INTRODUCTION
Parasites are typically found in communities where multiple species co-exist within the same host individuals (Esch et al. 1990; Sousa, 1994; Simberloff and Moore, 1997; Poulin, 1998; Lello et al. 2004) and in this respect represent ideal models for studies on specialization and life-history divergence. Several parasites also have complex life-cycles including multiple host species (Choisy et al. 2003; Parker et al. 2003) through which parasites have to pass for completion of the cycle. In many cases, life-cycles of different parasite species overlap as they share the same host species. This is particularly obvious in closely related species where similar evolutionary pressures maintain similar life-history traits. However, use of common resources is predicted to promote specialization, for instance, because of resource competition (Doebeli and Dieckmann, 2000; Schluter, 2000), resulting in apparent co-existence of species where fine scale niche divergence reduces the overlap in life-history characteristics. Ultimately, these processes may lead to disruptive selection where sympatric host races with a common descent diverge into different species (Dieckmann and Doebeli, 1999; Kondrashov and Kondrashov, 1999; Drossel and McKane, 2000).
In complex parasite life-cycles, multiple hosts with distinct parasite developmental stages in each form a complex net of interactions where parasite specialization may occur at each step as use of (1) different host species or (2) niche within a host (Adamson and Caira, 1994). Since evolutionary pressures at one stage have direct consequences for the overall parasite fitness, i.e. the whole life-cycle can be seen as a target of selection, untangling these interactions and the underlying evolutionary mechanisms requires simultaneous focus on several host phases in the life-cycle. However, specialization in host use by parasites has rarely been considered in multiple life-cycle stages. In this study, we focused on the life-histories of the sympatric trematode parasites Diplostomum spathaceum and D. gasterostei.
Both D. spathaceum and D. gasterostei have complex life-cycles including avian definitive host and 2 intermediate hosts, snail and fish. Parasites mature in the intestine of fish-eating birds and release eggs to infect the first intermediate snail hosts. Subsequent asexual reproduction in the snails gives rise to cercariae, which infect the lens (D. spathaceum) and vitreous body (D. gasterostei) of fish (Williams, 1966; Valtonen et al. 1997; Kennedy, 2001). Because of close morphological resemblance of the metacercariae (Williams, 1966), differentiation between the species in fish is usually made according to the site of infection. Indeed, morphological similarities, and consequent difficulties in species determination and nomenclature, are characteristic to the whole genus Diplostomum (e.g. Valtonen and Gibson, 1997 and references therein). Therefore, any information on the ecology of these species may also be helpful in resolving their taxonomy.
Our aim in the present work was to investigate how similarity of the life-cycles is reflected to the life-history characteristics of D. spathaceum and D. gasterostei. First, we determined the infection in snail and fish populations. We were particularly interested if the parasites used different intermediate host species, which could be expected because of overlap in the life-cycles. Second, to verify if the parasite species infect different locations in fish eye (lens and vitreous body) because of evolutionary specialization, we conducted exposure experiments using rainbow trout as a novel fish host for both parasites thus excluding possible effects of co-evolutionary history between the parasites and fish on the observed site selection. Third, we compared cercarial production from snail hosts (indirect measure of the rate of host exploitation), and cercarial characteristics such as activity and mortality rate in relation to age, between the parasite species. Since these parasites have very similar life-cycles, we expected to find similarities in cercarial characteristics. We discuss the results in relation to life-history divergence, transmission and the complex taxonomy of these parasites.
MATERIALS AND METHODS
Sampling of snail and fish populations
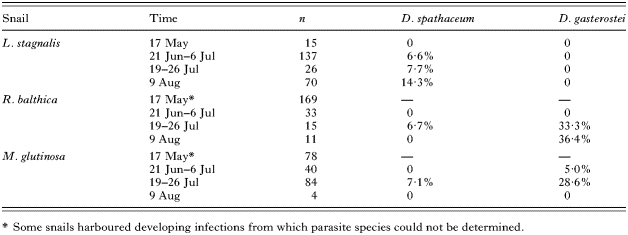
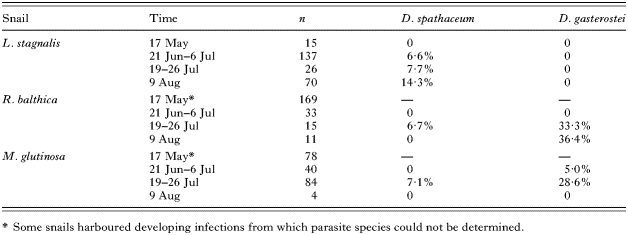
Samples of snails were collected by snorkelling from the lake Konnevesi in Central Finland 4 times in May–August 2004 (Table 1). Sampling was conducted in a 200×100 m shallow water area with a mixed bottom substrate of sand and small rocks. The species under focus were pulmonate snails Lymnaea stagnalis, Radix balthica (formerly known as Lymnaea ovata (Bargues et al. 2001)) and Myxas glutinosa from which all encountered individuals were collected so that the sampling procedure gave a rough estimate of the relative abundance of the snail species. Each time, snails were brought to the laboratory and placed individually in 2 dl of water for a few hours such that it was easy to separate individuals shedding cercariae. When no shedding was observed, snails were dissected under a microscope for pre-patent infections. Cercarial types were separated and identified according to their behaviour and morphology in vivo. Essentially, D. spathaceum cercariae hung downwards with furcae apart and formed a 90 ° angle with the tail stem (see Niewiadomska, 1986). The behaviour of D. gasterostei was similar except that the tail stem was straight and there was a slight bend in the head of the cercariae. The resting stage was followed by a short swimming burst in both species after which the cercariae again returned to the resting stage for a few seconds.
Samples of 34 roach (Rutilus rutilus) and 35 perch (Perca fluviatilis) of roughly equal size and age were caught from the lake using fish traps and brought to the laboratory where they were euthanized. Lens and vitreous body of the eyes were then dissected for parasites. Based on the common occurrence of both fish species and their relatively high abundance of Diplostomum parasites in Finnish water systems (e.g. Valtonen and Gibson, 1997; Valtonen et al. 1997, 2003), they were considered as principal fish host species for these parasites. Samples were taken in winter during the ice cover, when water temperature falls as low as 1 °C and the transmission of Diplostomum parasites is seized. This excluded the possible effects of differences in the timing of transmission during the summer months on the observed parasite numbers in fish.
Experimental exposure of rainbow trout
Cercariae of D. gasterostei were extracted from infected M. glutinosa by allowing individual snails to produce cercariae in a small amount of water. Cercariae were then used in exposure trials where young rainbow trout (Oncorhynchus mykiss) were exposed to a high number of cercariae from each snail in groups of 5–10 fish in aerated, room tempered water for 30–45 min. Different exposure procedures and doses were used, but this did not affect the result since the purpose was merely to determine the place of infection in fish. After the exposures, fish were maintained in a larger water volume at room temperature for several days to allow parasite establishment and early development. After this, fish were euthanized and eyes (lens and vitreous body separately), brains and the viscera of each fish were dissected for parasites. Cercariae of D. spathaceum released from L. stagnalis have earlier been used in a trout (Karvonen et al. 2003, 2004a,b,c, 2005), which have shown unquestionably that D. spathaceum cercariae establish only in the lens of the eye. Therefore, exposure trials with D. spathaceum were not conducted in the present study.
Cercarial production and characteristics
Cercarial production (used here as an indirect measure of the rate of host exploitation), life-span and activity, were compared between D. spathaceum and D. gasterostei. Cercarial production of D. gasterostei was followed from 15 M. glutinosa individuals (mean shell length 11·9±0·3 mm) in the laboratory until death of the snails. Each snail was maintained in 2 dl of water at room temperature with natural light rhythm and provided with lettuce in excess. Every day, snails were removed to another similar container and the number of cercariae released from each snail during the previous 24 h was determined by taking five 1 ml samples. Data for D. spathaceum cercarial production were taken from Karvonen et al. (2004a), where the cercarial release was determined from 21 L. stagnalis (mean shell length 37·8±0·8 mm) by taking 1 subsample daily from each snail. Although that methodology was different compared to the present study, 1 sample corresponded well to density estimation based on multiple samples (Karvonen et al. 2004a), and therefore provides a reasonable estimate for the between-species comparison. Since the snail host species M. glutinosa and L. stagnalis are of different size and shape (spherical and conical, respectively), and thus provide different volumes for the parasite sporocysts, we corrected cercarial production with the volume of the snails. A rough estimate of the volume of M. glutinosa was calculated from the shell length (h) as 4/3π(0·5 h)3. Similarly, we estimated the shell width of L. stagnalis to be 50% of length and calculated the volume as 1/3π(0·5 h/2)2 h. The number of released cercariae was then calculated per unit of snail volume. It should be noted here that all snails harboured fully developed infections (i.e. were filled with parasite sporocysts when dissected at the end of the experiment) and thus were considered to produce cercariae at maximum rate in relation to the age of infection.
The life-time of D. gasterostei cercariae was determined by following individual cercariae obtained from 10 M. glutinosa. Twenty cercariae from each snail (total 200 cercariae) were placed on well plates, 1 cercaria in each well containing 200 μl of water. Cercariae were then observed every 4 h at room temperature under a microscope until all cercariae had died. Survival data for D. spathaceum cercariae were taken from Karvonen et al. (2003). Although the methodology in that paper was different compared to the present study (i.e. survival of a large number of cercariae was estimated at room temperature in 1·5 l containers every 3 h), we expected that this would not affect the pattern of mortality in the between-species comparison. This seems reasonable since the water volume available for each cercaria at the beginning of the experiment in Karvonen et al. (2003) was significantly lower compared to this study (11·4 μl and 200 μl, respectively), but no density-dependent effects on cercarial mortality were observed (see also Anderson and Whitfield, 1975).
We also determined the activity of 3 D. gasterostei cercariae from each of the 10 M. glutinosa (total 30 cercariae), randomly selected from the 200 cercariae on the well plates (see above). The activity was assessed by observing each cercaria for 3 min during which time the number of swimming bursts and their duration in seconds were recorded. The activity of D. spathaceum cercariae was determined by retrieving 5 cercariae from 10 L. stagnalis to well plates from which 3 randomly selected individuals from each snail (total 30 cercariae) were followed as described above. Five randomly selected cercariae were originally retrieved from each snail to ensure that a sufficient number of cercariae would survive through the experiment. To obtain a detailed insight into differences in the cercarial activity between the parasite species, activity was assessed twice when the cercariae were 8 and 28 h old. However, due to cercarial mortality during this time, activity could not be measured from the same individuals at 8 and 28 h. Also, the number of cercariae examined fell from 30 (at 8 h) to 11 (at 28 h) in both parasite species.
RESULTS
A total of 682 snails were studied consisting of 248 L. stagnalis (36·4%), 228 R. balthica (33·4%) and 206 M. glutinosa (30·2%). D. spathaceum was observed in all 3 snail species (Table 1), but it was mainly associated with L. stagnalis, which harboured 21 of 28 D. spathaceum infections (75·0%). D. gasterostei, on the other hand, was found only from R. balthica and M. glutinosa in which species it was the dominant parasite comprising 35 of the 42 infected cases (83·3%). There was also strong seasonality of the infection in both parasites (Table 1). Infections were not detected in May, although 14·9% of the snails harboured immature furcocercarial infections from which the species identification could not be made. The prevalence of infection increased with summer and was highest in both species in July–August (Table 1). Other parasite species observed were xiphidocercariae (20·2%, overall prevalence in all hosts during the study) found from all 3 snail species and Trichobilharzia sp. (0·7%) found from R. balthica and L. stagnalis.
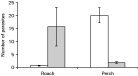
In the fish populations, roach harboured significantly higher numbers of D. spathaceum metacercariae in the lens compared to perch (t-test on log-transformed data: t67=6·513, P<0·001) whereas the opposite was true for the numbers of D. gasterostei in the vitreous body (t-test on log-transformed data: t67=−18·757, P<0·001; Fig. 1). However, numbers of D. spathaceum in the lens of roach did not differ from the numbers of D. gasterostei in the vitreous body of perch (t-test on log-transformed data: t67=−0·560, P=0·577; Fig. 1).

Fig. 1. Mean number of Diplostomum gasterostei in vitreous body (open bars) and D. spathaceum in lens (filled bars)±S.E. of roach (n=34) and perch (n=35) caught from Lake Konnevesi, Central Finland.
All rainbow trout became infected following the experimental exposure to D. gasterostei cercariae and no fish died during the exposures or maintenance. Parasites were found in the vitreous body of the eye, which in most cases contained tens to hundreds of metacercariae (mean 123·5, range 10–345). No parasites were detected from other parts of the eye or elsewhere in fish.
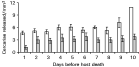
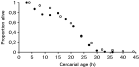
Absolute mean cercarial production in D. spathaceum was noticeably higher compared to D. gasterostei during the 10-day period [11966±2066 and 4471±314 (mean±S.E.) for D. spathaceum and D. gasterostei, respectively]. However, when cercarial numbers were corrected with snail volume, the mean production during the 10-day period was significantly higher in D. gasterostei (t-test: t34=2·084, P=0·045; Fig. 2). The number of cercarial swimming bursts did not differ between the species [t-test on log-transformed data: t58=1·354, P=0·181 (8 h), t20=0·176, P=0·862 (28 h); Fig. 3). The same was true for the duration of swimming bursts (t-test on log-transformed data: t58=0·572, P=0·570 (8 h), t20=0·734, P=0·471 (28 h); Fig. 3]. The pattern of cercarial life-span was very similar between the species (Fig. 4); less than 5% of the cercariae of both species were alive after 36 h from shedding. Maximum life-time of D. spathaceum cercariae was 37 h whereas very few cercariae of D. gasterostei survived until 44 h.

Fig. 2. Mean number of cercariae released per unit of snail volume (mm3)±S.E. from 15 Myxas glutinosa infected with Diplostomum gasterostei (open bars) and 21 Lymnaea stagnalis infected with D. spathaceum (filled bars) as a function of days to host death. Data for D. spathaceum were taken from Karvonen et al. (2004a).

Fig. 3. Mean number of swimming bursts±S.E. (A) and mean duration of bursts±S.E. (B) made by 8- and 28-h-old Diplostomum gasterostei (open bars) and D. spathaceum (filled bars) cercariae during a 3-min period in standardized laboratory conditions. Numbers of cercariae followed were 30 (at 8 h) and 11 (at 28 h) for both species.

Fig. 4. Survival of Diplostomum gasterostei (open circles) and D. spathaceum (filled circles) cercariae in standardized laboratory conditions in relation to age of cercariae. Data for D. spathaceum were taken from Karvonen et al. (2003).
DISCUSSION
Comparative studies on life-histories of closely related species may help to understand evolutionary pressures responsible for present-day life-cycle characteristics and factors underlying their divergence. In this study, we compared the life-history characteristics of two complex life-cycle trematodes, D. spathaceum and D. gasterostei. This group of parasites has been notorious for its taxonomical difficulties and nomenclature and therefore our aim was also to enlighten the complex taxonomy of these parasites. We observed that cercarial characteristics were very similar between the species. However, in host use, where competition for resources between closely related sympatric species could be expected, parasites had diverged as they used mainly different intermediate host species and in controlled experiments infected different regions of the eye of a novel fish host.
The two Diplostomum species infected mainly different snail host species. The infection of D. spathaceum was concentrated to L. stagnalis, which harboured the majority of the infected cases. On the other hand, D. gasterostei was found only in R. balthica and M. glutinosa. This supports the idea that the parasites have diverged in their use of different snail species in completing their life-cycle, which could reduce the overlap in life-histories. Previous studies have suggested that trematodes show high specificity in their mollusc intermediate hosts (e.g. Gibson and Bray, 1994) although this paradigm has been questioned in recent studies (Donald et al. 2004). In general, parasites should specialize to common host genotypes or species (e.g. Lively and Jokela, 1996; Lively and Dybdahl, 2000), which might facilitate transmission and increase overall parasite fitness. However, if two or more host species are equally abundant, this may generate conditions for parasite divergence in host use especially if interactions between the parasite species in the principal host species decrease their fitness. In this study, relative numbers of the collected snails gave us a rough estimate of snail abundance indicating that all three species were somewhat equally abundant at the sampling location although in larger scale this is undoubtedly affected by the different habitat requirements of the species. Details of how the parasite species might differentiate between the hosts are unclear, but it is probable that the miracidia, which actively seek out their host using chemical signals (reviewed by Haas and Haberl, 1997), can recognize desired host species thus promoting specialization.
In fish caught from the same area, D. spathaceum was found mainly in roach whereas the abundance of D. gasterostei was significantly higher in perch. Since roach and perch commonly co-exist in the same habitats (Koli, 1990), it is likely that they are exposed to both parasite species. This suggests that there are differences between the parasite species in their ability to infect these hosts, which supports host specialization. However, diplostomids generally show low host specificity in their fish intermediate hosts; D. spathaceum, for instance, is known to infect a wide variety of fish species worldwide (e.g. Chappell et al. 1994; Valtonen and Gibson, 1997). This may be related to the uncertainty of the transmission process; individual cercariae are not able to infect the host from long distances (Karvonen et al. 2003) and perform mainly upward movements in the water column while waiting for the host to arrive. Under such circumstances, it would be advantageous to be less specific and accept more host species to ensure that some transmission takes place (Adamson and Caira, 1994). Parasite specificity is generally determined not only by the number of host species a parasite can infect but also by the relative infection levels in these hosts (Poulin and Mouillot, 2003). Thus, it is likely that some hosts with higher infection levels, such as roach and perch in the present study, play more important roles in the parasite life-cycles. The parasite species also infected different regions in the eyes of roach and perch, which supports the idea of evolutionary site specialization by the parasites. To verify this, we conducted experimental exposures using rainbow trout as a novel fish host, which excluded possible effects of co-evolutionary history between the parasites and fish on the site selection. These exposures indicated similar site selection by the parasites thus supporting true site specialization.
In accordance with our expectations, cercarial activity and life-span were very similar between the parasite species, which may reflect similarity in the requirements to infect the next fish host species. Indeed, roach and perch, taken here as principal fish hosts for these parasites, have very similar ecology (Koli, 1990). Thus, there may be little pressure on the parasites to diverge in terms of characteristics of the infective stages. Interestingly, the magnitude of cercarial release per unit of snail volume was higher in D. gasterostei, which suggests differences in strategies of host exploitation between the parasite species. However, the level of infection in fish, D. spathaceum in the lens of roach and D. gasterostei in the vitreous body of perch, was similar and factors underlying this, such as total cercarial output from the snail populations, require further work.
To summarize, we found evidence of divergence in the use of intermediate host species between D. spathaceum and D. gasterostei, which corroborate the prediction that closely related parasite species competing for the same resources should have diverged in their life-history characteristics and host use. However, very little is still known about the infections in the avian definitive hosts where crosses between closely related sympatric taxa are likely. For instance, it is not known if these parasite species have specialized to use different definitive host species or if they inhabit different locations within the intestine of birds (see Karvonen et al. 2006). Furthermore, although our results indicate that divergence has taken place between D. spathaceum and D. gasterostei, it is unclear how this is seen in the genetic architecture of these species. Studies investigating the genetics in diplostomids between different species, host species and populations are just now beginning to appear (Galazzo et al. 2002; Reusch et al. 2004; Rauch et al. 2005; see also Criscione et al. 2005) and they provide foundations for future studies on host specialization and taxonomy of diplostomids.
We thank Kosti Valtonen for assistance in snail collection, and Sanna Taskinen, Katri Liljeroos and Satu Paukku for their help in the laboratory. John Holmes gave valuable comments on the manuscript. This study was supported by Kone Foundation, Ella & Georg Ehrnrooth Foundation and the SUNARE research programme of the Academy of Finland.