INTRODUCTION
Honey bees are well-known beneficial insects. Most importantly, honey bees serve as pollinators for various fruit and field crops, as well as countless environmentally important wild plants. Pollination services and honey production are valued annually, in the US alone, at over 15 billion dollars (Morse and Calderone, 2000). However, the health of honey bees is under threat from a parasitic mite, the varroa mite (Varroa destructor), one of the major contributing factors to heavy bee losses worldwide (de Jong et al. 1982; Mobus and de Bruyn, 1993; Sammataro et al. 2000). Varroa mite was detected for the first time on A. mellifera in 1958 (Mikawa, 1986) and was first introduced into the US in 1987 (Morse and Flottum, 1997). The US has suffered a heavy bee loss because of varroa infestation, as illustrated by the bee colony loss percentages in the northeastern US from the winter of 1995 to the spring of 1996: Maine 80%, New Jersey 60%, New York 60–70%, and Pennsylvania 53% (Finley et al. 1996). As a result, varroa mites have endangered honey production and pollination services in the US.
How do varroa mites affect honey bees? Several avenues have been proposed. First, varroa mites immunosuppress honey bees (Yang and Cox-Foster, 2005). Second, the mites live on bee haemolymph, which may cause reduced adult body weight and protein content. Third, varroa mites are implicated in many bee diseases. The mites are blamed for causing a so-called parasitic-mite syndrome (Shimanuki et al. 1994). More importantly, the mites have been suggested (Camazine and Liu, 1997; Allen et al. 1986; Ball, 1988; Ball and Allen, 1988; Bailey and Ball, 1991; Bowen-Walker et al. 1999) and demonstrated (Shen et al. 2005a,b) to vector various bee viruses. Among these diseases, deformed wing virus (DWV) was proposed to cause the symptom of deformed wings (Ball, 1988; Ball and Allen, 1988; Bailey and Ball, 1991; Bowen-Walker et al. 1999) as indicated with serological methods. More recently, with the more sensitive RT-PCR method, DWV was detected in bees, especially those parasitized with varroa mites and with deformed wings (Yang, 2004; Shen et al. 2005b; Yue and Genersch, 2005). As indicated by real-time PCR, varroa-parasitized bees with deformed wing symptoms all carried high levels of DWV RNA (Yang and Cox-Foster, 2005).
There is a four-way intricate interaction among bees, bee viruses, varroa mites, and bacterial challenge. Bee viruses have multiple transmission routes (Shen et al. 2005a) and varroa mites activate the replication of DWV in bees (Shen et al. 2005b). Under varroa-free conditions, DWV exists at low but detectable levels within bees without causing disease symptoms (Yang, 2004; Yang and Cox-Foster, 2005). However, when bees are parasitized by varroa mites, combined with an exposure to microbes, DWV can replicate to a level of 105-fold higher within 10 h, which may directly cause bee death (Yang and Cox-Foster, 2005). In this research, we used RT-PCR to further confirm the incidence of DWV in relation to varroa infestation in a different group of bees.
It has become clear that varroa mites have a keystone role in this four-way interaction between bees, mites, viruses and microbes. Clarifying the role of varroa mites in bee survivorship is needed given this complex interaction. Is the death of bee colonies only due to the interaction between bee immunosuppression and pathogens or are impacts of the mite parasitization on physiological traits also involved? Thus, we hypothesized that mite-parasitized bees would exhibit reduced survivorship, especially when exposed to microbial challenges, due to both immunosuppression and effects on viral replication.
Varroa mites mainly feed on the bees at their pupal stage. Some of the varroa-parasitized pupae develop into adult bees with deformed wings (DW), and the rest the parasitized pupae develop into normal wing (NW) bees, regardless of the number of parasitic mites. Mite-free (MF) bee pupae develop only into healthy adults with normal wings. These 3 groups of bees have different pathological status (Yang and Cox-Foster, 2005; Shen et al. 2005b). It was reported previously that in observation hives the varroa-parasitized overwintering bees (probably equivalent to the NW bees in this research) survived a shorter time than varroa-free bees (Kovac and Crailsheim, 1988). In this research, we examined all 3 groups of bees, and correlated the survivorship with bee physiology and pathology. We injected live Escherichia coli to different groups of bees to test our hypothesis. The survivorship was measured over time from adult emergence (or treatment) to death under conditions mimicking those found in the hives.
Are other traits correlated with the bee death as related to mite parasitization? Previously we have found that the gene encoding a critical enzyme (phenol oxidase (PO)) in cellular immune functions in insects is expressed in newly-emerged worker bees; however, the expression of this gene is suppressed at transcriptional level in the varroa-parasitized bees (Yang and Cox-Foster, 2005). PO has been used as a classical enzyme for measuring the activity of the cellular immune response (Ashida and Yamazaki, 1990; Moret and Schmid-Hempel, 2001; Gillespie et al. 1997; Cox-Foster and Stehr, 1994). Examining PO enzymatic activity in the newly-emerged bees may help to explain any differences in survivorship of parasitized versus non-parasitized bees. PO in insect haemolymph is present as a pro-enzyme called pro-PO. It is activated through the action of a serine protease cascade (Tong et al. 2005; Ratcliffe et al. 1984). The cascade can be triggered in the haemolymph by biotic and abiotic substances, such as microbes, aberrant tissues, glass beads, etc. In this research, we injected immuno-elicitors into the bees to activate this cascade. We asked how the available PO activity is correlated with the survivorship at adult emergence. In conjunction we also asked if the impact of varroa mites on other physiological traits, such as bee body weight, is linked to bee survivorship. Overall bee health may be reflected in body weight that is a result of many factors such as parasitization, nutrition, and genetics. Using bees from a single colony, we have controlled for pre-adult differences in genetics, nutrition, and environment, allowing the impact of varroa parasitization to be detected.
MATERIALS AND METHODS
Preparation of bees and varroa mites
The bees were collected from a colony kept in the University Park Apiary on the Pennsylvania State University campus, University Park, Pennsylvania, USA. The colony was heavily parasitized with varroa mites. Adult bees were collected at the time of emergence from a single comb with uniform brood cells, and then weighed. The bees were divided into 3 groups according to the conditions of varroa infestation and wing deformity: mite-free bees with normal wings (MF), mite-parasitized bees with normal wings (NW), and mite-parasitized bees with deformed wings (DW).
Bacterial challenge of bees
For each group of bees, 4 treatments were conducted: (1) injection of live E. coli bacteria (JM101) suspended in sterile bee saline (8·3±0·03 μl/bee or approximately 6×106 cells/bee), (2) sterile bee-saline injection (8·3±0·03 μl/bee), (3) sterile-needle wounding and (4) non-treatment control. The bacteria were freshly cultured overnight in LB medium (1% Bacto tryptone, 0·5% Bacto yeast extract, 1% NaCl, pH 7·0) at 37 °C; then the bacterial cells were collected by centrifuging at 16000 g for 10 min at 4 °C. The cells were washed by resuspending pellets in sterile bee saline twice (0·156 M NaCl, 0·003 M KCl, 0·002 M CaCl2, pH 7·0) (Anderson and Gibbs, 1988). The cell density was measured spectrophotometrically at 600 nm using a SPECTRAMAX 250 (Molecular Devices) against a standard curve, and then the cell concentration was adjusted to 7·1×105 cells/μl for injection. The injection sites were treated with 75% ethanol to sterilize. Injections were made with a 30-gauge needle attached to a micro-injector, whose details were described previously (Yang and Cox-Foster, 2005). After the treatments, the bees were kept individually in queen cages (1·8×2·9×3·0 cm3) and provided with sugar and water at 35 °C and 50% RH. The bees were observed for survivorship on a regular interval until death occurred. For each treatment, no fewer than 16 bees were treated. A total of 299 bees was used, including 187 MF bees, 56 NW bees, and 56 DW bees.
Detection of DWV with RT-PCR
Total RNA was isolated from each newly-emerged worker bee by homogenizing in 500 μl of TRIZOL™ reagent (Life Technologies), and treated with the RQ1 RNase-Free DNase (Promega) according to the manufacturer's specifications. RNA concentration was determined spectrophotometrically (SmartSpec 300, BIO-RAD). Five μg of total RNA from each sample were used to make cDNA with the M-MLV Reverse Transcriptase kit (Promega) according to the manufacturer's specifications. Primers were designed for their specificity to DWV (forward 5′-ACGACACAACATCCTGTAG-3′, reverse 5′-TAAACTAGGTTGGACTGGAA-3′) based on DWV genomic sequence (GenBank Accession number NC_004830), which amplify a 621 bp amplicon. Honey bee actin gene (GenBank Accession number BI504901) was used as an internal control for the RT-PCR. Actin primers (forward 5′-ATGAAGATCCTTACAGAAAG-3′, reverse 5′-TCTTGTTTAGAGATCCACAT-3′) amplify an amplicon of 514 bp. A negative control lacking template cDNA was included each time. The PCR reaction was carried out in 1X PCR buffer (50 mM KCl, 10 mM Tris-HCl, 0·1% Triton X-100) containing 0·2 mM dNTPs, 2·0 mM MgCl2, 2 ng/μl of each primer, 0·05 units/μl of Taq DNA polymerase (Promega), and 1 μl of cDNA in a total reaction volume of 50 μl. The template cDNA was denatured for 5 min at 94 °C, after which 35 amplification cycles (94 °C for 20 s, 50 °C for 20 s, and 72 °C for 1 min) were carried out (GeneAmp PCR System 9700). The PCR products (5 μl/sample) were electrophoresed in a 1·5% agarose gel, and stained with ethidium bromide.
Measurement of PO activity
Newly-emerged bees were individually homogenized on ice with 500 μl of 0·1 M Tris-HCl buffer (pH 7·0) (Sigma) with a Teflon® homogenizer and then centrifuged at 16000 g for 10 min at 4 °C. L-3, 4-dihydroxyphenylalanine (L-DOPA) (0·02 M) (Sigma) was used as a substrate and PO activity was spectrophotometrically determined at 475 nm. Tyrosinase (2 units/μl, Sigma) was used as a positive control. The total protein concentration was determined by a modified Bradford method (Vincent and Nadeau, 1983). To determine if PO activity could be induced in newly-emerged bees, the bees (4–14 bees/treatment) were injected with several immune-elicitors, including heat-killed yeast, bee saline, and E. coli. These injections were handled the same as specified in the survivorship treatments. PO activity was determined 60 min post-injection.
Statistical tests
The STATVIEW 5.0.1 statistical package (SAS Institute, Cary, NC) was used to analyse the data by ANOVA, Kaplan-Meier survivorship analysis, and Mantel-Cox tests.
RESULTS
Detection of DWV in the bees
RT-PCR results confirmed that DWV was detectable in all the bees tested (Fig. 1). All the mite-parasitized NW and DW bees had highly intense DNA bands whereas the varroa-free MF bees had faint DNA bands. This result confirmed that the DW bees had DWV at high levels. Previous data indicate that the DW bees have 106-fold higher levels of DWV than normal wing bees (Yang and Cox-Foster, 2005).

Fig. 1. Detection of DWV RNA in the bees with RT-PCR. A 621 bp amplicon showed that all the bees tested contained DWV RNA and that mite-parasitized bees contained high DWV viral RNA titres. Actin gene was used as a control. The numbers in parentheses are the number of bees tested.
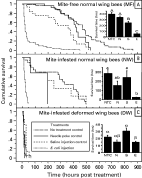
Effect of varroa parasitization on survivorship curves
Survivorship curves reveal how long the individuals of a group of organisms survive during a period of time or their entire life-span. There are basically 3 types of survivorship curves (Schowalter, 2000). In the non-treatment bees, the survivorship curves of the MF, NW and DW bees were significantly different (Mantel-Cox test, P<0·0001). The survivorship curve of the non-treatment MF bees was close to a Type-I curve (Fig. 2A), which represents a situation where individuals have high survival at an early stage, live out most of their expected life-span, and die in old age. The survivorship curve of the non-treatment NW bees exhibited a Type-II curve (Fig. 2B), in which individuals have a relatively constant death rate throughout their life-span. The survivorship curve of the non-treatment DW bees was a Type-III curve (Fig. 2C), in which most individuals die at a very early stage. Thus, varroa parasitism altered the types of survivorship curves of the bees, causing a shorter life-span in the parasitized bees.

Fig. 2. Varroa parasitism altered the types of survivorship curves of honey bees. NTC: non-treatment control; N, needle poke; S, saline injection; E, E. coli injection. Bars (mean±S.E.M.) with different letters are significantly different (ANOVA, P<0·001; pairwise comparison with Fisher's PLSD, P[les ]0·01)
In other control treatments (needle poke and saline injection), the patterns of survivorship curves were also different among different groups of bees. In the MF bees, survivorship curves of needle-poked and non-treatment groups were significantly different (Mantel-Cox test, P=0·031) although they were similar up to 400 h post-treatment, and the curve of needle-poke group was between types I and II; but the curve of saline-injected bees belonged to type II (Fig. 2A) and was significantly different from that of needle-poked bees (Mantel-Cox test, P=0·0063). In the NW bees, the survivorship curves of the 3 control groups were similar up to 200 h post-treatment and were Type-II curves (Fig. 2B). However, in the DW bees, the survivorship curves of all groups were Type-III curves. This suggested that the DW bees have been already naturally challenged possibly by a bacterium with a similar effect to E. coli before the experimental treatments.
All the E. coli-injected bees exhibited Type-III survivorship curves (Fig. 2). However, these type-III curves were not the same. The estimated survival times at 75% of cumulative death probability as determined by Kaplan-Meier survival analysis were significantly different (Table 1). Rank tests for the difference in survival functions were highly significant (Mantel-Cox test, P<0·0001).
Table 1. Estimated survival time at 75% of cumulative death probability in adult bees differing in mite parasitization and wing deformity after either non-treatment or E. coli injection (Estimated survival times determined by Kaplan-Meier survival analysis. Rank tests for the differences in survival times were highly significant (Mantel-Cox test, P<0·0001) between treatment groups and bee types.)

Interestingly, some MF individuals recovered from the E. coli challenge and survived for a similar amount of time to the needle-poked and saline-injected bees (Fig. 2A). There was no such evidence of recovery in the NW and DW bees. This suggested that the MF bees had a stronger immune system than the mite-parasitized NW and DW bees in fighting against the E. coli challenge.
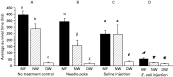
Comparison of survival time
Three comparisons can be made: the longest survival time, the estimated time to death of 75% the sample population, and the average survival time. The former reflects the survivor potential. While, the latter two reflect the average of the sample population.
For the longest survival time, under the laboratory conditions the non-treatment MF bees survived the longest, for 870 h (Fig. 2A). The non-treatment NW died sooner than the MF bees, with the longest survival time of 495 h (Fig. 2B), which was about 57% of that of the MF bees. The non-treatment DW bees died the fastest, and the longest survival time was only 67 h (Fig. 2C), which was only 7·7% of the longest survival time of the MF bees and 13·5% of the NW bees.
The estimated time to death of 75% of the sample population and average survival time of different groups of bees was related to both varroa infestation and treatments (Table 1, Figs 2 and 3). In the non-treatment controls, MF, NW, and DW bees had highly significantly different survival times with the MF and NW bees living about the same amount of time and the DW bees dying soon after emergence (Table 1 and Fig. 3A). Needle-poke treatment did not significantly change the survival time of the 3 groups of bees as compared to the respective non-treatment bees (Figs 2,3A and B). Saline injection only significantly reduced the survival time in the MF bees (Fig. 2A), but did not reduce the survival time in the NW and DW bees (Fig. 2B and C). Following E. coli injection, the bees lived for a significantly shorter time than saline-injected and non-treatment bees (Fig. 2). E. coli injected MF, NW, and DW bees only lived on average for 51·9±9·1, 24·0±2·0, and 14·8±1·3 h, respectively (Fig. 3D), and the estimated survival time of 75% of the MF, NW, and DW bees was 41·0±5·0, 27·9±2·0, 15·5±1·5 h respectively (Table 1). This decrease in survival time between the non-treatment and E. coli injection groups (Table 1) indicated that E. coli was pathogenic to the bees at the dosage of injection. After E. coli injection, the average survival time of the mite-parasitized NW and DW bees was significantly shorter than that of MF bees, indicating that these mite-parasitized bees had a significantly depressed ability to fight against the E. coli challenge as compared to the mite-free MF bees.

Fig. 3. Average survival time (mean±S.E.M.) of newly-emerged bees with different treatments. MF, mite-free bees with normal wings; NW, mite-parasitized bees with normal wings; DW, mite-parasitized bees with deformed wings. Bars with different letters are significantly different (ANOVA, P<0·001; pairwise comparison with Fisher's PLSD, P[les ]0·0389).
A comparison of average survival time of mite-parasitized bees showed that the survivorship of E. coli-injected NW bees was not significantly different from that of the DW bees without E. coli challenge.
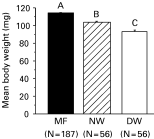
Effect of varroa mites on bee body weight and amount of soluble proteins
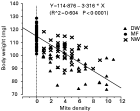
Varroa parasitization significantly reduced the body weight and amount of soluble bee proteins in newly-emerged worker bees. The MF, NW and DW bees weighed 113·6±0·4, 103·7±1·2, and 93·3±1·6 mg (Fig. 4). The varroa-free worker bees had an average of 7·8±0·1 μg of soluble proteins per μl of body extract; whereas, the mite-parasitized bees only had 6·4±0·2 μg protein per μl extract. That is, the mite-parasitized bees lost 18% of the total soluble proteins at the time of adult emergence as compared to the mite-free bees. It was likely that these differences were caused by the feeding activity of the mites since body weight was significantly negatively correlated with mite density (Fig. 5). Survivorship of NW and DW bees in the non-treatment control was not significantly correlated with body weight (P=0·703 and 0·595 respectively, linear regression), indicating a lack of relationship between body weight and survivorship in these bees.

Fig. 4. Varroa parasitization significantly reduced the body weight (mean±S.E.M.) in newly-emerged bees. Bars with different letters are significantly different (ANOVA, P<0·001; pairwise comparison with Fisher's PLSD, P<0·001).

Fig. 5. Body weight was significantly negatively correlated with the density of feeding mites. Linear regression was done on all the bees without distinguishing bee categories.
PO activity
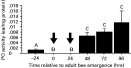
As measured in mite-free bees from late pupal stage to 4 days post-emergence from brood cells, worker bees lacked detectable PO activity early in their adult stage, even after injection with immune activators. No PO activity was detected in newly-emerged adult bees up to 24 h post-emergence (Fig. 6). Also, no PO activity could be detected in newly-emerged bees at 60 min following the injection of immuno-elicitors (bee saline alone, 0±1·2×10−7 EA/mg protein; E. coli in bee saline, 0±1·0×10−7 EA/mg protein; heat-killed yeast in bee saline, 0±0 EA/mg protein). Given the importance of PO in insect immunity, this lack of PO activity suggested that these bees were developmentally immuno-incompetent at this life stage. Thus, the stress of varroa parasitism and challenges by any bee pathogen may cause newly-emerged bees to die more easily during this immuno-incompetent period. PO activity became detectable in 48-h and older adult bees (Fig. 6).

Fig. 6. Phenol oxidase activity (mean±S.E.M.) from late pupal to early adult stages of worker bees. Time shown was related to bee emergence from the brood cell. Time 0 bees were collected when chewing out the brood cells. Arrows indicate the periods in which no phenol oxidase activity could be detected. Bees sampled were healthy and mite-free. Bars following the same letter are not significantly different (ANOVA, P<0·001; pairwise comparison with Fisher's PLSD, P<0·001).
DISCUSSION
This research demonstrated that varroa mites had a significant negative impact on bee survivorship, especially following pathogen challenge. Varroa mite parasitization shortened the survivorship of the bees as inferred from types of survivorship curves. In the non-treatment bees, the type of survivorship curve changed from long survival near-type I in MF bees, to medium survival type II in NW bees, and then to short survival type III in DW bees. This short survival type-III curve of the non-treatment DW bees may be related to high levels of DWV infection and the immunosuppression, since DW bees are highly infected with DWV and the expression of genes encoding antimicrobial peptides and immunity-related enzymes is suppressed in varroa-parasitized bees (Yang and Cox-Foster, 2005). In newly-emerged bees, the lack of PO activity required for cellular immune response may also have contributed to this reduction in life-span. The type-II survivorship curve of the non-treatment NW bees in this research agrees with the survivorship curve of the varroa-parasitized overwintering bees reported by Kovac and Crailsheim (1988).
The mite-parasitized NW and DW bees lived a significantly shorter time than the mite-free MF bees after they were challenged with live E. coli, indicating an impaired immune response of these mite-parasitized bees at whole-body level. Thus, the impairment at the transcriptional level of immune-related genes (Yang and Cox-Foster, 2005) is observed in impaired response to pathogens. In other words, varroa mites cause immunosuppression in the parasitized bees, which eventually leads to a reduced life-span. It is known that after exposure to a bacterial factor DWV in NW bees can rapidly replicate within 10 h to a similar level to that of DW bees (Yang and Cox-Foster, 2005). E. coli-challenged NW bees lived on an average of 1 day, approximately 50% as long as E. coli-challenged MF bees. Thus, this reduced survival time of the NW bees post-live E. coli injection was probably related to an induced high level of DWV infection combined with E. coli challenge. Moreover, the finding that the survivorship of E. coli-injected NW bees was not significantly different from that of the DW bees without E. coli challenge suggested that the short survivorship of the DW bees was mainly due to DWV infection. No increase in DWV titres was observed in DW and MF bees when injected with heat-killed E. coli (Yang and Cox-Foster, 2005), thus the decrease in survivorship of DW and MF bees post-injection of live E. coli as compared to the non-treatment bees in the respective group was probably due to E. coli infection alone.
Interestingly, saline injection had different effects on the survivorship of mite-free and mite-parasitized bees. Saline injection significantly reduced the survival time in MF bees; however, there was a tendency of increase in survival time both in NW and DW bees. These results suggested that the injection of bee saline might be beneficial to the mite-parasitized bees by regaining lost body fluid caused by varroa parasitism.
Given the importance of PO in insect immunity, the non-detectable PO activity in newly-emerged adult bees may cause a period of developmental immune deficiency. To our knowledge, this is an unusual finding in insects and has not been previously observed in other species. Under normal, mite-free conditions, this immune incompetency might be overcome by the feeding behaviour of adult bees. Nurse bees feed larval food to newly-emerged bees (Free, 1957). In honey bees, glucose oxidase (GOX) is expressed in the hypopharyngeal gland (Ohashi et al. 1999) and is secreted into larval food by nurse bees (Sano et al. 2004; Santos et al. 2005). GOX catalyses the oxidation of β-D-glucose by molecular oxygen to D-glucono-1,5-lactone and hydrogen peroxide, which provides a means to sterilize the food and is thought to prevent many larval diseases at colony level.
Beyond the effect upon the immune system of individual parasitized bees, mite parasitization within a colony may compound the susceptibility of newly-emerged worker bees by impacting indirectly the sterility of the food resources and colony level immunity. Mite-parasitized newly-emerged bees have reduced expression of the GOX gene (Yang and Cox-Foster, 2005). When these previously parasitized bees serve as nurse bees, they may have a reduced level of GOX secretion to brood food, allowing increased microbial contamination of the food fed to newly-emerged bees and causing a problem in overcoming the developmental immune incompetency due to lack of PO. Thus, any invading microbe may threaten the lives of newly-emerged bees in parasitized colonies and grow to a level in which contamination of varroa feeding sites can easily occur (Kanbar and Engels, 2003). Microbial contamination of the feeding site would provide the stimulus for highly increased DWV amplification (Yang and Cox-Foster, 2005) and may result in the deformed wing condition in these mite-infested bees.
Varroa mites reduced body weight of the parasitized bees. The body weight of a mite-free newly-emerged worker bee can range from 81 to 151 mg due to the difference in brood cell sizes and the strength of a colony (Winston, 1987). The average body weight of the bees tested in this research fell in this interval, and the comparison of body weight was restricted to highly uniform brood cells from a single comb. Mite parasitization caused a loss of body weight. However, the loss of body weight was not correlated with the life-span of adult bees previously parasitized at their brood stage. Mite parasitized bees do not differ in the duration and number of flights as compared to mite-free bees (Kovac and Crailsheim, 1988), indicating that there are no major changes in flight physiology or energy metabolism in varroa parasitized bees. Our results suggest that the primary cause of bee death and colony collapse may be due to the immunosuppression of the bees by the varroa mites and the continued impact on the adult bees. We are currently exploring the correlation between varroa mite parasitization, virus infections, and microbial exposure on colony health to determine the relationship.
We thank Dr Miaoqing Shen for helping to sample bees. We appreciate Dr Joachim de Miranda for providing E. coli. We want to especially thank Dr Scott Camazine for helping to initiate this research and input into experimental design. Dr Kelli Hoover and others provided insightful comments and suggestions for improvement of the manuscript. This research was supported by a grant from the Pennsylvania Department of Agriculture.