INTRODUCTION
The hepatic mixed function oxidase (MFO) system plays a vital role in the metabolism and disposal of a variety of xenobiotics and endogenous compounds. The system is of crucial importance in chemotherapy since drug efficacy is related to biotransformation and elimination.
Malaria infection, known to cause host hepatomegaly, disturbs the normal physiological functions of the liver (Srivastava et al. Reference Srivastava, Saxena, Pandey and Dutta1984), and hence it would be expected that alterations would occur in its MFO system. One of the important biochemical functions that is noticeably affected is the drug metabolizing or MFO system, which is comprised of 2 steps, i.e. Phase I (asynthetic reactions to make xenobiotics/endobiotics more polar) and Phase II (conjugation reactions with suitable biomolecules to reduce the toxicity of the polar substances) (Kato, Reference Kato1977). Almost all of the studies related to malaria and the MFO system, seem to be incomplete, as they present only the picture of Phase I drug metabolism, ignoring the Phase II reactions which are equally important for the host. Hepatic microsomal MFO system (cytochrome P-450) impairment has been reported in studies in rodent and primate malaria infection (Alvares et al. Reference Alvares, Veng, Scheibel and Hollingdale1984; Srivastava et al. Reference Srivastava, Sahni, Puri, Dutta and Pandey1991b, Reference Srivastava, Puri, Dutta and Pandey1993a, Reference Srivastava, Sharma, Shukla and Pandey1997; Srivastava and Pandey, Reference Srivastava and Pandey2000).
Glutathione-S-transferase(s) GST(s); (E.C.2.5.1.18), catalyse an important group of Phase II reactions of the MFO system and are helpful in eliminating undesirable electrophilic substances via conjugation reactions with glutathione (GSH). Much work on GST is related to its pharmacological aspects, but scanty information is available regarding its participation in diseased status (Peters et al. Reference Peters, Kock, Nagengast and Roelofs1970). The status of hepatic GST in mitochondrial, cytosolic and microsomal fractions during Plasmodium berghei infection, and after chloroquine treatment in Mastomys natalensis, has been studied by Srivastava et al. (Reference Srivastava, Arif and Pandey1995). Studies on the effect of the hepatoprotective drug Picroliv on soluble GST in liver during P. berghei infection have shown impairment of the enzyme as a result of infection (Chander et al. Reference Chander, Kapoor and Dhawan1992).
Mefloquine (Mf), a quinoline methanol derivative is related structurally to quinine. Mf is one of the drugs approved by the FDA for malaria chemoprophylaxis. Mf is also approved for the treatment of malaria and is widely used for this purpose in combination with artesunate (Dow et al. Reference Dow, Bauman, Caridha, Cabezas, Du, Gomez-Lobo, Park, Smith and Cannard2006).
Menadione (2-methyl-1,4-naphthoquinone, vitamin K) is a quinone-containing compound that has been utilized generally as a model for studies of oxidative damage. Menadione (Md) may cause depletion in intracellular GSH level by 3 different mechanisms (Di Monte et al. Reference Di Monte, Ross, Bellomo, Eklöw and Orrenuis1984). Md is also a known inhibitor of mammalian glutamate cysteine ligase and glutathione reductase. Md is known to induce phase I and II enzymes for metabolism of xenobiotics, drugs, and procarcinogens. The effect of menadione depends on its dose and duration of treatment (Sidorova and Grishanova, Reference Sidorova and Grishanova2004). Md has been used for various clinical purposes to prevent vitamin K deficiencies, as well as to treat malaria (Lopez-Shirley et al. Reference Lopez-Shirley, Zhang, Gosser, Scott and Meshnick1994).
It has been reported earlier that treatment with Mf and Md causes restoration in the levels of GSH as well as that of glutamate cysteine ligase (GCL) and glutathione reductase (GR) (Arora and Srivastava, Reference Arora and Srivastava2005). The rationale for treatment of malaria-infected host tissues with Mf and Md was to test the antimalarial efficacy of Mf and Md and to see whether treatment with both drugs had any effect on the cytosolic and microsomal GST(s) of the affected host tissues. The present study was aimed to investigate the status of GST(s) in cytosolic and microsomal fractions of hepatic and splenic tissues of the host during experimental infection with P. yoelii nigeriensis and after Mf and Md treatment in albino and db/+ mice. The study indicates that db/+ mice are equally susceptible to P. yoelii nigeriensis infection as compared to albino mice and that treatment with Mf and Md caused a restoration in the levels of both cytosolic and microsomal GST(s) of liver and spleen of infected mice. However, none of the drugs were found to have a 100% curative effect on the infected mice.
MATERIALS AND METHODS
Chemicals
GSH, mefloquine, menadione sodium bisulfite, potassium chloride (KCl) and sodium chloride (NaCl) were purchased from Sigma Chemical Co., USA. 1-chloro-2,4-dinitrobenzene (CDNB) was obtained from Spectrochem Pvt. Ltd, Mumbai, India. Folin & Ciocalteu's phenol reagent was purchased from Sisco Research Laboratories, Mumbai, India. Giemsa stain was from Qualigens India Ltd. All other chemicals used were of analytical grade.
Swiss albino and db/+ mice
In vivo maintenance of rodent strains of malarial parasites was carried out using Swiss albino (Mus musculus, out-bred strain) and db/+ mice. The phenotypic normal db/+ hybrid mice were obtained in a cross between normal db/+ parent strains. The albino and db/+ mice weighing 20–25 g, were housed in plastic cages with proper care according to the guidelines of our institutional animal house ethics committee, under standard laboratory conditions of temperature (22±1°C), humidity (50–60%) and maintained on commercially available pellet diet supplemented with soaked grains. Water was provided ad libitum. Breeding colonies of animals were maintained in an SPF (specific pathogen free) environment under standard housing conditions.
Parasites
The P. yoelii nigeriensis strain of rodent malaria parasites isolated from blood of infected Swiss albino and db/+ mice was employed for maintenance of infection as well as for enzymatic and chemotherapeutic studies.
Plasmodium yoelii nigeriensis infection in albino and db/+ mice
The laboratory isolates of P. yoelii nigeriensis parasites were routinely maintained by serial blood passage in albino and db/+ mice. For this, the infected blood from donor mice was collected through cardiac puncture and appropriately diluted with anticoagulant (sterile citrate buffer) so as to obtain nearly 2×107 parasitized RBCs/ml of diluted sample. Forty albino and 40 db/+ mice were inoculated with parasites by intraperitoneal passage of 0·5 ml of infected blood. The course of parasitaemia was monitored from day 1 post-infection (day 0) until day 10 in albino mice and day 10 in the case of db/+ mice by microscopical examination of Giemsa-stained thin blood smears. Results were obtained on the basis of mean±s.d. values of percentage parasitaemia and percentage mortality each day for 40 albino and 40 db/+ mice in 3 separate experiments.
Assessment of effect of mefloquine and menadione on P. yoelii infection in albino and db/+ mice
For this, 60 Swiss albino and 60 db/+ mice were divided into 6 groups comprising 10 animals each i.e., (I) normal (uninfected) controls, (II) normal+mefloquine (Mf)-treated controls, (III) normal+menadione (Md)-treated controls, (IV) infected controls, (V) infected+Mf-treated and (VI) infected+Md-treated. The drugs, Mf and Md were dissolved in triple distilled water. Albino and db/+ mice treatment groups (II, III, V and VI) were administered Mf (i.p.) at a dose of 5 mg/kg twice daily from day 1 p.i. on day 0 until day 10 whereas the menadione treatment groups were administered Md (i.p.) at a dose of 100 mg/kg twice daily from day 1 p.i. (day 0) until day 10. Parasitaemia was monitored from day 1 onwards in all the infected groups until day 10.
Three mice from each of the above groups (I–VI) were sacrificed by cervical dislocation on day 7 in case of albino mice and on day 5 in case of db/+ mice. The liver and spleen from each mice were excised, perfused and homogenized in ice-cold buffered KC1 (150 mm KCl, 50 mm Na2HPO4/KH2PO4, pH 7·4) using a Potter Elvehjem homogenizer. The homogenates were then subjected to subcellular fractionation at 1000 g for 15 min, 10 000 g for 30 min to obtain mitochondrial and post-mitochondrial fractions, and subsequently at 100 000 g for 60 min to obtain cytosolic and microsomal fractions. The cytosolic and microsomal fractions thus obtained were stored at 4°C and used as a source for GST estimation.
Measurement of GST(s) levels in liver and spleen of mice infected with P. yoelii nigeriensis
GST(s) activity in various dialysed cytosolic and microsomal fractions was determined spectrophotometrically at 340 nm with the standard substrate 1-chloro-2,4-dinitrobenzene (CDNB) and co-substrate GSH (Habig et al. Reference Habig, Pabst and Jakoby1974). All reactions were corrected for non-enzymatic conjugation, with reaction mixtures without enzyme serving as controls.
Under standard assay conditions the reaction mixture contained 100 mm phosphate, pH 6·5, 1·0 mm CDNB in 20 μl of ethanol, 1·0 mm GSH and enzyme protein unless stated otherwise. A unit of enzyme activity was expressed as the amount that catalyses the formation of 1 μmol S-2,4-dinitrophenyl-GSH adduct per min, using a molar extinction coefficient of 9·6 mm−1 cm−1 for CDNB. Protein was estimated by the method of Lowry et al. (Reference Lowry, Roseborough, Farr and Randall1951) using BSA as standard.
Data interpretation and statistical analysis
For assessing the effect of Mf and Md on P. yoelii infection in albino and db/+ mice, results were obtained on the basis of 3 identical experiments each comprised of the same number of albino and db/+ mice as described above. In a single experiment, mean±standard deviation of 3 separate observations within each of the 6 experimental groups was calculated and compared with those of the other 2 experiments. For differences between several mean values, an analysis of variance (ANOVA) was performed. Results were considered to have reached statistical significance when P<0·05.
RESULTS
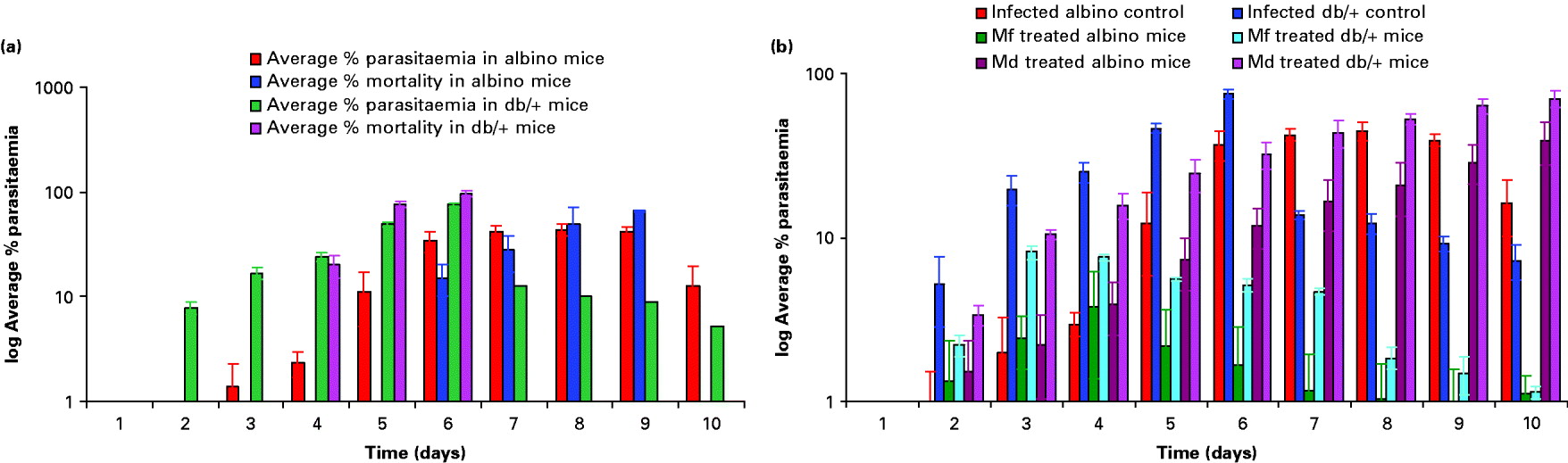
Swiss albino and db/+ mice were infected with P. yoelii nigeriensis and the percentage parasitaemia was ascertained as described in the Materials and Methods section. Fig. 1A is a depiction of the rise in average parasitaemia (P. yoelii nigeriensis) and average percentage mortality of mice with time in infected albino and db/+ mice. A progressive increase in average percentage parasitaemia was obtained in infected albino mice, with a maximum of 44% average parasitaemia by day 8. The average percentage mortality was found to be maximal by day 10 (75%). A relatively faster and lethal increase in average percentage parasitaemia was observed in infected db/+ mice, with a maximum of 75·2% average parasitaemia by day 6 as compared to albino mice. The average percentage mortality was found to be maximal by day 6 (96·7%). Fig. 1B shows the effect of Mf and Md treatment on the percentage parasitaemia in P. yoelii-infected albino and db/+ mice. Results obtained showed that Mf was able to suppress parasitaemia considerably by day 5 in the case of albino mice and by day 3 in the case of db/+ mice. However, it was not able to cure the mice completely as the average percentage parasitaemia obtained was approximately 1·0% even on day 10 in both cases (Fig. 1B). On the other hand, Md caused a delay in maturation of infection in both cases, but could not cure the mice.
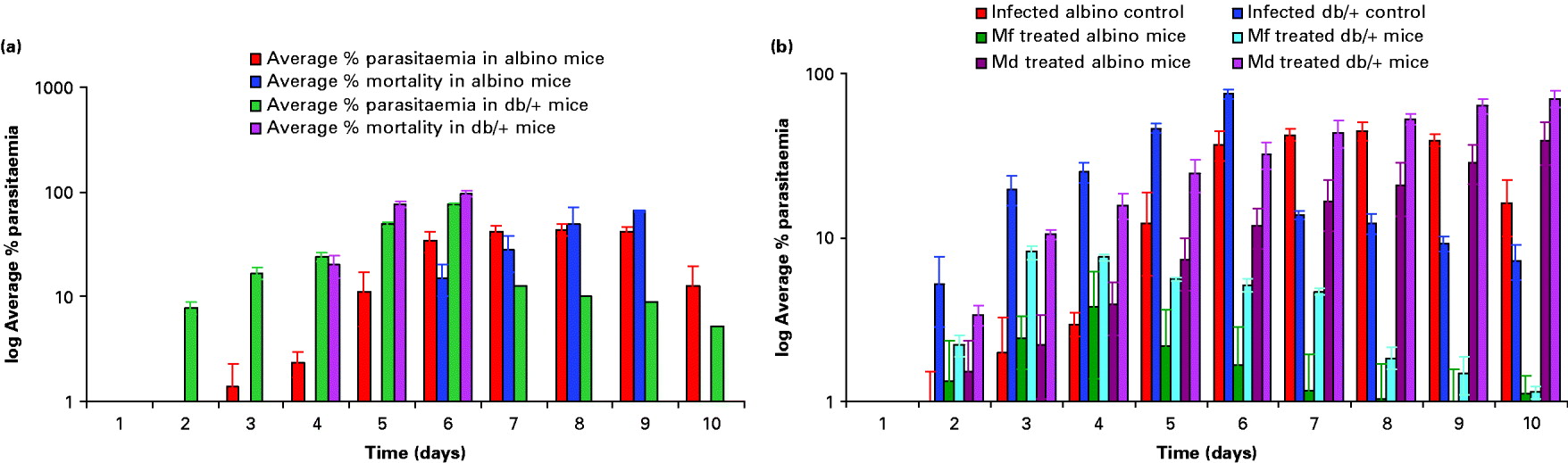
Fig. 1. Rise in parasitaemia and mortality with time in infected albino and db/+ mice (A). Effect of mefloquine (Mf) and menadione (Md) treatment on parasitaemia in Plasmodium yoelii-infected albino and db/+ mice (B). Values are mean±s.d. of 3 separate experiments. Dose: mefloquine 5mg/kg×10 days (i.p.) twice daily, menadione 100 mg/kg×10 days (i.p.) twice daily.
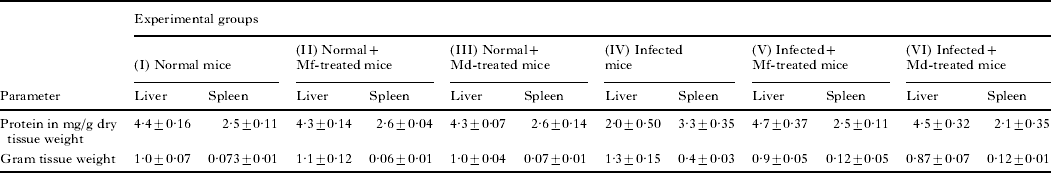
Table 1 depicts the gross changes in the liver and spleen of db/+ mice in acute P. yoelii infection and in the same tissues after treatment with Mf and Md. It is evident from Table 1 that Plasmodium infection caused a significant fall in liver protein concentration in db/+ mice (P=0·0007) on day 5. On the contrary, spleen registered a significant increase in its protein concentration during infection (P=0·0244). It may be seen from Table 1 that spleens and livers of Plasmodium-infected db/+ mice experienced a significant increase in their gross weight, the increase being much more marked in spleen (P<0·0001) as compared to liver (P=0·0008). Similar trends in protein content and tissue weight were observed in the case of infected and drug-treated albino mice (results not shown).
Table 1. Effect of parasitaemia on protein concentration and weight of liver and spleen of infected db/+ mice
(Values are mean±s.d. of 3 separate experiments.)

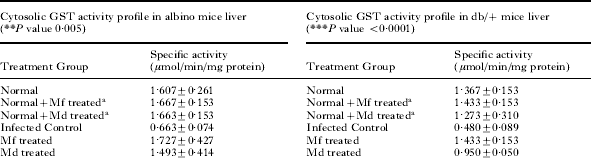
Hepatic and splenic GST(s) activity profiles on day 7 during P. yoelii infection in albino mice and db/+ mice are shown in Table 2. Plasmodium infection caused a significant fall in the activity of the drug metabolizing enzymes, GST(s). The results obtained show that the activity of GST(s) in all cases decreased with a rise in parasitaemia. Mf and Md treatment caused restoration of the levels of GST(s) albeit, to varying degrees. Mf caused approximately 100% protection on both cytosolic and microsomal hepatic GST activities of albino mice. Md, on the other hand, protected cytosolic and microsomal GST of albino liver by 87·9 and 36·8% respectively. Cytosolic and microsomal GST activities of albino spleen were protected to about 47·6 and 39·4% respectively by Mf, and to about 22·2 and 9·1% respectively by Md.
Table 2. Cytosolic and microsomal GST activity profile in Plasmodium yoelii-infected tissues of Swiss albino and db/+ mice before and after mefloquine and menadione treatment
(Values are mean±s.d. of 3 separate experiments. P<0·05 with respect to normal. aNot significant with respect to normal.)


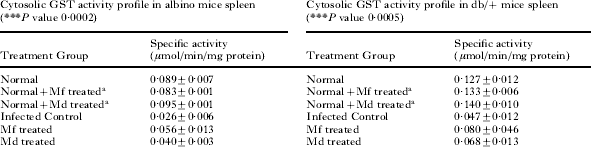

In the case of db/+ mice, Mf offered about 100 and 41·3% protection respectively on hepatic and splenic cytosolic GST(s) activities and 64·2 and 17·1% protection respectively on hepatic and splenic microsomal GST(s) activities. Md was able to afford lesser protection as compared to Mf. Md protected cytosolic GST(s) of liver and spleen by about 53·0 and 26·3% respectively and microsomal GST(s) from both sources by about 19·1 and 8·5% respectively in db/+ mice.
Mf and Md treatment had practically no effect on the hepatic and splenic GST(s) levels of uninfected albino and db/+ mice. Cytosolic and microsomal GST(s) levels of liver and spleen of normal mice remained more or less the same after Mf and Md treatment in both cases (Table 2).
DISCUSSION
The host-parasite relationship in malaria infection has been studied extensively (Von Brand, Reference Von brand1973). Protein metabolism in the infected animal has been the subject of a number of studies in the past (Von Brand, Reference Von brand1973; Siddiqi and Alhomida, Reference Siddiqi and Alhomida1999). In the present study, a significant fall in the protein level in the liver but a perceptibly reverse and statistically significant increase in the protein level in the spleen is suggestive of notable alterations in the protein status in the organs of the host. The net increase in the total protein content due to acute infection may be attributed either to the synthesis of fresh protein or to the accumulation of foreign bodies (i.e. macrophages and parasites etc.). Sharma et al. (Reference Sharma, Shukla, Singh and Sen1979) and Saxena et al. (Reference Saxena, Ghatak and Sen1981) also reported an increase in the protein content of spleen and liver due to P. berghei infection in mice and Mastomys natalensis. Hepatomegaly and splenomegaly was observed on days 5 and 6 post-infection. The mean liver weight was significantly increased as compared to that of healthy controls. The increase in the size and weight of liver and spleen has been attributed to the rise in both dry weight and moisture content (Aikawa et al. Reference Aikawa, Suzuki, Gutierrez and Krier1980). This may provide a favourable environment for the rapid proliferation of the parasite.
The decrease in both soluble and microsomal GST activity clearly indicates the reduced ability of the infected host to clear the undesirable metabolites generated during malaria infection. Depressed GST activity may cause less excretion of electrophiles during malaria. One of the important functions of GST is to protect cellular constituents from oxidative damage (Mosialou et al. Reference Mosialou, Ekstrom, Adang and Morgensiern1973). It has been reported that increased oxidative damage during malaria infection may be attributable to decreased GST (Clark et al. Reference Clark, Chaudhri and Cowden1989; Srivastava et al. Reference Srivastava, Puri, Dutta and Pandey1991a, Reference Srivastava, Puri, Dutta and Pandey1992). Electron microscopic studies have shown that the smooth endoplasmic reticulum that is involved in the metabolism of many foreign compounds becomes very sparse and vascular as a result of malaria infection (Rosen et al. Reference Rosen, Royeroft, Hans and Barry1967). Thus, the infection results in the loss of structural and functional integrity of the smooth endoplasmic reticulum.
The altered/disturbed physiology of Plasmodium-infected tissues as reported earlier (Gutierrez et al. Reference Gutierez, Aikawa, Fremount and Sterling1976; Sharma et al. Reference Sharma, Shukla, Singh and Sen1979; Tekwani et al. Reference Tekwani, Shukla and Ghatak1988) is in agreement with the present data showing a decrease in soluble and microsomal GST(s), which play a major role in detoxification. Emudianughe et al. (Reference Emudianughe, Bickel, Taylor and Andrews1985) have reported suppression of Phase II metabolism in P. berghei-infected mice. Decreased paracetamol disposition has been observed in rats following malaria infection (Mansor et al. Reference Mansor, Edwards, Roberts and Ward1991). The above reports, in accordance with the present data, indicate the involvement of Phase II as well as Phase I drug metabolism during malaria infection (Srivastava and Pandey, Reference Srivastava and Pandey1995, Reference Srivastava and Pandey1996; Srivastava et al. Reference Srivastava, Tripathi, Puri, Dutta and Pandey1991c).
Liver metabolism is adversely affected during infection with malarial parasites (McCarthy et al. Reference McCarthy, Furner, Van Dyke and Stitzel1970; Von Brand, Reference Von brand1973; Chander and Kapoor, Reference Chander and Kapoor1990; Srivastava et al. Reference Srivastava, Sahni, Puri, Dutta and Pandey1991b, Reference Srivastava, Puri, Dutta and Pandey1993b). Plasmodium infection, as expected (Sharma et al. Reference Sharma, Shukla, Singh and Sen1978; Srivastava et al. Reference Srivastava, Tripathi, Puri, Dutta and Pandey1991c, Reference Srivastava and Pandey1995), caused a significant fall in the activity of hepatic and splenic GST(s). The decrease in the activity of host GST(s) might have serious implications for the infected host in drug therapy, toxicity and overall metabolism of the drug. Thus, the efficacy of the drug or its metabolites would be expected to undergo alterations as a result of the biochemical changes in the host. The earlier studies on the metabolism, disposition and toxicity of the antimalarials have been done on normal animals (Thompson and Werbel, Reference Thompson and Werebel1972). These results have been applied directly without any consideration to the infected host for the treatment of infection. It is quite unsafe to follow the drug regimens for antimalarials based on their metabolism in healthy animals. It is felt that adverse effects of malaria infection on drug metabolizing enzymes, should caution against the repetitive use of antimalarials as such or in combined drug therapy (Brodie and Gillette, Reference Brodie and Gillette1971) where the pharmacological response or toxicity could be modified by a metabolic transformation of the drug.
The P. yoelii strain used in this study was found to be sensitive to Mf when it was administered to mice at a dose of 5·0 mg/kg twice daily×6 days via the i.p. route. In the present study, Mf was able to suppress the rise in parasitaemia in albino and db/+ mice but was unable to completely cure both mice models of infection. Mf treatment also caused a restoration in the levels of cytosolic and microsomal GST(s) of liver, as well as that of spleen in both cases. On the other hand, treatment with Md at a dose of 100 mg/kg twice daily×5 days caused a restoration in the levels of both cytosolic and microsomal GST(s) of liver and spleen in albino and db/+ mice. It caused a delay in infection maturation in both cases, but could not cure the mice completely.
The present study also describes an alternative host that is a mutant on C57BL/Ks strain for the maintenance of rodent malaria infection in addition to the routinely used albino mouse (Bray and York, Reference Bray and York1979). The study indicates that db/+ mice are equally susceptible to P. yoelii nigeriensis infection when compared to albino mice. The infection causes a decrease in the hepatic and splenic GST(s) levels as compared to the same tissues from the uninfected mice. Therefore, it can be concluded from the present study that malaria infection can depress hepatic and splenic GST functions in infected albino and db/+ mice and that the declined levels of microsomal and cytosolic GST are brought back to almost normal following Mf and Md treatment. Also, although not having a 100% curative effect, Mf has the potential to suppress and control P. yoelii nigeriensis infection in albino and db/+ mice, provided that the treatment with a dose of 5·0 mg/kg twice daily by i.p. route is continued for a period of 10 days.
This investigation received financial support from CSIR, New Delhi (India) in the form of a Senior Research Fellowship to R. A. and an ad hoc research grant to A. K. S. The authors are grateful to Dr S. K. Puri, Head, Parasitology Division, CDRI, Lucknow for providing the P. yoelii nigeriensis-infected albino mice for the present work.