Published online by Cambridge University Press: 01 March 2004
Atlantic salmon (Salmo salar) parr (age 0+), infected by the ectoparasite Gyrodactylus salaris, were exposed to aqueous aluminium (Al), copper (Cu), zinc (Zn), iron (Fe) and manganese (Mn), at 4 different concentrations. There was a negative correlation between G. salaris infections and metal concentrations in both Zn- and Al-exposed salmon. In the Zn-experiment, all 4 concentrations tested caused a decrease in the G. salaris infections, while in the Al-experiment the G. salaris infection did not decline at the lowest concentration. The number of G. salaris increased continuously during the experiments in all control groups, and in all groups exposed to Cu, Fe and Mn. At the highest concentration, however, copper seemed to impair the growth of G. salaris infection. The results show that aqueous Al and Zn are environmental factors of importance controlling the distribution and abundance of the pathogen G. salaris. Other pollutants might also have an influence on the occurrence of G. salaris. Finally, the results demonstrate that aqueous Al and Zn have a stronger effect on the parasite than on the salmonid host, suggesting that both metals may be used as a pesticide to control ectoparasites such as G. salaris.
Gyrodactylosis, caused by the monogenean ectoparasite Gyrodactylus salaris, has devastated natural Atlantic salmon (Salmo salar L.) populations in several of the some 40 rivers infected by the parasite in Norway (Heggberget & Johnsen, 1982; Johnsen & Jensen, 1991; Mo, 1994; Johnsen, Møkkelgjerd & Jensen, 1999). G. salaris is supposed to be ecdemic to Norway, most probably introduced by man on imported Baltic salmon in the early seventies (Bakke, Jansen & Hansen, 1990). Several methods have been applied in order to find a proper method to control and eliminate G. salaris from Norwegian rivers. The main strategy involves chemical treatment of the infected part of the rivers with the pesticide rotenone (Johnsen & Jensen, 1991), which effectively kills gill-breathing animals including fish (Almquist, 1959; Lindahl & Öberg, 1961; Morrison, 1977). However, two main problems with this method have been its negative influences on the gene pool of the resident fish species and its non-specific effect on the aquatic biota. In addition, rotenone treatment has also failed in several of the infected larger and more complicated river systems, in some even after 2 treatments. Therefore, there is a general and increasing concern that this method has to be replaced by more parasite specific methods reducing the negative environmental consequences of eliminating G. salaris.
Aquatic ectoparasites, in general, are affected by abiotic factors in the macroenvironment (Chubb, 1977), and several authors have mentioned the importance of water physico-chemical conditions for gyrodactylid monogeneans. Malmberg (1957, 1970) suggested that factors like humus content, degree of eutrophication, temperature and salinity could affect the occurrence of Gyrodactylus species. Koskivaara, Valtonen & Prost (1991) discussed the effects of pollution and oxygen content, Scott & Nokes (1984) demonstrated the importance of temperature, while Khan & Kiceniuk (1988) examined effects of petroleum hydrocarbon pollution. Regarding G. salaris, environmental factors such as water temperature (Jansen & Bakke, 1991, 1993a,b; Appleby & Mo, 1997), salinity (Soleng & Bakke, 1997; Soleng, Bakke & Hansen, 1998), as well as aqueous Al and water pH (Soleng et al. 1999) have been shown to influence population dynamics, range extension and dispersal.
In 1999, Soleng et al. reported that aqueous Al was able to eliminate G. salaris from infected salmon in the laboratory, without killing the fish during the exposures (Soleng et al. 1999). These results suggest that abiotic environmental factors such as water quality in general and dissolved metals in particular, might have important influences upon parasite reproduction and survival and, hence, could function as a specific parasiticide for fish ectoparasites. In this respect it is worth noticing that the Norwegian areas, in which G. salaris has been suggested to be responsible for the reduction in Atlantic salmon populations, are outside the areas influenced by freshwater acidification (Soleng et al. 1999). Usually, freshwater acidification is associated with elevated concentrations of aqueous Al (Gensemer & Playle, 1999). In addition to acidification, river systems affected by mining activities seem not to be colonized by G. salaris.
The main objective of the present study was to confirm the previous results obtained by Soleng et al. (1999) under slightly different conditions, and to extend the study by incorporating other metals with the potential to have major influences on the occurrence and survival of G. salaris in Norway, and on fish ectoparasites in general. The metals selected were: aqueous aluminium (Al), copper (Cu), zinc (Zn), iron (Fe) and manganese (Mn), all associated with mining and freshwater acidification in Norway.
Atlantic salmon (Salmo salar) parr (age 0+, mean length 6·1±0·9 cm, range 4·0 to 9·0 cm) of the River Lærdalselva stock, were obtained from the local hatchery in Lærdal, Western Norway. The fish were transported by car from the hatchery to the National Veterinary Institute (NVI), Oslo, in a large oxygenated tank. At NVI, the fish were equally distributed in two 1000 l holding tanks, and acclimatized at 8 °C for a minimum of 2 weeks prior to the experiments. The holding tanks received activated-charcoal filtered and dechlorinated Oslo City tap water (Table 1), at a flow rate of approximately 2·0 l/min. The naïve fish had previously not been exposed to gyrodactylids, but were routinely disinfected with formalin against ectoparasites and fungus from the hatchery. The G. salaris used in the experiments originated from naturally infected salmon parr caught in the River Lærdalselva. These fish were kept in a separate 200 l holding tank before they were used to infect the naïve fish.

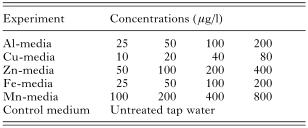
The present study was performed as 5 separate experiments. In each experiment, salmon were exposed to 4 different concentrations of a metal; aluminium (Al), copper (Cu), zinc (Zn), iron (Fe) and manganese (Mn). The test media (Table 2) were prepared by addition of stock solutions of metal sulphates to the tap water. The concentrations used are comparable with what is found in acidified waters or water draining from mining areas in Norway. For each experiment, 4 stock solutions of various concentration of the given metal were prepared, and added to the tap water by means of peristaltic pumps. Untreated tap water always acted as control medium.
Six flow-through systems were used in the experiments, 4 for the metal exposures and 2 for the controls (Fig. 1). Each metal exposure system consisted of a mixing tank and an exposure tank of equal size (50×50×80 cm), where the fish were kept during the experiments. No mixing tanks were used for the two control systems (Fig. 1). The metal solutions were added to the tap water entering the mixing tanks. Water flow rate through the systems was 2·0 l/min. The water flow rate was kept constant in the exposure systems by use of a 500 l reservoir tank placed above the other tanks (Fig. 1). The fish were sheltered from disturbances by non-transparent lids covering the tanks. All experiments were run at a temperature between 6 and 10 °C.

Fig. 1. Schematic presentation of the experimental setup.
Ninety naïve salmon were infected with G. salaris by exposure for 30 min in a small tank (5 l of water) to fins from the naturally infected salmon. Thereafter they were transferred to a larger tank (30 l of water) to increase the intensity of the infrapopulations to a minimum of 50 parasites. Ten fish were then transferred to each of the 6 exposure tanks, and the experiment started. Each experiment lasted between 11 and 15 days. If all parasites were eliminated from the fish, the exposure was terminated by stopping the chemical addition. During acclimation, the fish were fed un-medicated pellet food, but kept unfed during the experiments.
During the experiments, water temperature, conductivity and pH were measured daily in each exposure tank, and the chemical dosage and water flow through the tanks were controlled. During each experiment, 3 water samples were taken from each exposure tank for later analyses of aqueous metals and major chemical compounds. The intervals between fish examinations depended on the rate of infection changes, but occurred on a daily basis at start of the experiments.
Water pH, temperature and conductivity were measured directly in the laboratory. pH was measured by a Radiometer PHM 210 pH-meter, while water temperature and conductivity were measured by an YSI Model X conductivity meter. In addition, water samples were taken for later analyses of: alkalinity (potentiometric titration with hydrochloric acid to pH 4·5), Ca2+, Mg2+, Na+ (induced coupled plasma atomic-emission spectrometry (ICP)), K+ (atomic absorption flame spectrophotometry (AAS)), total Al3+, Cu2+, Fe2+/3+, Mn2+ and Zn2+ (ICP-Mass Spectrometry (ICP-MS)), SO42− and Cl− (ion chromatography (IC)), NO3− and total N (spectrophotometry) and total organic C (TOC) (Wet Chemical Oxidation IR-detection).
The growth and survival of infrapopulations of G. salaris were measured by counting the G. salaris specimens on the skin and fins of each fish under a stereomicroscope on anaesthetized fish (0·05% chlorobutanol) placed in a Petri dish (see Bakke, Jansen & Hansen, 1991; Soleng et al. 1999) with the same water as in the exposure tanks. Observations from each day were analysed separately by one-way ANOVA. The type-I error was adjusted according to the Bonferroni procedure to take into account the series of analyses of variance done within each experiment (Underwood, 1997). In case of significance in the overall test, Tukey-Kramer HSD was used for pair-wise comparisons.
The physico-chemical properties of the tap water were stable throughout the experimental period (Table 1). There were some differences in pH between the various test media, however, because the different metal salts affect pH to varying degrees. In the Al-exposures, the pH was 6·1, while in the other metal exposures, the pH varied from 6·5 to 6·8 (Table 3). Apart from the Al-exposures, the pH in the other metal exposures and the control medium was approximately the same throughout the experiments (Table 3). The metal analyses revealed that the amounts of metals added in each experiment corresponded fairly well to the nominal concentrations (Tables 2 and 3). Thus, in all experiments, we obtained the decided concentration gradients (for exact values, see Table 3).

The number of G. salaris increased continuously in both control groups, to approximately twice that of the initial infection, without any significant difference between them (Tukey-Kramer HSD). In the Al-experiment, however, the G. salaris infection was eliminated after 3 days in the Al 200 exposure and after 8 days in the Al 100 exposure (Fig. 2). In the Al 50 exposure, the G. salaris infection declined to 12% of the initial infection after 14 days. After a slight decrease in the G. salaris infection the first 2 days in the Al 25 exposure, the infections returned to approximately the same rate of increase as in the controls although a decline could be observed at the end of the experiment (Fig. 2). Statistically, there were no differences between the Al 25 group and the 2 controls (Tukey-Kramer HSD). In the 2 exposures with parasite elimination (Al 100 and 200), the exposure media were exchanged with untreated control water after the elimination. At the end of the experiment (day 14), one G. salaris specimen was found in the Al 100 group and 2 specimens on 1 fish in the Al 200 group.

Fig. 2. Number of Gyrodactylus salaris on Atlantic salmon parr exposed to 4 different concentrations of aqueous Al (mean±S.E., n=10).
The number of G. salaris increased continuously during the experiment in both control groups, without any significant difference between them (Tukey-Kramer HSD; 1 control group was terminated after 9 days due to technical difficulties (Fig. 3). Also in the Cu-exposures (Cu 10, Cu 20, Cu 40, and Cu 80), G. salaris infections increased continuously throughout the experiment (Fig. 3). No significant difference was observed between the Cu-exposures and the controls (Tukey-Kramer HSD).

Fig. 3. Number of Gyrodactylus salaris on Atlantic salmon parr exposed to 4 different concentrations of aqueous Cu (mean±S.E., n=10).
The number of G. salaris increased continuously during the experiment in both control groups, without any significant difference between them (Tukey-Kramer HSD). However, G. salaris was eliminated after 3 days in the Zn 400 exposure. In the Zn 200 exposure, the G. salaris infection declined rapidly during the first 4 days to persist thereafter at very low levels during the rest of the experiment (Fig. 4). After an initial increase in number of G. salaris in the Zn 100 exposure, the infection declined continuously during the rest of the experiment, but persisted at low levels (Fig. 4). In the Zn 50 exposure, after an initial decrease, the infection of G. salaris persisted at an approximately constant level through the experimental period of 15 days. However, at the end of the experiments, the infection levels of G. salaris in all 4 Zn-exposures were significantly reduced compared with the controls (F5,50=218·9, P<0·0001).

Fig. 4. Number of Gyrodactylus salaris on Atlantic salmon parr exposed to 4 different concentrations of aqueous Zn (mean±S.E., n=10).
The infection of G. salaris in the 2 controls increased continuously during the experimental period, and all Fe-exposure groups showed a corresponding increase in the infections throughout the experiment (Fig. 5), and the trend in parasite increase in all the Fe-exposures was similar. However, in the Fe 100 group, a slight decline in the number of G. salaris at the end of the experiment was observed. There were no significant differences between any of the groups throughout the experiment (Tukey-Kramer HSD).

Fig. 5. Number of Gyrodactylus salaris on Atlantic salmon parr exposed to 4 different concentrations of aqueous Fe (mean±S.E., n=10).
The infection of G. salaris in the 2 controls increased continuously during the experimental period, as did the G. salaris infection in all the Mn-exposures (Fig. 6). However, in the Mn 200 exposure group there was a restricted increase in the level of infection. No significant difference was observed between the Mn-exposures and the controls (Tukey-Kramer HSD).

Fig. 6. Number of Gyrodactylus salaris on Atlantic salmon parr exposed to 4 different concentrations of aqueous Mn (mean±S.E., n=10).
Limited fish mortality was observed in all exposures and control groups, except in the Cu-experiment. Highest mortality was observed in the Al-experiment (3 fish died in Al 25, 4 in Al 50, 3 in Al 100, and 1 in Al 200). However, mortality was also observed in the control groups in the Al-experiment (5 and 6). In the Zn-experiment 1 fish died in the Zn 50 and Zn 100 group, and 2 fish in the Zn 400 group. During the Fe-experiment, 1 fish died in each of the Fe 25, 50 and 200 groups, and in 1 of the controls, while 3 fish died in the Fe 100 group. In the Mn-experiment, 2 fish died in 1 of the controls.
The present results on the influence of different metal solutions on previously untested G. salaris and Atlantic salmon strains kept under physical conditions different from previous experiments (Soleng et al. 1999), confirm the observations made by Soleng et al. (op. cit.) that aqueous Al has a marked negative effect on G. salaris, and may eliminate or reduce infections. The effect of the metal solution was found to be dependent on the concentrations used. However, the present results also reveal that aqueous Zn has a similar effect on G. salaris infection as Al, and that the other metals tested, Cu, Mn and Fe, had no significant effect on G. salaris infections at the concentrations tested. However, aquatic pollutions by other heavy metals may still be of significance in controlling G. salaris occurrence and population dynamics. Besides the influence of aqueous Al and Zn, previous studies have shown that environmental factors such as water temperature, pH and salinity also influence the reproductive rate and survival, and hence population dynamics, dispersal and the distribution range of gyrodactylids (see review by Bakke, Harris & Cable, 2002).
The present results on Al-exposed G. salaris correspond with the results previously obtained by Soleng et al. (1999). They reported a complete elimination of the parasite when the nominal additions of Al to the test water were 100 and 200 μg/l, while 50 μg/l reduced the number of parasites without eliminating them. Field studies from Nova Scotia, Canada, show comparable results as parasite species richness was significantly higher in yellow eels (Anguilla rostrata) from rivers with pH above 5·4 compared with more acidic rivers (Cone, Marcogliese & Watt, 1993; Marcogliese & Cone, 1996, 1997). Halmetoja, Valtonen & Koskenniemi (2000) also reported that the mean number of metazoan parasite specimens on perch (Perca fluviatilis) was markedly lower at pH 5·3–5·9 compared to pH 6·4. Even though the authors did not refer to Al, the effects of acidification are most probably due to aqueous Al alone or in combination with low pH in the most acidic lakes. Elevated concentrations of aqueous Al are typical for acidified freshwater systems (Gensemer & Playle, 1999). Soleng et al. (1999) reported that pH as low as 5·0 was needed to eliminate G. salaris from salmon without the presence of aqueous Al because, at pH 5·2, no effect of acidity alone on G. salaris was observed.
Some fish mortality was observed in the Al-experiment. It is well documented that aqueous Al at pH below 6·0 has negative effects on Atlantic salmon (see review by Gensemer & Playle, 1999). In the present study, however, the mortality of fish was highest in the two controls as also observed by Soleng et al. (1999), indicating that the mortality observed was independent of Al.
In the Zn-experiment, all 4 Zn-concentrations tested resulted in a clear impairment of the G. salaris population during the experiment as the number of parasites decreased compared to an approximately 4-fold increase in the controls. This is the first record of the influence of aqueous Zn on a monogenean. However, previously it has been reported that aqueous Zn has a toxic effect on free-living stages of digeneans (Mecham & Holliman, 1975; Asch & Dresden, 1977; Evans, 1982a,b; Morley, Crane & Lewis, 2001). It should be noted that Morley et al. (2001), also reported that low concentrations of aqueous Zn could increase survival time of the free-living stages of digeneans when water temperature was relatively low. Munkittrick & Dixon (1988) found significantly lower prevalence and intensity of infection of acanthocephalans in the guts of white suckers (Catostomus commersoni) in lakes polluted with Zn and Cu, compared to control lakes. However, the macroinvertebrate fauna of the polluted lakes was also reduced, indicating that reductions in the intermediate host community may also have contributed to the effect of metals on the indirectly transmitted acanthocephalans (Munkittrick & Dixon, 1988).
It is reasonable to believe that animals infected with parasites could be more sensitive to pollution than non-infected animals. Boyce & Yamada (1977) reported that sockeye salmon (Oncorhynchus nerka) smolts infected with intestinal cestodes were significantly more susceptible to aqueous Zn than non-infected smolts. McCahon, Brown & Pascoe (1988), however, demonstrated that freshwater amphipods (Gammarus pulex) infected with acanthocephalans were no more sensitive to lethal concentrations of cadmium (Cd) than uninfected individuals. In addition, Guth, Blankespoor & Cairns (1977) found that freshwater snails (Lymnea stagnalis) infected with trematodes were more sensitive to aqueous Zn than uninfected snails. The present results show that G. salaris is much more sensitive to aqueous Zn than the host, which may be a feature characteristic for ectoparasites directly exposed to the polluted medium, as opposed to endoparasites. Therefore, a cost-benefit situation occurs for salmon in which the negative effect of Zn-exposure is less than the positive effect of the observed reduction in the level of ectoparasitic infections. We can only speculate if this was the case in our Al-experiment where controls showed a higher mortality than Al-exposed fish.
The present study demonstrated no significant effect of aqueous Cu on the G. salaris infections of Atlantic salmon despite the significant effect of Zn. This is in contrast to previous results on parasites as Evans (1982a,b) reported that aqueous Cu (as well as Zn) has a toxic effect on the free-living stages of digeneans. Both the bio-availability and toxicity of Cu are dependent on pH, which may explain the difference between the present results and the results reported by Evans (1982a,b). These authors did not give any data on water pH, while G. salaris and the host were exposed to aqueous Cu at pH 6·6 in the present experiment. At this pH, Cu-toxicity is highly correlated with the ionic Cu2+-concentration (Meador, 1991). Cu is considered to be acutely toxic to sensitive teleost species in soft water at concentrations between 10 and 20 μg/l. In the present study, however, no mortality of salmon was observed in any of the Cu-exposures. Thus, we cannot exclude the possibility that the amount of Cu2+ in our test media was too low to have any effect on the salmon, as well as the G. salaris populations. However, differences occurred between the four Cu-exposures. At the end of the experiments there was a negative correlation between the growth of infection and the level of Cu-exposure which could indicate an influence of aqueous Cu already at the concentrations used. On the other hand, no decline in the G. salaris infections was observed.
The present results demonstrate that aqueous Fe and Mn have no significant effect on the G. salaris populations on salmon, at least at the pH and metal concentrations tested. In addition to Al, elevated concentrations of Fe and Mn are associated with freshwater acidification. Our results, however, lead us to the conclusion that aqueous Al is the principal toxicant influencing the G. salaris populations on salmon in acidified waters.
The present results demonstrated that among the metals tested on G. salaris infestations of salmon, only Al and Zn showed a significant negative influence on parasite survival and hence have a potential as parasiticides to fish ectoparasites. Cu, Fe and Mn did not cause any decline in the G. salaris infections in our experiments. In the Cu-experiment, however, the highest levels of the metal seemed to impair the G. salaris populations although without any decline of the infections. Most chemotherapeutic agents used against fish parasites are organic compounds (see review by Schmahl, Taraschewski & Mehlhorn, 1989). Among inorganic compounds to our knowledge only Cu (CuSO4) has been used to any extent.
Generally, aquatic invertebrates are not as sensitive to aqueous Al and Zn as fish (Gensemer & Playle, 1999; Lydersen, Löfgren & Arnesen, 2002). The present results demonstrate that aqueous Al and Zn, however, have a stronger effect on the parasite than on the salmonid host despite the fact that the Atlantic salmon is regarded as one of the most sensitive freshwater fish species to pollutants in general, and to Al in particular (Grande, Muniz & Andersen, 1978; Poléo et al. 1997; Gensemer & Playle, 1999). In another comparative study, aqueous Al was also shown to have a similar effect on other fish ectoparasites (unpublished data), without killing any fish. Hence, the present results demonstrate that both Al and Zn, within the concentration levels used, are parasite-specific and also have the potential to be used as pesticides to control G. salaris infections. The Al-concentration used in the present study is within the range found in Scandinavian lakes (Lydersen et al. 2002). In fact, many limed salmon rivers in Norway have higher concentrations of total Al than 200 μg/l, which was used as the highest concentration in the present study. The natural concentrations of total Zn in Scandinavian freshwaters, however, are normally lower than 5 μg/l (Lydersen et al. 2002). Acute toxicity is reported for aquatic invertebrates from Zn >30 μg/l, and arthropods are found to be the most zinc-sensitive among the many tested invertebrates (Eisler, 1993). However, freshwater insects, such as many species of mayflies, damselflies, stoneflies and caddisflies, are relatively tolerant to aqueous Zn, with LC50 values usually >1·33 mg Zn/l (Lydersen et al. 2002). Fish can accumulate Zn from their surroundings, but the amount is strongly dependent on dose, duration of exposure, water chemistry, and several biological factors such as species, life-stage, and general condition (Eisler, 1993). Contrary to Zn, there is no evidence that Al biomagnifies in aquatic systems and toxicity is mainly external due to the interaction between aqueous Al and the gill surface (Poléo, 1995; Exley et al. 1996; Lydersen et al. 2002).
We appreciate the economic support from the Norwegian Research Council, The Norwegian Directorate for Nature Management and Mr Hans Rasmus Astrup and his colleagues. Also special thanks to Lars Flodmark, University of Oslo, for valuable help with the statistics.

Table 1. Chemical composition of Oslo City tap water used in the experiments (mean±S.D.)
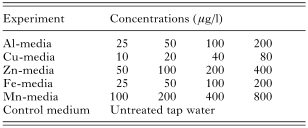
Table 2. The nominal concentrations of the various metals (aluminium (Al), copper (Cu), zinc (Zn), iron (Fe), manganese (Mn)) added to the tap water in each experiment
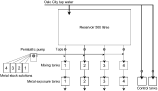
Fig. 1. Schematic presentation of the experimental setup.

Table 3. The concentrations (μg/l) of the various metals (aluminium (Al3+), copper (Cu2+), zinc (Zn2+), iron (Fe2+/3+), manganese (Mn2+)) to which the fish have been exposed

Fig. 2. Number of Gyrodactylus salaris on Atlantic salmon parr exposed to 4 different concentrations of aqueous Al (mean±S.E., n=10).

Fig. 3. Number of Gyrodactylus salaris on Atlantic salmon parr exposed to 4 different concentrations of aqueous Cu (mean±S.E., n=10).

Fig. 4. Number of Gyrodactylus salaris on Atlantic salmon parr exposed to 4 different concentrations of aqueous Zn (mean±S.E., n=10).

Fig. 5. Number of Gyrodactylus salaris on Atlantic salmon parr exposed to 4 different concentrations of aqueous Fe (mean±S.E., n=10).

Fig. 6. Number of Gyrodactylus salaris on Atlantic salmon parr exposed to 4 different concentrations of aqueous Mn (mean±S.E., n=10).