatic levels indicated that males had significantly higher concentrations than females in both ligulosed and uninfected roach (Fig. 5). Healthy male and female 11-KT levels followed seasonal variations with lower values in autumn than in spring whereas no seasonal difference was observed in parasitized roach. Plasma 11-KT concentrations were 27-fold lower in infected male fish in spring whereas ligulosed females exhibited a 50% decrease in their plasma levels in spring.

Fig. 5. Concentrations of plasma 11-keto-testosterone (11-KT) in male (♂) and female (♀) roach uninfected (black bars) or infected (grey bars) with the cestode Ligula intestinalis. Results are given as mean±C.I. Significant differences are indicated (*P<0·05; **P<0·01).
Male plasma E2 values were significantly 2-fold lower in ligulosed fish when compared to levels measured in non-parasitized roach in autumn (Fig. 6). The parasitized female roach were also characterized by an inhibition of the E2 circulating levels with concentrations respectively 1/2 and 1/5 of the E2 concentration measured in the non-parasitized roach in autumn and spring.

Fig. 6. Concentrations of plasma 17-β-estradiol (E2) in male (♂) and female (♀) roach uninfected (black bars) or infected (grey bars) with the cestode Ligula intestinalis. Results are given as mean±C.I. Significant differences are indicated (*P<0·05; ***P<0·001).
Aromatase activity
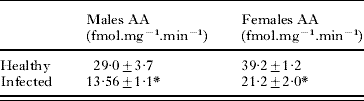
Gonadal aromatase activity was very low and below detection limits in all analysed samples. On the contrary a high aromatase activity could be measured in the brain (Table 2). Comparison between ligulosed and non-ligulosed roach revealed a significant 50% decrease in aromatase activity for both sexes.
Table 2. Brain aromatase activity (fmol.mg−1.min−1) in male and female roach uninfected or infected with the cestode Ligula intestinalis
(Significant differences are indicated (*P<0·05).)
DISCUSSION
Here we report that L. intestinalis was present at a low prevalence in a roach population living in a low polluted site. Of the studied population, 8% was infested with a similar percentage for both sexes and an impact of parasitization ranging from 3 to 13%. L. intestinalis appeared to be responsible for impaired gonadal growth of infested fish and the inhibition of gametogenesis of the reproductive cells as previously reported (Arme and Owen, Reference Arme and Owen1968). These effects were associated with inhibition of steroid synthesis. Male 11-keto-testosterone, female 17-β-estradiol and progesterone plasma concentrations of both genders were, respectively, 27-, 5- and 3-fold lower in ligulosed fish when compared to their non-infected counterparts. Progesterone levels were negatively correlated with the parasitization index in females suggesting a direct link between the presence of the parasite and the impaired steroid synthesis. The steroid pathway was also altered in the brain as aromatase activity of infected roach was reduced to 50% of that of the non-infected fish in this organ.
Endocrine disrupting compounds have also been reported to be responsible for impaired gonadal growth, reduced steroid synthesis and inhibition of aromatase activity (Jobling et al. Reference Jobling, Beresford, Nolan, Rodgers-Gray, Brighty, Sumpter and Tyler2002; Martin-Skilton et al. Reference Martín-Skilton, Lavado, Thibaut, Minier and Porte2006). However, this study was conducted at a low polluted site and effects appeared specific of the infested population. The absence of VTG synthesis in the male roach population is in accordance with the low occurrence of xeno-estrogens. Several reviews (MacKenzie, Reference MacKenzie1999; Sures, Reference Sures2004) and meta-analyses (Blanar et al. Reference Blanar, Munkittrick, Houlahan, MacLatchy and Marcogliese2009) have discussed the effects of pollution on fish and their parasites. The outcome with regards to the prevalence and importance of parasitism in a given fish population varies greatly depending on the sensitivity of the parasite or the fish species. Lafferty and Kuris (Reference Lafferty and Kuris1999) showed that pollutants may increase parasitism by either increasing host susceptibility or by increasing the abundance of intermediate hosts and vectors. However, pollution can also decrease the number of parasitized fish when the parasite is more sensitive to a pollutant than its host (Sures, Reference Sures2004). When considering L. intestinalis, Hecker and Karbe (Reference Hecker and Karbe2005) observed an increase of the prevalence of ligulosed bream in several polluted sites. Up to 82% of infested bream were found at polluted sites whereas only 7·9% of parasitized fish were observed at the reference site. In the present study, the prevalence of the cestode was 8% in the studied roach population living at a low contaminated site. This can be compared with a previous report where low prevalence of infected roach (5%) in polluted sites in the River Seine (close to the sampling areas of this study) were measured (Minier et al. Reference Minier, Caltot, Leboulenger and Hill2000), suggesting no effect of pollution on the prevalence of L. intestinalis in roach. This discrepancy may be attributed to either the different fish species or the pollutants they are exposed to, although the Seine River is characterized by its high pollutant burden of both industrial and urban origin (Claisse, Reference Claisse1989; Minier et al. Reference Minier, Abarnou, Le Guellec, Jaouen-Madoulet, Tutundjian, Bocquené and Leboulenger2006). Furthermore, a number of potentially confounding factors, including season, fish age and length or sampling site may also affect parasitism (Kennedy and Burrough, Reference Kennedy and Burrough1981).
No correlation between the length of fish and occurrence of parasitism was found in the present study. In addition, no difference in weight and only a slight effect on condition index were assessed between parasitized and non-parasitized fish. However, since the aim of the work was to look at mature fish, only fish longer than 14 cm were collected, so parasitic impact in young roach could not be investigated. In 1968, Arme and Owen observed that roach from the age group of 2+ were more affected in their growth and weight that older (3+ and more) fish. This was also later confirmed by Carter et al. (Reference Carter, Pierce, Dufour, Arme and Hoole2005). L. intestinalis had a small inhibitory effect on body length, weight and condition index in young fish (i.e. 2 years old) but this could not be seen with older roach. An explanation for this may arise from the inhibition of gonad development. Infected roach might be able to invest more energy in their body development as reproduction is stopped thus counterbalancing the lost energy due to parasitism. Nevertheless, parasites may not be the only reason for adult roach failing to differentiate reproductive cells. In our study, 6·5% of the non-parasitized roach did not mature in spring. Another possible explanation might be related to the individual resilience which may allow the more resistant roach to survive the infestation and become adult (Kennedy and Burrough, Reference Kennedy and Burrough1981; Kennedy et al. Reference Kennedy, Shears and Shears2001). These fish with greater resilience may therefore perform in a manner similar to the general population of roach even if they are infested.
One remarkable observation from this study is the occurrence of 1 male and 1 female roach that, although infested by L. intestinalis, reached sexual maturity based on histological examination. This indicated that, in rare cases, ligulosed roach can achieve complete gametogenesis. This has not been previously reported in roach although it has been observed in ligulosed gudgeon (Gobio gobio) (Arme and Owen, Reference Arme and Owen1968). A possible explanation could be that these roach were parasitized only recently, or shortly following puberty, thus limiting the effect of the plerocercoid. In their important study in the British Isles, Arme and Owen (Reference Arme and Owen1968) reported that although intake of L. intestinalis is possible throughout the roach life, it rarely occurs in fish whose age exceeds 3 years. This might be related to the predominantly copepod diet of young fish which leads to a higher probability of parasite infestation. In contrast, older fish might be less parasitized because of the relatively insignificant number of ingested copepods. In the present study, the length of the L. intestinalis (>10 cm, in both cases) does not support a recent infestation and this hypothesis might be excluded. Another explanation for the occurrence of mature roach may be that some roach can be resistant enough and less affected by L. intestinalis so that they could undergo gonad development even in the presence of the parasite.
This study indicated that all steroid production measured was affected by the presence of the plerocercoid in the body cavity. In addition to strong inhibition of sex-specific 11-KT and E2, P synthesis was also significantly reduced, indicating a general inhibition of steroid synthesis. Inhibition of 11-KT and E2 were reported in bream (Hecker and Karbe, Reference Hecker and Karbe2005) showing that L. intestinalis could have a specific action on sex steroid production in both species. Inhibition of P synthesis is reported here for the first time. As P is involved in the onset of annual gonadal development (Schulz et al. Reference Schulz, de França, Lareyre, Legac, Chiarini-Garcia, Nobrega and Miura2009), this may explain the inhibitory effect on gonad maturation generally observed in ligulosed fish. Nevertheless, other effects have been reported such as inhibition of gonadotrophin (Carter et al. Reference Carter, Pierce, Dufour, Arme and Hoole2005) or GnRH production (Arme, Reference Arme1997). Moreover, our data indicated that brain aromatase activity in parasitized roach decreased by half compared to the non-infected roach in both sexes suggesting that neuroendocrine production is also altered in ligulosed roach. Similarly, in ligulosed male bream, brain aromatase activity was inhibited and negatively correlated with the prevalence of L. intestinalis infection (Hecker et al. Reference Hecker, Sanderson and Karbe2007). Multiple endocrine parameters are affected by the presence of this tapeworm and it is difficult to identify the primary and specific effect of L. intestinalis on the roach endocrine system.
CONCLUSIONS
The present study demonstrated significant negative effects on the reproductive function of wild roach infected by the tapeworm L. intestinalis collected from a reference site in Normandy. All the studied steroid parameters were affected. This could be responsible for the inhibition of VTG synthesis and the suppression of gonad maturation. This work also indicates that ligulosed fish should be excluded when measuring hormonal levels, aromatase activity and gonadal development in order to study pollutant-related endocrine disrupting effects. However, VTG induction might still be a good indicator of xenoestrogen exposure although it could generate false negative results.
ACKNOWLEDGMENTS
The authors thank the Pisciculture de Venables, especially the Hoydrie family for their valuable help in the fish collection. The authors are grateful to Dr Jeanette Rotchell (University of Sussex) and Dr Jenny Shaw (Plymouth Marine Laboratory) for their advice and correction of the manuscript.
FINANCIAL SUPPORT
This study is part of the RAED (project no. 008) and DIESE (project no. 040) research projects supported by grants awarded by the European Interreg IIIA and IVA France (Channel) England programmes.










