Published online by Cambridge University Press: 09 October 2003
The present study was designed to assess the role of inulin and sugar beet fibres (SBF) on adult O. dentatum in growing pigs. Four experimental diets were formulated based on barley flour with added insoluble fibre from oat husk (Diet 1), a pure carbohydrate source inulin (Diet 2), soluble fibre from sugar beet fibre (SBF) with a high proportion of soluble fibre components (Diet 3) or inulin plus SBF (Diet 4). Thirty-two 10-week-old pigs were divided randomly into 4 groups each of 8 pigs. After 3 weeks adaptation on Diet 1 all pigs were infected with a single dose of 6000 L3O. dentatum. At week 7 p.i. one group was switched to Diet 2, another group to Diet 3 and another group to Diet 4. The remaining 8 pigs continued on Diet 1 until the end of the experiment and served as controls. At week 13, all pigs were necropsied and their worm burdens determined. The worm recoveries from the pigs on the inulin supplemented diet (Diet 2) were reduced by 97% compared to the controls (Diet 1). Further, the inulin-fed pigs exhibited markedly reduced faecal egg counts. The pigs on inulin plus SBF diet (Diet 4) and on SBF diet (Diet 3) had 86% and 70% adult worm reductions compared with the controls, respectively. The results from this study indicate that highly degradable and rapidly fermentable carbohydrates such as dietary inulin have a profound deworming effect on O. dentatum infection.
Intestinal helminth infections continue to be a major problem affecting more than 2 billion humans and animals worldwide (Keusch & Farthing, 1995). Control of gastrointestinal parasites in monogastric mammals such as pigs and humans is based largely on use of anthelmintics. However, problems such as drug resistance, food residues and environmental pollution have arisen (Waller, 1993) and this has stimulated research into alternative control methods which do not contain substances such as fixatives, preservatives or hormones, but are easily degradable, and do not lead to bioaccumulation in the host's body or the environment (McCorkie, 1995). Among active approaches are the exploitation of the genetic resistance of animals (Stear & Murray, 1994), biological control through either nematophagous fungi that entrap the parasite larvae (Larsen, 2000) or vaccination (Knox & Smith, 2001), herbal medicines (Hammond, Fielding & Bishop, 1997) and selected nutrition or diets (Coop & Kyriazakis, 1999).
There is convincing evidence that host nutritional factors can have an important influence on the establishment and development of parasites (Coop & Kyriazakis, 2001; Petkevičius et al. 1999, 2001). Pigs are often used as host models for human nutrition because of similarities in many aspects of digestive anatomy and physiology (Miller & Ullrey, 1987). The advantage of using the pig as an animal model is that it permits experimental manipulation of diet and parasites which would be unacceptable or impossible in human subjects. Oesophagostomum spp. are very common in pigs in many parts of the world and these parasitic infections have shown a high prevalence in man in certain tropical regions (Polderman & Blotkamp, 1995). Recent studies in pigs experimentally infected with O. dentatum demonstrate that diets containing carbohydrates with contrasting physico-chemical and digestibility properties can have a profound impact on the parasite establishment, persistence and localization (Petkevičius et al. 1997, 1999). Carbohydrates such as inulin and sugar beet fibre which are resistant to degradation in the small intestine, but easily fermentable in the large intestine, can decrease the establishment and fecundity of O. dentatum (Petkevičius et al. 2001), while carbohydrates resistant to degradation in the small and large intestine favoured establishment and fecundity of O. dentatum.
We report here our recent results on the influence of dietary carbohydrates as a novel means for the control of parasites. In the present experiment we investigated the effects of inulin and sugar beet fibre, used as single and/or mixed components of the diet, on O. dentatum in pigs. Special emphasis was given to the effect of inulin and sugar beet fibre on already established infection as a chemotherapeutic approach.
Four experimental diets were formulated based on barley flour with added insoluble fibre from oat husk (Diet 1), a pure carbohydrate source inulin (Diet 2), sugar beet fibre (SBF) which has a high proportion of soluble fibre components (Diet 3) and inulin plus SBF (Diet 4). The diets were supplemented with soybean meal, synthetic lysine, vitamins and minerals to balance the concentrations of essential nutrients (Table 1). The net energy (Feed Units for pigs, FUp) was 0·99, 1·36, 1·29 and 1·32 FUp per kg dry matter, respectively and the amount of digestible protein 118–138 g/FUp.
Table 1. Composition of the plant material and chemical composition of the experimental diets (LMW sugars, low molecular weight sugars; NSP, non-starch polysccharides; NCP, non-cellulosic polysaccharides; FUp, Feed Units for pigs. Values in parentheses are soluble NSP.)

Thirty-two Landrace/Yorkshire/Duroc cross-breed hogs were purchased from a specific pathogen-free farm (Sjælland III, Denmark). The animals were approximately 10 weeks old on arrival and were divided into 4 groups by stratified random sampling each of 8 pigs, according to bodyweight and sex as follows: 4 littermates were distributed with 1 animal in each of the groups, respectively. The average live weight of the pigs at the start of the experiment was 20·4 kg. The pens were thoroughly disinfected and dried prior to introduction of the pigs and at regular intervals during the study period. Excreta was removed twice daily and pigs were kept without bedding. Experimental pigs had free access to water via drinking nipples. All pigs were initially maintained for 3 weeks on Diet 1, and then were inoculated with a single dose of 6000 O. dentatum infective larvae (week 0). Seven weeks post-infection (p.i.) 3 groups of 8 pigs were switched to alternative diets, either Diet 2, 3, or 4; the remaining 8 pigs were maintained on Diet 1, as controls. At week 13 p.i. all pigs were slaughtered for worm recoveries and intestinal content analysis.
The pigs were weighed on arrival at the stable, and at every other week, and at slaughter, weighing occurred at the same time of the day. Faecal samples were collected from the rectum for worm egg counts and evaluation of faecal consistency the day after arrival, the day before larval inoculation, and once a week thereafter. Faecal egg counts were monitored every week p.i. using a modified McMaster technique (Roepstorff & Nansen, 1998) with minimum sensivity of 20 eggs per gram (e.p.g.) faeces. Faecal egg counts (e.p.g.) were determined weekly until slaughter. The pigs were killed 3 h after the morning feed by intravenous injection of phenobarbital. The gastrointestinal tract was quickly removed and samples taken from the gut content at various sites along the intestine. Samples of content taken from the caecum (Ce) and 5 segments of the colon and rectum (Co1–5) were processed for worm recovery (from 100% sample). Oesophagostomum dentatum was collected from digesta and washings of the caecum and colon using a modified agar-gel method described by Slotved et al. (1996) and the worms counted. The developmental stages of O. dentatum were identified using the criteria of Goodey (1926). The sex of adults of both species was recorded. Samples for the chemical analysis and short-chain fatty acid determinations were taken by sampling from the rectum 4, 3 and 2 days before slaughter.
All analyses were performed in duplicate. Chromic oxide and short-chain fatty acids determinations were performed on wet materials; other analyses were performed on freeze-dried materials. Protein was determined by the Kjeldahl method using a Kjeltec autosampler system 1035 (Foss Tecator, Höganäs, Sweden), ash analysed by the AOAC method (Association of Official Analytical Chemists, 1990), fat (hydrochloric acid-fat) was extracted with diethyl ether after acid hydrolysis (Stoldt, 1952) and chromic oxide was determined using the method of Schürch, Loyd & Crampton (1950). Feed units for pigs (FUp) were estimated in vitro as described by Boisen & Fernandez (1998). Starch was analysed by an enzymatic colorimetric method (Bach Knudsen, 1997), non-starch polysaccharides (NSP) by an enzymatic-chemical method (Bach Knudsen, 1997) and fructans by the method described by Bach Knudsen & Hessov (1995). Short-chain fatty acids (SCFA) were determined by the method described by Jensen, Cox & Jensen (1995).
The diet effect on egg counts was assessed by analysis of variance assuming that the transformed counts log10 (no. of eggs+10) for each pig are normally distributed with mean (log10 (no. of eggs+10)ijk)=αi+βj, where αi is the diet main effect, βj is the main effect of week and index k represents the pig. The analysis was, finally, confined to observations at weeks 10–13, when shedding of eggs had stabilized. Repeated measures on a given pig were assumed (auto-) correlated. Instead of the standard deviation a (skew) 95% reference interval is given, roughly covering 95% of (average) egg counts from pigs on a given diet. The SAS programme was used for analysis (Version 8.2, SAS® Institute Inc.).
The diet effect on worm burdens was contrasted for each intestinal section and analysed by analysis of variance (ANOVA) on the transformed counts log10 (no. of worms+10). The basic datum was the sex-specific count in a given section of a given pig. These transformed counts were thus classified according to sex, section and diet, and grouped according to pig. This allowed for the simultaneous assessment of the influence of these three factors. Pig was retained in the model as a random effect.
The mean position of the O. dentatum worms along the large intestine in each pig was calculated by one-way ANOVA (Petkevičius et al. 1995), by simply using the section number (1–5) as a proxy for geometrical position along a line. The effects of diet, sex and section on worm abundance were estimated by ANOVA on log10-transformed worm-counts. The estimates obtained were interpreted as relative abundances, choosing a reference level for each factor. Section 4 was chosen in the case of section, because of its intermediate status.
The effect of diet on the chemical composition of faecal materials was analysed by a one-way ANOVA and significant differences identified by the Student–Newman–Keuls multiple comparison procedure (Keuls, 1952).
The experiment was designed so as not to cause higher parasite loads on the animals than under average natural conditions. The infection course was subclinical. The experiment was approved by the Danish Animal Ethical Committee (Experimental animal permission licence: 2000/561-321). Meetings were held with the agricultural and laboratory technicians to explain the purpose of the experiment and what was required from the persons handling the pigs.
There were no clinical signs of parasitic disease in any of the pigs during the experiment. All pigs had a normal appetite during the experiment; daily feed allowance was completely consumed, and weight gains and general appearance was similar in all different diet groups. The average live weight (S.D.) of the pigs at the start of the experiment was 20·4 kg (1·7). There were no significant differences in the weight gain of pigs between all groups due to diet or sex of the pigs (P>0·05). In all experimental groups, the average weight (S.D.) of pigs increased gradually at 3, 5, 7, 9, 11 and 13 weeks p.i. and reached weights of 28·5 kg (3·3), 33·7 kg (4·5), 38·8 kg (5·8), 49·6 (7·4), 60·4 (7·5) and 73·8 kg (8·6), respectively.
The faecal consistency was softer and more ‘fatty’ in pigs fed Diets 2–4 than when fed Diet 1. This was also reflected in the concentration of dry matter and the composition of faecal materials, where the concentration of marker, protein, fat and SCFA were higher and of NSP lower in pigs consuming Diets 2–4, compared to Diet 1. The faecal composition of pigs on Diet 2 with the high level of inulin had the highest concentration of marker and protein, while the concentration of fat was intermediate to that of Diet 1 and Diets 3 and 4, respectively (Table 2).
Table 2. Composition (g/kg dry matter or mmol/kg digesta) of digesta materials from the rectum of pigs fed Diets 1–4 (NSP, non-starch polysaccharides; NCP, non-cellulosic polysaccharides; SCFA, short chain fatty acids; t, trace.)

The pH in the caecum and colon sections of pigs on Diet 2 (inulin) was significantly lower than in pigs on the other diets (Fig. 1). In the caecum (Ce) the pH for pigs on inulin was 5·0, compared to 5·8–5·9 for pigs on any of the other diets. The concentration of SCFA in faeces was 39–55 mmol/kg digesta higher in pigs fed diets containing the fermentable carbohydrates compared to Diet 1 (Table 2). The high-level inulin diet further influenced the molar proportions of SCFA significantly; acetate and butyrate were lower, while propionate and valerate were higher than for any of the other diets (Table 2).

Fig. 1. pH in caecum and colon of pigs fed experimental diets: oat hull meal (Diet 1), inulin (Diet 2), sugar beet fibre (Diet 3) and inulin plus sugar beet fibre (Diet 4). Sections of the large intestine: Ce, caecum; Co1, colon 0–20%; Co2, colon 21–40%; Co3, colon 41–60%; Co4&5, colon and rectum 61–100%.
The geometric mean faecal O. dentatum egg counts and worm burdens for all groups are presented in Tables 3 and 4. Eggs first appeared in the faeces of all pigs between weeks 3 and 4. After diet switching at week 7, O. dentatum faecal egg counts for pigs on Diets 2, 3 and 4 changed dramatically. At week 9, group 2 pigs on the inulin-supplemented diet (Diet 2) exhibited an 87% decline in egg excretion levels compared to the controls; in 5 of these 8 pigs the counts dropped to zero. By week 10, only 2 of the 8 pigs in the Diet 2 group excreted eggs, which continued at low levels to the time of slaughter. By the end of the experiment (week 13 p.i.) the overall egg counts for the pigs on inulin (Diet 2) were reduced by 99% compared with controls (Diet 1) (P<0·0001) (Table 3).
Table 3. Geometric mean of Oesophagostomum dentatum egg counts at week 13 p.i. (In each column, estimates with different superscripts differ statistically at the 5% significance level.)

Table 4. Geometric mean of Oesophagostomum dentatum worm burdens (In each column, estimates with different superscripts differ statistically at the 5% significance level.)

The geometric mean of O. dentatum worm burdens, and locations of worms, in the sections of the large intestine are shown in Table 4. The sex ratio in sections Co 2+3 is given, as well as the average location of worms in each diet group. Sections Co 2 and 3 harboured the majority of worms, therefore the other sections were excluded in the calculation of the sex ratio in order to avoid ‘boundary effects’. The worm recoveries from pigs on Diet 2 (inulin supplemented) had 97% fewer O. dentatum adults compared to controls (Diet 1) (P<0·0001). Four of the Diet 2 pigs were completely free of worms, 2 pigs had only male O. dentatum worms, and the remaining 2 pigs had low mixed sex worm burdens. The pigs on inulin plus SBF diet (Diet 4) had 86% fewer total O. dentatum worms compared with controls (Diet 1) (P<0·001).
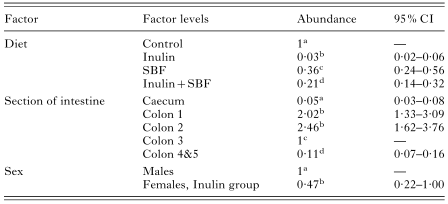
The effects of type of diet, worm location and worm sex on O. dentatum abundance are shown in Table 5. The numbers given are estimates of relative abundance, based on counts of male and female worms in 5 sections of the intestine in each of 32 pigs. Colon 3 was chosen as reference section and the oat husk diet (control) as reference for diet.
Table 5. The effects of diet, sex and section on Oesophagostomum dentatum worm abundance (In each column estimates with different superscripts differ statistically at the 5% significance level.)
This experiment demonstrated that a highly fermentable carbohydrate, such as inulin, can have a marked anti-worm effect. While SBF also exhibited an anti-parasitic effect, the impact was significantly less than that of inulin. These results confirm our previous findings that the type and levels of dietary carbohydrates markedly influence the establishment, gut location and fecundity of O. dentatum in the intestinal tract of pigs (Bjørn, Roepstorff & Nansen, 1996; Petkevičius et al. 1995, 1997, 1999, 2001). In general, diets that consist of insoluble fibre, and which are resistant to fermentation in the large intestine (e.g. from oat-hull meal or wheat bran) provide favourable conditions for the establishment of O. dentatum and worm fecundity (Petkevičius et al. 1999). The opposite occurs, however, with diets containing such fermentable carbohydrate sources as inulin alone or in combination with sugar beet fibre (Petkevičius et al. 2001).
The mechanisms responsible for this effect are not clear, but several possibilities are considered. Dietary carbohydrates can influence the digestion and absorption of nutrients in various parts of the gastrointestinal tract (Bach Knudsen, Jørgensen & Canibe, 2000; Bach Knudsen & Jørgensen, 2001), and exert important influences on the secretory response of the gut to feed intake (Low, 1989), volume flow (Bach Knudsen, Jensen & Hansen, 1993), mucosal architecture (Jin et al. 1994; Brunsgaard, 1998), activity of the gut flora (Jensen & Jørgensen, 1994), and the morphological development of the gastrointestinal tract (Jørgensen, Zhao & Eggum, 1996). Non-digestible carbohydrates, because of their chemical characteristics, resist digestion in the small intestine of monogastric animals and humans, and are, therefore, potential substrates for microflora in the large intestine (Cummings et al. 1997). Inulin, a polymer of fructose with β-(2–1) glycosidic linkages is not hydrolysed by the endogenous digestive enzymes of humans, and is, therefore, nearly completely recovered (∼90%) in the ileal effluents (Bach Knudsen & Hessov, 1995). Because pigs harbour a more active microflora in the stomach and small intestine, the recovery of inulin at the end of the small intestine is consequently lower, with recoveries at the terminal ileum of approximately 60% (Bach Knudsen, 2001). Nevertheless, inulin is also a colonic feed for the microflora of the pigs' large intestine and causes of the changes in microbial population and the rapid generation of organic acids (SCFA and LA), which lower the pH of the luminal contents. It is likely that this lowered gut pH may create an unsuitable environment for the mature and immature helminths. The close association between the increased levels of SCFA and low pH with expulsion of O. dentatum suggests that the physico-chemical changes associated with increased fermentation are important in this therapeutic effect.
Among the physical changes that could also affect O. dentatum survival at the larval stage are alterations in the epithelial lining of the large intestine, which could adversely effect nodule formation and larval development (Theodoropoulos et al. 2001; Petkevičius et al. unpublished observations). Although immunity to O. dentatum is considered to be relatively weak in normal infections (Stewart & Gasbarre, 1989; Roepstorff & Nansen, 1994) there is evidence that inulin diets in humans increase immune responses (Pratt et al. 1996; Kelly-Quagliana et al. 1998). Future investigations must also assess these potential factors, on O. dentatum development, persistence and fecundity.
In conclusion, the results from this study indicate that highly and rapidly degradable carbohydrates such as inulin can have a profound influence on O. dentatum infection in pigs, offering an approach to ‘nutritional deworming’. The results showed that there were no significant differences in liveweight gain between the diets. This is a positive factor to reinforce the anthelmintic effect of diet containing inulin. Our results have potential value for parasite control for both conventional and ecological pig production systems. Consideration should also be given to the application of this nutritional approach for control of nematode parasites in humans.

Table 1. Composition of the plant material and chemical composition of the experimental diets

Table 2. Composition (g/kg dry matter or mmol/kg digesta) of digesta materials from the rectum of pigs fed Diets 1–4

Fig. 1. pH in caecum and colon of pigs fed experimental diets: oat hull meal (Diet 1), inulin (Diet 2), sugar beet fibre (Diet 3) and inulin plus sugar beet fibre (Diet 4). Sections of the large intestine: Ce, caecum; Co1, colon 0–20%; Co2, colon 21–40%; Co3, colon 41–60%; Co4&5, colon and rectum 61–100%.

Table 3. Geometric mean of Oesophagostomum dentatum egg counts at week 13 p.i.

Table 4. Geometric mean of Oesophagostomum dentatum worm burdens

Table 5. The effects of diet, sex and section on Oesophagostomum dentatum worm abundance