Published online by Cambridge University Press: 27 September 2004
In an earlier study it was shown that Cryptocotyle lingua cercariae, matured in Littorina littorea from a polluted marine lagoon, displayed slower horizontal swimming rates, and reduced longevity compared to cercariae released by periwinkles from a cleaner environment. This work investigated whether the pollution-induced reduction in swimming rates was due to an inefficient swimming action or the adoption of a less direct swimming path. In addition, cercariae from L. littorea that had been transferred from an ‘unpolluted’ to a ‘polluted’ site for 1 month provided information on the speed with which pollutants affect cercariae through their intermediate hosts. Results indicated that, in general, horizontal swimming rates were reduced due to slower swimming rather than disorientation and longer swimming pathways. Effects of host transplantation to a polluted site were clearly evident after 1 month. Evidence suggested that the pollutants accumulated by the cercariae via their first intermediate host affected the neuromusculature associated with swimming performance rather than sensory structures. Bearing in mind the reduced viability of C. lingua cercariae in polluted sites it is assumed that high prevalence of this digenean in gastropods (at such sites) must be due to their continual introduction by infected birds attracted to these habitats from other areas.
The potential of parasites as biological indicators of pollution has received much interest since the early 1990s. Recent reviews highlight a wide range of pollutant-induced effects on various parameters of parasitic health (Morley, Irwin & Lewis, 2003a; Williams & Mackenzie, 2003; Pietrock & Marcogliese, 2003; Sures, 2004). In particular, the free-living larvae of Digenea have been highlighted as potentially good indicators of heavy metal pollution (Lafferty, 1997) and laboratory experiments have generally shown that increasing concentrations of pollutants bring about a reduction in cercarial survival and infectivity (Evans, 1982; Cross, Irwin & Fitzpatrick, 2001; Morley, Crane & Lewis, 2001). It is surprising therefore that the viability of cercariae released from hosts found naturally in polluted sites and those released from hosts from unpolluted (clean) sites does not appear to have received much attention.
The trematode Cryptocotyle lingua (Creplin 1825) is a ubiquitous parasite of sea birds. Its rediae are common in the littoral gastropod Littorina littorea (L.) found on rocky shores around the UK. Cercariae released from L. littorea are strong swimmers and they locate and attach to inshore fish on which they form metacercarial cysts. When the fish are eaten by marine birds the metacercariae are released in the hosts' digestive tracts where they develop into adult flukes. Following liberation from L. littorea, C. lingua cercariae respond to light detected with 2 pigmented photoreceptors, by swimming towards it. They propel themselves through the water in a 3-dimensional figure of eight pattern using a tail powered by large glycogen reserves (Chapman & Wilson, 1973). Cross et al. (2001) showed that C. lingua cercariae that had matured in L. littorea from a polluted marine lagoon were of poorer quality and had reduced fitness (i.e. they displayed slower horizontal swimming rate (HSR) and reduced longevity) compared to control cercariae from a cleaner environment. The present study adds to that work. It investigates if pollution-induced reduced swimming rates are due to less efficient swimming action or disorientation that causes a less direct (and therefore longer) path-length swum. In addition, by transplanting specimens of L. littorea infected with C. lingua from an unpolluted (clean) to a polluted site, the swimming behaviour and longevity of cercariae was compared against those liberated from L. littorea maintained at ‘polluted’ and ‘clean’ habitats. The results therefore provide information on the effects of pollutants on cercariae developing inside their molluscan hosts and the mechanisms by which they reduce the viability of the cercariae following their release.
L. littorea from a known ‘polluted’ site (Evans, Irwin & Fitzpatrick, 2001; Cross et al. 2003) at Whitehouse Lagoon (Lat. 54°39′N, Long. 5°55′W) were collected in September 2001. Contemporarily, a sample was collected from Muck Island which is a ‘clean’ site (Lat. 54°50′N, Long. 5°40′W). Periwinkles were chilled at 4 °C for 24h then placed individually into separate plastic beakers containing cooled seawater and shaken on an orbital shaker (80 rpm) under illumination. After 30 min each beaker was placed under a light microscope and the seawater observed for cercariae. Periwinkles infected with C. lingua were identified by the presence of their cercariae. Rea & Irwin (1991) developed this method of encouraging C. lingua cercarial release from infected L. littorea. Infected periwinkles from each group were then isolated and maintained until approximately 60 ‘clean’ and 30 ‘polluted’ specimens were collected.
Two batches of 30 ‘clean’ and a single batch of 30 ‘polluted’ infected periwinkles were placed into separate ‘Oyster’ bags constructed from 5 mm plastic mesh. One batch (referred to as clean-to-polluted) of ‘clean’ periwinkles was transplanted to the ‘polluted’ Whitehouse Lagoon site and anchored to the shore by a length of rope and left for approximately 1 month. The other two bags, containing infected periwinkles, were left for the same length of time at the original site from which they were collected i.e. the one containing periwinkles from Whitehouse Lagoon was left there and the other, containing periwinkles from Muck Island was left at that site. These two bags contained the periwinkles that would act as controls in the subsequent experiments. Seaweed, collected on the immediate shoreline, was placed in each bag at the beginning of the experiment and replaced periodically as a food source. (A fourth bag containing periwinkles from Whitehouse Lagoon was left at Muck Island, i.e. ‘polluted-to-clean’. Unfortunately it was washed away in a storm and lost from the experiment.)
C. lingua cercariae were encouraged to shed from infected periwinkles as previously described (Rea & Irwin, 1991). Horizontal Swimming Rates (HSR) of individual cercariae towards a cool light source were measured and recorded using the methods outlined by Cross et al. (2001). Each experiment was repeated 5 times using 5 cercarial releases from 5 individual periwinkles. The 5 fastest cercariae to swim a marked 50 mm distance were recorded on videotape, for each replicate trial. The time taken to swim between the marks was recorded (mm/sec) with an accuracy of 0·04 sec. In addition, path length swum (PLS) and turning frequency (TF) were recorded for the fastest cercaria in each replicate.
Tracings of cercarial swimming events were taken from a television monitor and analysed to provide information on actual distance travelled between the 2 marks which were 50 mm apart. Needles were stuck at each point of directional change on a photocopy of the tracing of each individual cercarial swimming event. A length of cotton was then positioned to follow the line created by the pins. The length of cotton between one end of the pathway and the other was measured as it represented the actual distance (PLS) travelled by the cercaria. HSRs were calculated using the measured path lengths and recorded swimming event times and provided the speed at which each cercaria was actually swimming.
As the above experiment demonstrated that C. lingua cercariae did not swim directly towards the light source, the number of turns per swimming event (TF) by each cercaria was counted. For practical reasons only turns greater than 90° away from the source of light were considered as turns.
Measurements were performed on ‘clean’ and ‘polluted’ cercariae at the start and end of the field experiment as well as on ‘clean-to-polluted’ cercariae at the end. All behavioural experiments were carried out in artificial seawater prepared by adding Instant Ocean (Aquarium Systems) to distilled water to achieve a salinity of 30·2 g NaCl/l which is comparable to natural seawater at Muck Island.
The longevity of cercariae was calculated from 10 replicates of 10 cercariae from 3 different periwinkles. Each group of 10 cercariae was isolated in individual wells and kept in the dark at 15 °C and observed periodically until death. For a more comprehensive description see Cross et al. (2001).
The HSR, PLS, TF and longevity of cercariae released by periwinkles from the ‘clean-to-polluted’ site were compared to those of cercariae from the ‘clean’ site at the start of the experiment.
Nested ANOVAs compared HSR, PLS and TF between ‘clean’ and ‘polluted’ cercariae. Replicates were nested within periwinkle and periwinkle nested with sampling time and between the start and end of the experiment.
Longevity data were compared by nested proportional hazards, survival models with periwinkle (nested within sampling time) and time effects.
The experiment involved cercariae that emerged from three distinct groups of L. littorea. The terms ‘clean’ and ‘polluted’ are applied to cercariae from periwinkles that were not transplanted from their original habitats. The term ‘clean-to-polluted’ is applied to cercariae from periwinkles that were transplanted from Muck Island (clean) to Whitehouse Lagoon (polluted).
‘Clean’ cercariae swam faster than those released by periwinkles from the ‘polluted’ habitat (Fig. 1). The HSR of all the cercariae significantly changed over the period of study, although only ‘polluted’ cercariae had replicate differences between individual periwinkles (Table 1). ‘Clean-to-polluted’ cercariae were the most significantly affected followed by ‘clean’ and then ‘polluted’ cercariae (compare F ratios (start/end), Table 1).

Fig. 1. Mean HSR (mm s−1) of ‘clean’ and ‘polluted’ Cryptocotyle lingua cercariae in artificial seawater. Black line shows the HSR of ‘clean’ transplanted cercariae at the beginning of the experiment and after transplantation of their host to a ‘polluted’ site (clean-to-polluted). Error bars show standard error.
Table 1. A comparison of swimming speed (HSR) of Cryptocotyle lingua cercariae from ‘clean’, ‘polluted’ and ‘clean-to-polluted’ periwinkles (‘Clean-to-polluted’ refers to periwinkles that were transplanted from a ‘clean’ habitat to a ‘polluted’ habitat for approximately 1 month. Results are nested ANOVAs, replicates nested by periwinkle and periwinkle nested by time (start/end of experiment).)

There was no significant difference in the distances swum between ‘clean’ or ‘clean-to-polluted’ cercariae between the start and end of the experiment at (P=0·098 and 0·1368 respectively) (Fig. 2). However, ‘polluted’ cercariae swam significantly further at the start of the experiment (F=27·06, P=<0·0001) (Fig. 2). ‘Polluted’ cercariae also exhibited significant periwinkle effects i.e. cercariae shed from different periwinkles swam different distances between the two marks 50 mm apart in the swimming chamber (F=70·393, P=<0·0001). The periwinkle effect was more marked than the difference observed between the distances swum between the start and end of the experiment (compare F ratios).

Fig. 2. Mean path lengths (mm) swum by ‘clean’ Muck Island and ‘polluted’ Whitehouse Lagoon Cryptocotyle lingua cercariae in artificial seawater. Black line shows the path length swum by ‘clean’ transplanted cercariae at the beginning of the experiment and after transplantation of their host to a ‘polluted’ site (clean-to-polluted). Error bars show standard error.
Although all the cercariae turned more frequently at the end of the experiment (P>0·001), only ‘polluted’ cercariae turned significantly more (F=8·0, P=0·0104) (Fig. 3).

Fig. 3. Mean number of turns made by ‘clean’ and ‘polluted’ Cryptocotyle lingua cercariae in artificial seawater. Black line shows the number of turns made by ‘clean’ transplanted cercariae at the beginning of the experiment and after transplantation of their hosts to a ‘polluted’ site (clean-to-polluted). Error bars show standard error.
The longevity of ‘clean’ cercariae was significantly shorter at the end of the experiment (Table 2). However, the longevity of ‘polluted’ cercariae was not significantly affected (Table 2). ‘Clean-to-polluted’ cercariae had greatly reduced longevity after their host periwinkles were moved from a ‘clean’ site to a ‘polluted’ habitat. The reduction in ‘clean’ cercarial longevity was not as great as the effect caused by the transplantation of infected periwinkles (compare LRχ2 ratio, Table 2; Fig. 4). Systematic differences between the longevity of cercariae shed by different periwinkles collected from the same site were observed. With the exception of cercariae from ‘polluted’ periwinkles, the periwinkle effect was not as significant as the temporal effect (compare P values, Table 2).

Fig. 4. (A) Mean longevity (h) of ‘clean’ and ‘polluted’ Cryptocotyle lingua cercariae in artificial seawater. Black line shows the longevity of ‘clean’ transplanted cercariae at the beginning of the experiment and after transplantation of their hosts to a ‘polluted’ site (clean-to-polluted). Means were calculated from longevity of cercariae from 3 periwinkles (100 cercariae per periwinkle). Error bars show standard error. (B) Kaplan-Meier estimated survival plots for ‘clean’, ‘polluted’ and ‘clean-to-polluted’ Cryptocotyle lingua cercariae: ‘Polluted’ cercariae at the start ([utrif ]) and end ([dtri ]) of the experiment; ‘clean’ cercariae at the start (□) and end ([squf ]) end of the experiment and ‘clean-to-polluted’ () cercariae at the end of the experiment.
Table 2. A comparison of Cryptocotyle lingua cercarial longevities from ‘clean’, ‘polluted’ and ‘clean-to-polluted’ periwinkles (‘Clean-to-polluted’ refers to periwinkles that were transplanted from a ‘clean’ habitat to a ‘polluted’ habitat for approximately 1 month. Results are nested proportional hazards survival analysis. Likelihood-ratio χ2 and probabilities are shown; periwinkle is nested by time (start/end of experiment).)

The results presented here confirm the conclusion of Cross et al. (2001) that C. lingua cercariae liberated from periwinkles found naturally in a polluted habitat are less well equipped to achieve transmission to their next host than those from an unpolluted environment.
Such cercariae swim more slowly, swim less directly, turn more frequently and live for shorter periods. In addition, specimens from different ‘polluted’ hosts display greater variation in these fitness criteria than those from unpolluted waters. Fluctuations in quantifiable characteristics of cercarial fitness over time and over geographical areas have not been reported previously and may have implications for future behavioural studies.
As all experiments carried out in this study involved cercariae swimming in the same artificial sea water, the differences in activity observed must have been due to factors that influenced the development of the cercariae while inside their L. littorea hosts. Whether or not the effects observed were due to the elevated levels of a number of heavy metals known to occur in periwinkles from Whitehouse Lagoon (Evans et al. 2001; Cross et al. 2003) is open to conjecture. However, other studies have reported changes in swimming behaviour of free-living cercariae in response to toxic substances during their intramolluscan development. Juvenile Fasciola hepatica accumulated radio-isotope labelled selenium via their rediae in vivo after its assimilation by their molluscan hosts (Lymnaea columella) (Hsu, 1986). Morley, Crane & Lewis (2003b) observed behavioural changes in Echinoparyphium recurvatum cercariae after development in rediae inside a metal-contaminated host (Lymnaea peregra). A change in encystment rates of the E. recurvatum cercariae was attributed to the absorption of cadmium (Cd) during development in the metal-contaminated host tissue. These studies clearly show that developing cercariae can acquire and display the effects of metal pollutants accumulated by their hosts.
The fact that cercariae did not swim as fast after their gastropod hosts were transplanted from a ‘clean’ habitat to a ‘polluted’ habitat is very important. The accumulation of pollutants by the molluscan host appears to have affected cercarial development in a way that impairs their swimming performance. As there was no significant difference in the turning frequency or path length swum by ‘clean’ and ‘clean-to-polluted’ cercariae the reduction in HSR appears to have been a result of slower swimming speed. This could have been due to the suppression of muscular activity. C. lingua propel themselves through the water in a 3-dimensional figure of eight pattern by means of a dilation adjacent to the body and a fin along the edge of their tail (Chapman & Wilson, 1973). Longitudinal muscles with large glycogen reserves and numerous mitochondria provide the high-energy requirements to power the tail (Rea & Irwin, 1992). There are many potential toxins in Whitehouse Lagoon that could affect this system. For example manganese, a known pollutant at Whitehouse Lagoon (personal observation), when accumulated by Norway lobsters (Nephrops norvegicus) affected the neuromusculature of their tail resulting in reduced swimming velocity (Baden & Neil, 1998). Direct exposure of Cd and zinc (Zn) to free-living Diplostomum spathaceum cercariae causes a rapid reduction in their swimming activity (Morley, Crane & Lewis, 2003c). A similar reduction in the efficiency of the C. lingua cercarial tail could have caused the observed effects, although this is purely speculative.
The fact that there was no significant difference between the distances swum or the frequency of turning in cercariae derived from periwinkles transferred from clean to polluted sites is of interest. Rees (1975) was of the opinion that C. lingua cercariae swam a straight course towards a light source by alternately exposing their paired photoreceptors to the light. Morley et al. (2003b) showed that the orientation behaviour (geo- and photo-taxis) of E. recurvatum cercariae was significantly affected by direct exposure to Zn and Cd and they suggested that this might be due to the binding of metals to selected sensory receptors on the outer tegument. A similar, though not significant, effect was observed in this study but it was subsidiary to the reduction in swimming speed observed in the same ‘clean-to-polluted’ cercariae. It would appear that pollutants acquired by cercariae while developing in a molluscan host affect the efficiency of the functional anatomy related to swimming performance rather than the sensory structures involved in maintaining swimming direction.
Although factors measured (HSR, PLS and TF) in each of the experiments produced remarkably consistent results by cercariae from different periwinkle individuals, ‘polluted’ cercariae always displayed significant ‘periwinkle effects’. L. littorea accumulates many of its pollutants through their diet of algae. It would appear that in the polluted conditions of Whitehouse Lagoon some L. littorea accumulated more contaminants than others. Cross et al. (2003) showed that the concentrations of iron, copper and Zn in L. littorea infected by C. lingua from Whitehouse Lagoon varied between months and also between sexes. As all the experiments in the present study were carried out at the same time, the ‘periwinkle effects’ observed may have been associated with the sexes of the L. littorea.
Differences in the longevity of cercariae released by different periwinkles were evident for all the groups. Siddall & des Clers (1994) found an inherent natural variability in the survival and susceptibility of Zoogonoides viviparous to sewage sludge. Variations in assimilation rates of food by periwinkles would expose developing cercariae to differing amount of nutrients and pollutants. In the present study the ‘clean-to-polluted’ cercariae displayed the greatest reduction in longevity. Perhaps the sudden change to a polluted environment caused the periwinkles concerned to exhibit a stress response that affected cercarial development within their rediae. Krist and colleagues showed that the starvation of Microphallus sp. infected gastropods (Potamopyrgus antipodarum) affected cercarial development (within the intermediate gastropod host) and that successful transmission was more likely to occur from individual cercariae that had developed in healthier/fed hosts (Krist et al. 2004). Gastropods have been shown to withdraw into their shells due to exposure to a toxic contaminant and under those conditions they are unable to feed (Hughes, Chapman & Kitching, 1987). This reduces the nutritional reserves available to the rediae in which the cercariae are maturing. As cercariae do not feed during their brief free-living existence any reduction in their finite energy resources may affect their longevity.
As C. lingua cercariae penetrate the skin of marine fish and encyst subcutaneously their swimming behaviour has been selected for effective dispersion and host attachment rather than for host penetration and internal migration within their intermediate host. Consequently a reduction in swimming rates and longevity will have grave consequences for cercarial transmission success. The present study and that of Cross et al. (2001) have shown that C. lingua cercariae are particularly vulnerable to pollutants in the water in which they swim and to pollutants acquired through their molluscan hosts. It is surprising therefore that they should remain prevalent in littorines in polluted habitats. The reason for their continued presence is probably because gulls, their final hosts, appear to be attracted to polluted habitats (see Morley et al. 2003a) and they continually infect the periwinkles that survive in such places.
We are grateful to D. H. Saville for his technical assistance. This work contributes to the INTAS funded project 011.0210.
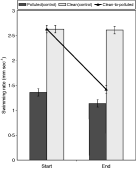
Fig. 1. Mean HSR (mm s−1) of ‘clean’ and ‘polluted’ Cryptocotyle lingua cercariae in artificial seawater. Black line shows the HSR of ‘clean’ transplanted cercariae at the beginning of the experiment and after transplantation of their host to a ‘polluted’ site (clean-to-polluted). Error bars show standard error.

Table 1. A comparison of swimming speed (HSR) of Cryptocotyle lingua cercariae from ‘clean’, ‘polluted’ and ‘clean-to-polluted’ periwinkles
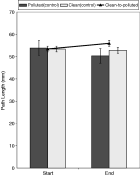
Fig. 2. Mean path lengths (mm) swum by ‘clean’ Muck Island and ‘polluted’ Whitehouse Lagoon Cryptocotyle lingua cercariae in artificial seawater. Black line shows the path length swum by ‘clean’ transplanted cercariae at the beginning of the experiment and after transplantation of their host to a ‘polluted’ site (clean-to-polluted). Error bars show standard error.
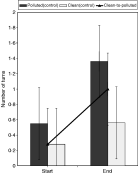
Fig. 3. Mean number of turns made by ‘clean’ and ‘polluted’ Cryptocotyle lingua cercariae in artificial seawater. Black line shows the number of turns made by ‘clean’ transplanted cercariae at the beginning of the experiment and after transplantation of their hosts to a ‘polluted’ site (clean-to-polluted). Error bars show standard error.
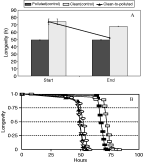
Fig. 4. (A) Mean longevity (h) of ‘clean’ and ‘polluted’ Cryptocotyle lingua cercariae in artificial seawater. Black line shows the longevity of ‘clean’ transplanted cercariae at the beginning of the experiment and after transplantation of their hosts to a ‘polluted’ site (clean-to-polluted). Means were calculated from longevity of cercariae from 3 periwinkles (100 cercariae per periwinkle). Error bars show standard error. (B) Kaplan-Meier estimated survival plots for ‘clean’, ‘polluted’ and ‘clean-to-polluted’ Cryptocotyle lingua cercariae: ‘Polluted’ cercariae at the start ([utrif ]) and end ([dtri ]) of the experiment; ‘clean’ cercariae at the start (□) and end ([squf ]) end of the experiment and ‘clean-to-polluted’ () cercariae at the end of the experiment.

Table 2. A comparison of Cryptocotyle lingua cercarial longevities from ‘clean’, ‘polluted’ and ‘clean-to-polluted’ periwinkles