INTRODUCTION
The liver fluke, Fasciola hepatica is an extremely important parasite of livestock throughout the world (Fairweather, Reference Fairweather2005). The prevalence of the disease is high in the UK and Ireland: a recent study in Ireland revealed the presence of liver fluke in 57% of cattle, with a further 9% of the livers examined having pathological damage attributed to Fasciola hepatica (Murphy et al. Reference Murphy, Fahy, Mc Auliffe, Forbes, Clegg and O'Brien2006). Fascioliasis is also emerging as a major zoonosis and is considered to be a serious problem in some countries (Mas-Coma et al. Reference Mas-Coma, Bargues and Valero2005).
Triclabendazole (TCBZ), a benzimidazole derivative, has been the drug of choice for treating liver fluke infections in livestock for over 20 years due to its high efficacy against both adult and juvenile stages (Fairweather, Reference Fairweather2005). However, resistance to TCBZ has emerged in fluke populations and since the mid-1990s, when resistance was first discovered in Australia, it has been reported in a number of European countries (Fairweather, Reference Fairweather2005; Alvarez-Sanchez et al. Reference Alvarez-Sanchez, Mainar-Jaime, Perez-Garcia and Rojo-Vasquez2006). The heavy reliance on a single drug to maintain productivity and animal health puts treatment strategies at risk and, if resistance to TCBZ develops further, it could severely compromise its future use.
To date, the mechanism of resistance to TCBZ is unknown. In contrast to the situation in nematodes and other benzimidazoles, studies have yet to confirm that resistance is associated with any mutations in the β-tubulin molecule of the fluke, specifically, the phenylalanine-to-tyrosine switch at position 200 (Robinson et al. Reference Robinson, Trudgett, Hoey and Fairweather2002; Ryan et al. Reference Ryan, Hoey, Trudgett, Fairweather, Fuchs, Robinson, Chambers, Timson, Ryan, Feltwell, Ivens, Bentley and Johnston2008). More recently, attention has focussed on changes in the uptake and/or metabolism of TCBZ. The enhancement of oxidative metabolism of TCBZ in TCBZ-resistant isolates of F. hepatica has been suggested as a possible mechanism of resistance within the fluke. Recent work by Alvarez et al. (Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005) has shown marked differences in the capacity of F. hepatica to oxidize TCBZ into the sulphoxide (TCBZ.SO) metabolite between isolates and similarly the metabolism of TCBZ.SO to TCBZ sulphone (TCBZ.SO2) has also been shown to be greater in TCBZ-resistant flukes (Robinson et al. Reference Robinson, Lawson, Trudgett, Hoey and Fairweather2004).
The aim of the present study was to investigate whether the action of TCBZ is altered in the presence of methimazole (MTZ), an inhibitor of the flavin monooxygenase (FMO) system (Tynes and Hodgson, Reference Tynes and Hodgson1983). Through the use of metabolic inhibitors, it has been shown that the FMO system probably is the main pathway for the metabolism of TBCZ to TCBZ.SO (Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005). In the present study, MTZ was used to block the metabolic pathway in the fluke, in order to determine whether a TCBZ-resistant isolate could be made more sensitive to TCBZ action. Incubations were carried out on whole flukes rather than microsomal fractions (as used by Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005). Changes to surface architecture were assessed by scanning electron microscopical examination of surface changes to the fluke. The tegument is the main route of entry for TCBZ compounds into the fluke (Mottier et al. Reference Mottier, Alvarez, Ceballos and Lanusse2006a; Toner et al. Reference Toner, McConvery, Brennan, Meaney and Fairweather2008) and therefore is likely to manifest the result of any altered drug metabolism.
MATERIALS AND METHODS
Adult male Sprague-Dawley rats were each infected with 20 metacercarial cysts of either the Oberon TCBZ-resistant or Cullompton TCBZ-susceptible isolate of F. hepatica. The Oberon isolate originated on a farm where TCBZ resistance was suspected, in Oberon, New South Wales, Australia in 1999, and has since been maintained in the laboratory. The isolate has been shown to be resistant to TCBZ action as the drug has limited efficacy against Oberon isolates in vivo and in vitro (Walker et al. Reference Walker, McKinstry, Boray, Brennan, Trudgett, Hoey, Fletcher and Fairweather2004; Keiser et al. Reference Keiser, Utzinger, Vennerstrom, Dong, Brennan and Fairweather2007). The Cullompton isolate originated from a field isolate in Cullompton, Devon, England. This isolate has been shown to be susceptible to TCBZ action in vivo and in vitro (Robinson et al. Reference Robinson, Trudgett, Hoey and Fairweather2002; McCoy et al. Reference McCoy, Fairweather, Brennan, Kenny, Ellison and Forbes2005; Halferty et al. Reference Halferty, Brennan, Hanna, Edgar, Meaney, McConville, Trudgett, Hoey and Fairweather2008a, Reference Halferty, Brennan, Trudgett, Hoey and Fairweatherb).
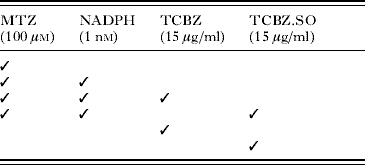
Adult flukes (at least 12 weeks old) were removed from the bile ducts of rats under sterile conditions in a laminar flow cabinet and washed repeatedly in warm (37°C) NCTC 135 culture medium (pH 7·4) containing antibiotics (penicillin 50 IU/ml; streptomycin 50 μg/ml). The flukes were transferred to fresh culture medium containing MTZ (1×10−4m) for 2 h at 37°C. After the pre-incubation period, the flukes were transferred to fresh culture medium for 22 h at 37°C containing one of a number of drug and inhibitor combinations (Table 1). A stock solution of methimazole was initially prepared at a concentration of 1×10−1m in distilled water. A stock solution of nicotinamide adenine dinucleotide phosphate (NADPH) was initially prepared at a concentration of 1×10−3m in distilled water. The concentrations of MTZ and NADPH used were chosen to be similar to those used by Alvarez et al. (Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005) in a previous study on F. hepatica. The concentrations of TCBZ and TCBZ.SO used correspond to the maximum blood level of TCBZ.SO reached in vivo (13·3 μg/ml following a therapeutic dose of 10 mg/kg TCBZ in sheep) (Hennessy et al. Reference Hennessy, Lacey, Steel and Prichard1987). The two compounds were initially prepared as stock solutions in dimethyl sulphoxide (DMSO) and added to the culture medium to give a final solvent concentration of 0·1% (v/v). Controls were prepared by incubating whole flukes in NCTC 135 medium for 24 h at 37°C. Controls at 0 h were also prepared. After incubation, the flukes were fixed and processed for scanning electron microscopy (SEM). A minimum of 4 flukes were prepared for each treatment.
Table 1. Drug and inhibitor combinations
(MTZ, methimazole; NADPH, nicotinamide adenine dinucleotide phosphate; TCBZ, triclabendazole; TCBZ.SO, triclabendazole sulphoxide.)

Tissue preparation for SEM
Flukes were lightly flat-fixed for 1 h at room temperature in 4% (w/v) glutaraldehyde in 0·1 m sodium cacodylate buffer (pH 7·4) containing 3% (w/v) sucrose. The flukes were subsequently free-fixed in fresh fixative for a further 3 h at 4°C. The flukes were washed overnight at 4°C in 0·1 m sodium cacodylate buffer (pH 7·4) containing 3% (w/v) sucrose. After post-fixation in 1% osmium tetroxide for 1 h, the tissues were washed several times in fresh buffer, dehydrated through a series of ethanol, dried in hexamethyldisilazane, mounted on aluminium stubs, sputter-coated with gold-palladium and viewed in an FEI Quanta 200 scanning electron microscope operating at 10 keV.
RESULTS
Given the large number (16) of experiments carried out in this study, a detailed description of surface changes observed for each one is not practicable. Instead, the text will focus on the main features of the response to each treatment and will be illustrated by appropriate micrographs.
Cullompton isolate treated with TCBZ+MTZ+NADPH
Swelling and furrowing of the inter-spinal tegument was observed over all the body surface and obscured the spines (Fig. 1A). In the midbody region, the tegument covering the spines was also swollen and some disruption was observed to the spine tips (Fig. 1B). In the tail region, a layer of fine blebs carpeted the surface (Fig. 1C).

Fig. 1. Scanning electron micrographs (SEMs) of the tegumental surface of the liver fluke, Fasciola hepatica (Cullompton isolate) following in vitro treatment for 24 h with MTZ+NADPH+TCBZ (A–C) or MTZ+NADPH+TCBZ.SO (D–E). (A) Ventral surface of the midbody region showing swelling of the inter-spinal tegument (arrow) and disruption to the spine tips (double arrow). (B) A high-power image of the ventral surface of the midbody region showing disruption to the spine tips (arrow). (C)) High-power image of the ventral surface of the tail region showing furrowing (F) and blebbing (B) on the tegumental surface. (D) Dorsal surface of the midbody region showing slight swelling of the tegument (arrow). (E) High-power image of the dorsal surface of the tail region showing blebbing (B) and swelling (arrow) of the inter-spinal tegument. S, spine.
Cullompton isolate treated with TCBZ.SO+MTZ+NADPH
Slight swelling of the tegument between and covering the spines was observed in the midbody and tail regions (Fig. 1D). Blebbing of the surface membrane was conspicuous in the tail region (Fig. 1E).
Oberon isolate treated with TCBZ+MTZ+NADPH
There was a general swelling of the tegument, especially in the midbody and tail regions, so that the spines were partially or completely submerged (Fig. 2A and B). Disruption to the spine tips was visible at higher magnification (Fig. 2A inset). In the tail region, the tegumental surface displayed a ‘roughened’ appearance, due to the presence of microvillus-like projections (Fig. 2B). Localized areas of blebs were seen in all body regions (Fig. 2B inset).

Fig. 2. Scanning electron micrographs (SEMs) of the tegumental surface of the liver fluke, Fasciola hepatica (Oberon isolate) following in vitro treatment for 24 h with MTZ+NADPH+TCBZ (A–B) or MTZ+NADPH+TCBZ.SO (C–E). (A) Dorsal surface of the midbody region showing swelling of the inter-spinal tegument (arrow) and of the tegument covering the spines (double arrow). S, spine. Inset shows a higher-magnification image of swelling to the tegument between the spines (arrow), together with blebbing (B) and disruption associated with the spines tips (double arrow). (B) Dorsal surface of the tail region showing swelling (arrow) and furrowing (F) of the surface tegument. The surface appears roughened, due to the presence of microvillus-like projections (MV). Inset shows a higher-power image of the tegumental surface in the dorsal oral cone region, highlighting an area of burst blebs (B). (C) Ventral surface of the midbody region, showing swelling of the inter-spinal tegument (arrow) and of the tegument covering the spines (double arrow). S, spine. (D) A high-magnification image of the ventral surface of the tail region, showing extensive blebbing (B) on the tegumental surface. (E) Dorsal surface of the apical cone region, showing swelling (arrow), blebbing (B) and sloughing (double arrow) of the apical tegument membrane.
Oberon isolate treated with TCBZ.SO+MTZ+NADPH
Severe swelling and furrowing of the inter-spinal tegument was evident in all regions of the body (Fig. 2C). The tegumental covering of the spines was also swollen (Fig. 2C). Blebbing was extensive, occurring in all body regions, but mainly in the tail region (Fig. 2D). Areas where the apical plasma membrane had been removed (thus exposing the cytoplasm of the syncytium) were observed in the oral cone and midbody regions (Fig. 2E).
Cullompton and Oberon isolates treated with MTZ
A predominantly normal morphology was observed over the whole body surface of both isolates following 24 h in vitro incubation in methimazole (Fig. 3A).

Fig. 3. Scanning electron micrographs (SEMs) of the tegumental surface of the liver fluke, Fasciola hepatica (Cullompton and Oberon isolates) following in vitro incubation for 24 h in methimazole (A), in NCTC 135 medium without drug (control, B) and in MTZ+NADPH (C, D). (A) Ventral surface of the apical cone region, Cullompton isolate, showing the surface morphology of the tegument and spines (S) which appear normal. (B) Oberon isolate 24 h control. Ventral surface of the oral cone region, showing the morphology of the spines (S) and general body surface. (C) Dorsal surface of the midbody region, Cullompton isolate, showing swelling (arrow) and furrowing (F) of the inter-spinal tegument and disruption to the spine tips (double arrow). S, spine. (D) Dorsal surface of the tail region, Cullompton isolate, showing swelling (arrow) and furrowing (F) of the inter-spinal tegument. The spines (S) appear to be sunken due to the swelling of the tegument (double arrow) around them.
Controls
The surface architecture of the control specimens appeared normal (Fig. 3B). For further images of normal morphology, the reader is referred to the papers by Bennett (Reference Bennett1975) and Fairweather et al. (Reference Fairweather, Threadgold, Hanna and Dalton1999).
Cullompton and Oberon isolates treated with MTZ+NADPH
Limited evidence of disruption was seen in the two isolates. Swelling and furrowing of the tegument was observed in all body regions, but were more marked in the Cullompton isolate (Fig. 3C and D). Disruption to the spine tips was also seen in the Cullompton, but not the Oberon, isolate (Fig. 3C).
Oberon isolate treated with TCBZ
A more detailed description will be given for this treatment, to complement previous descriptions of the effect of TCBZ.SO against the Oberon isolate (Walker et al. Reference Walker, McKinstry, Boray, Brennan, Trudgett, Hoey, Fletcher and Fairweather2004) and of TCBZ and TCBZ.SO against the Cullompton isolate (Halferty et al. Reference Halferty, Brennan, Trudgett, Hoey and Fairweather2008b). Following in vitro incubation for 24 h in TCBZ, a predominantly normal morphology was observed on both the ventral and dorsal surfaces of the apical cone region (Fig. 4A and B). On the ventral surface of the midbody region, general swelling of the inter-spinal tegument was observed (Fig. 4C), with some disruption to the spines visible at higher magnification (Fig. 4C inset). On the dorsal surface in this region, general swelling of the inter-spinal tegument was also observed (Fig. 4D). At a higher magnification, microvillus-like projections could be seen on the swollen tegument covering the spines (Fig. 4D inset). On the ventral surface of the tail region, a patch of localized disruption was observed. The inter-spinal tegument was extremely swollen and furrowed (Fig. 4E). When viewed at a higher magnification, swelling of the inter-spinal tegument could be seen so that the spines appeared partially submerged by the surrounding tegument (Fig. 4E inset). On the dorsal surface, areas of swelling and furrowing of the inter-spinal tegument were observed (Fig. 4F). At a higher magnification, spines appeared to be sunken due to the tegument swelling around them (Fig. 4F inset).

Fig. 4. Scanning electron micrographs (SEMs) of the tegumental surface of the liver fluke, Fasciola hepatica (Oberon) following in vitro treatment for 24 h in TCBZ. (A) Ventral surface of the oral cone region, showing the normal appearance of the spines and the tegumental surface. OS, oral sucker. Inset shows a higher-power image of a spine (S). (B) Dorsal surface of the oral cone region, showing the normal appearance of the spines and the tegumental surface. Inset shows a higher-magnification image of a spine (S). (C) Ventral surface of the midbody region, showing slight swelling of the inter-spinal tegument (arrow). S, spine. Inset shows a higher-power image of the slight disruption (arrow) to the tegument covering a spine. (D) Dorsal surface of the midbody region, showing swelling and flattening of the inter-spinal tegument (arrow). The spines (S) lie flat against the body surface. Inset shows a higher-magnification image of the swelling of the tegument covering a spine (arrow). The surface appears roughened due to the presence of microvillus-like projections (MV). (E) Ventral surface of the tail region, showing a patch of localized disruption (arrow). Inset shows a higher-power image of inter-spinal swelling (arrow) of the tegument. The spine (S) is partly submerged by the swollen tegument around it. (F) Dorsal surface of the tail region, showing areas of swelling and furrowing (arrow). Inset shows a higher-magnification image of a spine (arrow) that appears to be sunken due to the swelling of the tegument around it.
Oberon isolate treated with TCBZ.SO
Following in vivo incubation for 24 h in TCBZ.SO, disruption to the tegument was similar to that reported previously (Walker et al. Reference Walker, McKinstry, Boray, Brennan, Trudgett, Hoey, Fletcher and Fairweather2004, Figs. 17–24). Briefly, swelling of the tegument was observed in all regions of the body, obscuring the spines, with a focus towards the anterior end of the fluke. Blebbing occurred in patches in the oral cone and tail regions, but was more extensive in the midbody region. In the midbody and tail regions, the surface had a ‘roughened’ appearance, due to the presence of microvillus-like projections.
Cullompton isolate treated with TCBZ
Changes to the surface were similar to those described by Halferty et al. (Reference Halferty, Brennan, Trudgett, Hoey and Fairweather2008b) (Figs. 1B, 2A, D and 3B). Briefly, swelling of the inter-spinal tegument was observed on both surfaces of the apical cone region, so that the spines appeared submerged. In the midbody region, swelling of the tegument was so severe that the spines were not visible and the surface was carpeted with blebs; this was particularly evident on the ventral surface. In the tail region, general swelling and furrowing of the inter-spinal tegument was observed, with patches of blebs along the lateral margins; the spines were submerged.
Cullompton isolate treated with TCBZ.SO
Changes to the tegument were similar to those described by Halferty et al. (Reference Halferty, Brennan, Trudgett, Hoey and Fairweather2008b) (Figs. 1C, 2B, E and 3C). Briefly, limited swelling of the inter-spinal tegument was observed on the surface of the apical cone region. Disruption to the spine tips was evident, with isolated blebs associated with a number of the tips. In the midbody region, the tegument was extremely swollen, which obscured the spines. Blebbing was also observed and was more extensive on the ventral than dorsal surface. Swelling and furrowing of the tegument was evident in the tail region, with severe blebbing visible on the surface.
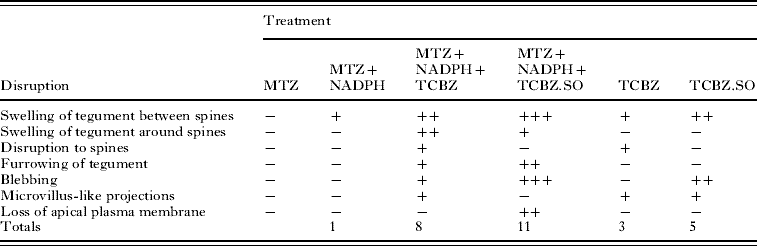
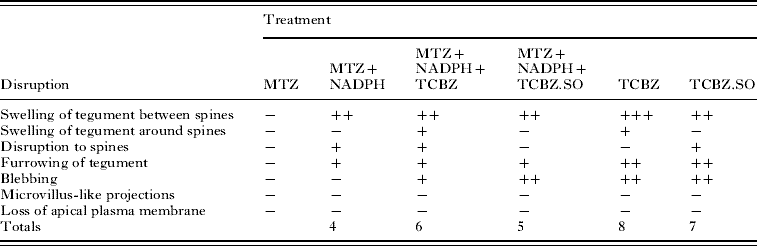
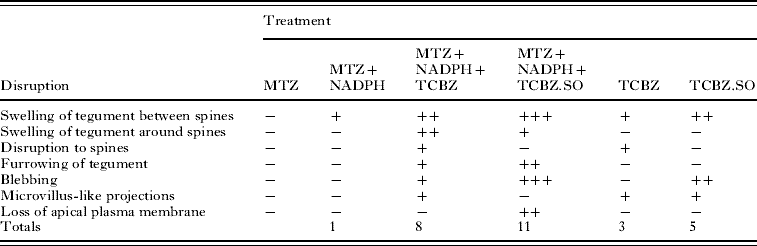
The main changes brought about by drug action and the relative severity of the changes are summarized in Tables 2 and 3.
Table 2. Summary of results for the Oberon isolate
−, no noticeable disruption; +, general/ mild disruption; ++, severe disruption; +++; very severe disruption; MTZ, methimazole; NADPH, nicotinamide adenine dinucleotide phosphate; TCBZ, triclabendazole; TCBZ.SO, triclabendazole sulphoxide.
Table 3. Summary of results for the Cullompton isolate
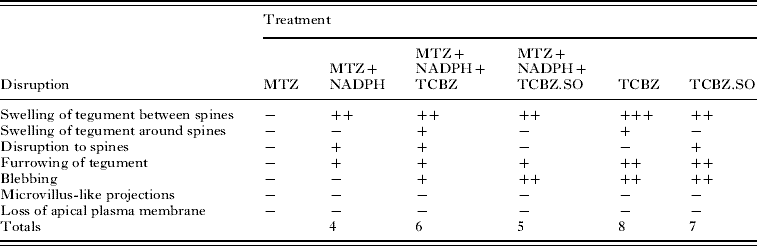
−, no noticeable disruption; +, general/mild disruption; ++, severe disruption; +++, very severe disruption; MTZ, methimazole; NADPH, nicotinamide adenine dinucleotide phosphate; TCBZ, triclabendazole; TCBZ.SO, triclabendazole sulphoxide.
DISCUSSION
From the results presented in this paper, it is evident that treatment with TCBZ or TCBZ.SO alone resulted in more severe disruption to the triclabendazole-susceptible Cullompton isolate than the triclabendazole-resistant Oberon isolate. When the FMO inhibitor, methimazole was included in the drug incubation medium, disruption to the Oberon isolate was greater than that after either drug on its own. MTZ treatment alone caused no change to the surface morphology of either isolate, although in the presence of NADPH it induced some swelling of the tegument and disruption to the spine tips, more so in the Cullompton flukes. However, the changes observed were not as great as with the combinations containing either TCBZ or TCBZ.SO.
With the Oberon isolate, treatment with TCBZ resulted in few surface changes, apart from general swelling of the tegument and some disruption to the spine tips. When combined with MTZ, there was far greater swelling around and between the spines, together with blebbing of the apical plasma membrane. TCBZ.SO-treated flukes were more severely affected than those treated in TCBZ, in that there was widespread swelling and blebbing; the results for TCBZ.SO confirmed the previous findings of Walker et al. (Reference Walker, McKinstry, Boray, Brennan, Trudgett, Hoey, Fletcher and Fairweather2004). When flukes were incubated in TCBZ.SO in the presence of MTZ, there was more severe disruption than with TCBZ.SO treatment alone. Thus, the level of blebbing was greater and there was some sloughing of the apical plasma membrane. So, for this isolate, there was greater disruption with TCBZ.SO than with TCBZ; MTZ co-incubation lead to greater disruption with both drugs; and the combination of MTZ+TCBZ.SO was the most damaging of all treatments.
With the Cullompton isolate, incubation in TCBZ alone resulted in general swelling of the tegument and limited patches of blebs. Addition of MTZ to the incubation medium did not result in a greater level of disruption. If anything, there was more severe disruption with TCBZ alone, which induced a greater degree of swelling and blebbing. This may be due to the reduced conversion of TCBZ to its sulphoxide and sulphone metabolites. Treatment with TCBZ.SO alone caused widespread tegumental swelling and severe blebbing, particularly in the more posterior region of the body. These changes were not exacerbated by co-incubation with MTZ: in fact, they were less severe. So, for the Cullompton isolate, there was slightly greater surface disruption with TCBZ than TCBZ.SO; MTZ co-incubation induced less disruption; and the most disruptive treatment was with TCBZ. The latter observation was in agreement with that of Halferty et al. (Reference Halferty, Brennan, Trudgett, Hoey and Fairweather2008b), although in that study the internal changes induced by TCBZ were less than those caused by either TCBZ.SO or TCBZ.SO2.
It appears that inhibition of drug metabolism has a relatively greater effect in TCBZ-resistant than -susceptible flukes. It has been shown that, in another TCBZ-resistant isolate, the Sligo isolate, there is a greater capacity to metabolize TCBZ to TCBZ.SO (Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005) and TCBZ.SO to TCBZ.SO2 (Robinson et al. Reference Robinson, Lawson, Trudgett, Hoey and Fairweather2004) than in the TCBZ-susceptible Cullompton isolate. This may be as a result of the over-expression of the FMO enzyme system in triclabendazole-resistant flukes, which would make them particularly sensitive to enzyme inhibition. MTZ has a relatively greater impact on TCBZ sulphoxidation in Sligo than in Cullompton flukes: 43% inhibition as against 34% (Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005). The uptake of TCBZ and TCBZ.SO by the Sligo fluke is also significantly reduced (Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005), possibly as a result of increased activity of P-glycoprotein (Pgp)-linked drug efflux pump activity. Co-incubation with the Pgp substrate/inhibitor ivermectin decreased efflux of TCBZ and TCBZ.SO in Sligo flukes, so that levels of the drug present were comparable to those in the Cullompton isolate (Mottier et al. Reference Mottier, Alvarez, Fairweather and Lanusse2006b).
A number of experiments have been carried out in host animals to determine whether co-administration of MTZ with benzimidazole anthelmintics can alter the pharmacokinetics of the anthelmintic. The idea behind this strategy is to enhance the bioavailability and extend the active life span of the anthelmintic, thus increasing its efficacy: this would be particularity useful in the treatment of drug-resistant parasites. The strategy has been successful in sheep and cattle, enhancing the plasma concentrations of different benzimidazoles (Lanusse and Prichard, Reference Lanusse and Prichard1992a, Reference Lanusse and Prichardb, Reference Lanusse and Prichard1993; Lanusse et al. Reference Lanusse, Gascon and Prichard1992, Reference Lanusse, Gascon and Prichard1995). It has also been applied to TCBZ, both in vivo and in vitro using sheep liver microsomes (Virkel et al. Reference Virkel, Lifschitz, Sallovitz, Pis and Lanusse2006, Reference Virkel, Lifschitz, Sallovitz, Ballent, Scarcella and Lanusse2008). In a separate study, it has been shown that the efficacy of albendazole against the parenteral stages of the nematode, Trichinella spiralis in mice was increased by co-incubation with methimazole (López-García et al. Reference López-García, Torrado, Torrado, Martínez and Bolás1998). As well as host metabolism, it is now known that parasites such as F. hepatica can metabolize anthelmintics, so the strategy could be extended to include the parasite, to manipulate the interaction of the drugs with the parasite.
In conclusion, the present study has shown that it is possible to reverse the TCBZ-resistant state of the liver fluke to a more susceptible condition by the co-administration of the FMO inhibitor, MTZ. The results of this morphological study are consistent with the metabolic data published by Alvarez et al. (Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005) and support the concept of altered drug metabolism in TCBZ-resistant flukes. The previous study was carried out on isolated microsomal fractions of fluke. Interestingly, the current observations provide a morphological manifestation of what the inhibition of drug metabolism by the fluke can lead to. There is a need to carry out a parallel drug/metabolite concentration analysis and morphological study on the same flukes. As the two sets of data relate to two separate TCBZ-resistant isolates, the concept may be a general feature of TCBZ-resistant flukes, indicating that it may contribute to the development of drug resistance. The potential role of other drug metabolism pathways in drug resistance needs to be evaluated and it remains to be seen whether this type of information could be used to manipulate drug pharmacokinetics for the treatment of TCBZ-resistant flukes.
This work was supported by a DARDNI Post-graduate Studentship to C. D. It was also partially supported by a grant from the European Union (DELIVER grant, no. FOOD-CT-200X-023025) and by a BBSRC/Defra grant (C00082X/1).