Introduction
Echinococcosis refers principally to two severe chronic helminthic diseases, cystic echinococcosis and alveolar echinococcosis, caused by the cystic growth or the intrahepatic tumour-like growth of Echinococcus granulosus (E. granulosus) sensu lato and Echinococcus multilocularis, respectively (McManus et al., Reference McManus, Gray, Zhang and Yang2012; Singh et al., Reference Singh, Dhand, Ghatak and Gill2014; Gottstein et al., Reference Gottstein, Soboslay, Ortona, Wang, Siracusano and Vuitton2017; Wen et al., Reference Wen, Vuitton, Tuxun, Li, Vuitton, Zhang and McManus2019). Human cystic echinococcosis (hydatid disease) most commonly occurs in internal organs, especially the liver and lung (da Silva, Reference da Silva2010). Hydatid disease continues to be a substantial cause of morbidity and mortality in many parts of the world and is challenging to be eliminated (BelhassenGarcía et al., Reference BelhassenGarcía, Romeroalegria, Velascotirado, AlonsoSardón, Lopezbernus, Alvelasuarez, Del Villar, Carpioperez, Galindoperez and Corderosanchez2014). At present, the early diagnosis of echinococcosis is not precise, and the prevention effect is low. Surgical operation is the primary treatment for hydatidosis, but the injury is severe. Moreover, the cysts are easy to rupture during the surgery, and the protoscoleces in the cysts may enter the tissue and cause re-infection (Toumi et al., Reference Toumi, Noomen, Salem, Rabeh, Jabra, Korbi, Bannani, Nasr, Zouari, Mondher and Hamdi2017). Therefore, further exploring the interaction between E. granulosus sensu lato and the host in the early stage of infection is of great significance for developing novel immunotherapies.
Parasitic infections induce host immune responses that eliminate the invading parasites. However, parasites have evolved to develop effective strategies to evade host immune attacks and survive in a hostile environment for long periods (Shao et al., Reference Shao, Sun, Chen, Zhan and Zhu2019). Echinococcus granulosus sensu lato could escape host immunosurveillance by interfering with monocyte differentiation and DC maturation (Wang et al., Reference Wang, Wang, Lv and Zhang2015a, Reference Wang, Zhou, Shen, Wang, Wu, Liu, Yuan, Xu, Hu and Caob). In addition to the fibrous cyst's physical barrier, components in the E. granulosus cyst fluid (EgCF) are involved in the escape from the host's immune response (Li et al., Reference Li, Ju, Wang, Zhang, Li, Zhu and Zhao2013; Zhang et al., Reference Zhang, Wang, Ali, Li, Bi, Wang, Lu, Shao, Vuitton, Wen and Lin2016). Hydatid fluid is a complex mixture of components derived from both the parasite and the host (Ahn et al., Reference Ahn, Han, Bae, Ma, Kim, Cai, Yang, Kang, Wang and Kong2015). Proteomic analysis of hydatid cyst fluid from different anatomical locations revealed variations in protein expression and the extensive exchange of proteins between the metacestode and surrounding host environment cross the cyst wall through unknown mechanisms (Monteiro et al., Reference Monteiro, de Carvalho, Zaha and Ferreira2010; Zeghir-Bouteldja et al., Reference Zeghir-Bouteldja, Polome, Bousbata and Touil-Boukoffa2017; Zhou et al., Reference Zhou, Wang, Cui, Shi, Ma, Yu, Zhao and Zhao2019). Cyst fluid is a massive reservoir of immune-modulators, such as serodiagnostic antigens, low molecular mass metabolites and extracellular vesicles (Diaz, Reference Diaz2017; Zhou et al., Reference Zhou, Wang, Cui, Shi, Ma, Yu, Zhao and Zhao2019). Besides protoscoleces, various immunomodulatory effects have been attributed to EgCF (Mokhtari Amirmajdi et al., Reference Mokhtari Amirmajdi, Sankian, Eftekharzadeh Mashhadi, Varasteh, Vahedi, Sadrizadeh and Spotin2011; Wang et al., Reference Wang, Wang, Lv and Zhang2015a; Zhou et al., Reference Zhou, Wang, Cui, Shi, Ma, Yu, Zhao and Zhao2019; Yang et al., Reference Yang, Wu, Fu, Yan, Li, Guo, Zhang, Wang, Shen, Cho and Zheng2021). We have previously demonstrated glycomolecules in EgCF could interfere with the Toll-like receptor 4 (TLR4)-mediated activation of DCs and the downstream inflammatory factors (Hou et al., Reference Hou, Li, Dong, Wang, Wang, Yang, Xu, Chen, Wu and Chen2020). Additionally, EgCF could promote T lymphocytes' differentiation into Treg cells and induce transforming growth factor-β secretion, subsequently impairing NK cell effector function (Yin et al., Reference Yin, Chen, Zhang, Xu, Hou, Wu and Chen2014). Nevertheless, how EgCF modulates the inflammatory response in macrophages remains poorly understood.
Macrophages play a critical role in the initiation of inflammation by releasing inflammatory mediators and pro-inflammatory cytokines. Macrophages recognize microorganisms via a limited number of pattern recognition receptors, including TLRs, which play crucial roles in innate immune responses (Iwasaki and Medzhitov, Reference Iwasaki and Medzhitov2015; Browne, Reference Browne2020). Recognition of pathogen components such as lipopolysaccharides (LPS) by TLR4 on macrophages results in the activation of mitogen-activated protein kinases (MAPKs) and the transcription factor nuclear factor-κB (NF-κB) signalling pathways, leading to the production of pro-inflammatory cytokines (Schmid et al., Reference Schmid, Gruber, Piskaty, Woehs, Renner, Nagy, Kaltenboeck, Wasserscheid, Bazylko and Kiss2012; Vidya et al., Reference Vidya, Kumar, Sejian, Bagath, Krishnan and Bhatta2018). Tumour necrosis factor receptor-associated factors (TRAFs) form a family of adapter molecules, coupling the tumour necrosis factor receptor (TNFR) superfamily to intracellular signalling events (Yang and Sun, Reference Yang and Sun2015; Park, Reference Park2018). Among them, TRAF6 plays a pivotal role in the TLR4-mediated signalling pathway. TRAF6 forms a homo-dimer and catalyses K63-linked ubiquitination; it can also be modified by K48-linked ubiquitination and proteasomal degraded to block the inflammatory process (Walsh et al., Reference Walsh, Lee and Choi2015). For instance, Leishmania donovani infection impairs K63-linked ubiquitination of TRAF6 by induction of host deubiquitinating enzyme A20 and suppresses the production of interleukin (IL)-12 and TNF-α (Srivastav et al., Reference Srivastav, Kar, Chande, Mukhopadhyaya and Das2012). Death-associated protein kinase-related apoptosis-inducing kinase 1 inhibits the pro-inflammatory signalling pathway by targeting TRAF6 for degradation, thereby suppressing inflammatory signalling-mediated tumour growth and metastasis in advanced cervical cancer cells (Park et al., Reference Park, Pang, Park, Hong, Lee, Ooshima, Kim, Cho, Han, Lee, Song, Park, Yang and Kim2020). However, the role of TRAF6 in macrophages during E. granulosus sensu lato infection remains poorly understood.
In this study, we speculated that EgCF might regulate inflammation response by targeting TRAF6 protein. Using three macrophage models, we provided evidence that EgCF could promote the proteasomal degradation of TRAF6 and suppress the downstream activation of NF-κB and MAPK signalling pathways and subsequent cytokine production.
Materials and methods
Cells and cell culture
Mouse peritoneal macrophages were isolated from C57BL/6 female mice (6–8 weeks, 16–25 g). Briefly, mice were sacrificed by cervical dislocation. Subsequently, mice were injected intraperitoneally with Dulbecco's modified Eagle's medium (DMEM) using a 5 mL syringe. The abdomen was gently massaged without removing the needle, then most of the fluid from the peritoneum was slowly withdrawn with the syringe (You-Nian et al., Reference You-Nian, Liu, Meng, Anesthesiology and Hospital2016). Collected peritoneal exudate cells were washed with phosphate-buffered saline (PBS) three times and centrifuged at 1500 rpm for 5 min. Cells were resuspended and plated in six-well plates at a density of 1 × 106 cells per well in DMEM containing l-glutamine supplemented with 10% fetal bovine serum (FBS) according to previous reports (Chen et al., Reference Chen, Fuller, Dunlap and Loros2020; Gao et al., Reference Gao, Wei, Chen, Chen, Ding, Ding, Wu, Du and Cao2020; He et al., Reference He, Jiang, Song, Riezman, Tontonoz, Weston, Guagliardo, Kim, Jung, Heizer, Fong and Young2020). After overnight culture, the non-adherent cells were removed, and the attached cells (enriched in peritoneal macrophages) were treated with LPS (1 μg mL−1) or LPS and EgCF (2.15 mg mL−1) for the indicated periods as a previous report (Hou et al., Reference Hou, Li, Dong, Wang, Wang, Yang, Xu, Chen, Wu and Chen2020).
The mouse macrophage cell line RAW 264.7 and human monocytic cell line THP-1 were purchased from the Cell Bank of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. RAW 264.7 cells were cultured in DMEM (Gibco) supplemented with 10% FBS, 1% penicillin–streptomycin (Gibco) in a humidified incubator with 5% CO2 at 37°C following the provider's instructions. THP-1 cells were maintained in RPMI 1640 medium (Gibco) containing 10% FBS, 1% penicillin–streptomycin and 0.05 mm β-mercaptoethanol (Gibco). THP-1 cells were differentiated into macrophages by 150 nm PMA treatment for 24 h.
Preparation of Echinococcus granulosus cyst fluid
Sheep liver hydatid cysts containing protoscoleces were acquired from a slaughterhouse in Shihezi, Xinjiang, China. After sterilizing the E. granulosus sensu lato-infected sheep liver with 75% alcohol, cyst fluid in the non-calcified translucent vesicles was extracted using a 50 mL sterile syringe. The upper layer mixture after standing was collected and centrifuged at 3000 rpm for 10 min. The resulting supernatant from different sheep livers was mixed, filtered through a 0.22-μ m filter, quantified for protein concentration and stored in a −80°C refrigerator for later use.
Quantitative real-time polymerase chain reaction (PCR)
RNA was extracted using a total RNA kit (Omega) and converted into cDNA using the RevertAid RT Reverse Transcription Kit (Thermo Scientific). The primer sequences are listed in Table 1. Quantitative real-time PCR was performed on a CFX96 Touch detection system (Bio-Rad) using the TB Green Premix Ex Taq II (Tli RNaseH Plus; TaKaRa). Relative expression levels of target genes were calculated by normalizing to β-actin using the 2−ΔΔCt method and expressed as fold-changes to that of the control group.
Table 1. Primer sequences for real-time PCR

Enzyme-linked immunosorbent assays (ELISAs)
Cell culture medium from mouse peritoneal macrophages or RAW 264.7 cells treated with LPS with or without EgCF was collected and centrifuged at 3000 rpm for 10 min. The supernatants were collected and used for detecting TNF-α, IL-6 and IL-10 concentrations by mouse ELISA kits according to the manufacturer's instructions (Multisciences Biotech).
Western blot analysis
Cells were harvested at indicated times in the RIPA lysis buffer. Samples were electrophoretically separated on a 10% polyacrylamide gel, transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% bovine serum albumin for 2 h at room temperature, followed by incubation at 4°C overnight with primary antibodies specific to phospho-NF-κB p65 (p-p65, #3033), NF-κB p65 (#3987), phospho-p38 MAPK (p-p38, #9211), p38 MAPK (#9212), phospho-extracellular signal-regulated kinase (ERK)1/2 (p-ERK1/2, #4370), ERK1/2 (#4695) from Cell Signalling Technology, TRAF6 (#ab137452) from Abcam and β-actin (#TA-09) from ZSGB-BIO. Following washing three times for 10 min with 0.1% Tween-20 in TBST, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. After washing, the immune complexes were detected by an enhanced chemiluminescence assay (Thermo Fisher). β-Actin was served as a loading control.
Immunofluorescence assay
Cells grown on glass coverslips were fixed in 4% paraformaldehyde for 30 min and permeabilized with 0.1% Triton X-100. Then cells were washed three times with PBS and blocked with 1% bovine serum albumin (BSA) for 1 h at 25°C. Samples were incubated with the primary antibody against p65 in PBST and 1% BSA overnight at 4°C. Subsequently, cells were washed three times in PBS, incubated with Rhodamine-conjugated Goat anti-Rabbit IgG (H + L) Secondary Antibody (ZSGB-BIO #ZF-0316) for 1 h at room temperature, and counterstained with diamidino-2-phenylindole. The images were observed under a fluorescence microscope (Olympus).
Statistical analysis
All experiments were independently repeated with similar results. Data are represented as mean ± s.e.m. of at least three replicates. Statistical analyses were performed using GraphPad Prism version 8.0 software. Comparison between groups was made using Student's t test or one-way analysis of variance. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
Results
EgCF inhibits the LPS-induced inflammatory response in mouse peritoneal macrophages
Production of inflammatory mediators is a crucial response of macrophages to LPS stimulation. To figure out whether EgCF exerts an inhibitory effect on LPS-induced macrophage activation, primary mouse peritoneal macrophages were treated with EgCF and LPS. Real-time PCR analysis showed that LPS highly induced the mRNA levels of TNF-α, IL-12 and IL-6. In contrast, EgCF exposure significantly attenuated the expression of the inflammatory cytokines evoked by LPS at 1 h post-treatment and lasted to 6 h post-treatment but enhanced IL-10 mRNA levels compared to the LPS alone group (Fig. 1A–D). Furthermore, we measured these cytokines in the cell culture supernatant by ELISA. The LPS-induced secretion of TNF-α and IL-6 protein in the cell culture supernatant was also dramatically inhibited, whereas IL-10 protein secretion was elevated in the presence of EgCF (Fig. 1E–G). Taken together, these results indicated that EgCF caused a shift from pro-inflammatory to anti-inflammatory cytokine production in mouse peritoneal macrophages.

Fig. 1. EgCF inhibits LPS-induced inflammatory response in mouse peritoneal macrophages. Mouse peritoneal macrophages were treated with EgCF (2.15 mg mL−1) and LPS (1 μg mL−1) for the indicated time points. The mRNA expression levels of TNF-α (A), IL-12 (B), IL-6 (C) and IL-10 (D) were analysed by real-time PCR. Mouse peritoneal macrophages were incubated with LPS in the presence or absence of EgCF for 24 h. Secretion of TNF-α (E), IL-6 (F) and IL-10 (G) in the cell culture supernatants was measured by ELISA.
EgCF inhibits the LPS-induced inflammatory response in RAW 264.7 cells
To further validate the immunomodulatory effect of EgCF in macrophages, the RAW 264.7 cell line was also treated with EgCF and LPS. Results showed that EgCF could dramatically inhibit the LPS-induced mRNA expression of IL-6 and TNF-α (Fig. 2A and B). In contrast, the mRNA expression of IL-10 significantly increased at 6 h upon exposure to EgCF in RAW 264.7 cells (Fig. 2C). Exposure with EgCF also suppressed the LPS-induced IL-6 protein production while enhancing the secretion of IL-10 in RAW 264.7 cells (Fig. 2D and E). These results confirmed that EgCF suppressed the production of pro-inflammatory factors and exerted an anti-inflammatory potential in mouse macrophages.

Fig. 2. EgCF suppresses LPS-induced inflammatory response in RAW 264.7 cells. RAW 264.7 cells were treated with EgCF (2.15 mg mL−1) and LPS (1 μg mL−1) for the periods indicated. The mRNA expression levels of IL-6 (A), TNF-α (B) and IL-10 (C) were analysed by real-time PCR. RAW 264.7 were incubated with LPS in the presence or absence of EgCF for 24 h. Secretion of IL-6 (D) and IL-10 (E) in cell culture supernatants was measured by ELISA.
EgCF suppresses the LPS-induced activation of NF-κB and MAPK signalling and promotes the proteasomal degradation of TRAF6 in mouse macrophages
It is well established that NF-κB and MAPKs have essential roles in the LPS-induced inflammation process. However, the signal transduction mechanisms involved in EgCF-exposed macrophages remain poorly understood. These cascades' activation was checked by detecting phosphorylation levels of NF-κB p65, p38 MAPK and ERK1/2 using immunoblot. Intriguingly, results showed that the LPS-induced NF-κB p65 phosphorylation was inhibited in RAW 264.7 cells post-EgCF treatment. Besides, EgCF also suppressed the LPS-induced phosphorylation of p38 MAPK and ERK1/2 in RAW 264.7 cells (Fig. 3A). Similar inhibition of the LPS-induced activation of p65, p38 and ERK1/2 signalling was also observed in mouse peritoneal macrophages (Fig. 3B). Furthermore, immunofluorescence assay indicated that the LPS-induced nuclear translocation of NF-κB p65 was abolished in EgCF-treated mouse peritoneal macrophages (Fig. 3C). These findings collectively suggested that EgCF suppressed the LPS-induced activation of NF-κB and MAPK pathways in mouse macrophages.

Fig. 3. EgCF inhibits LPS-induced NF-κB and MAPK signalling activation in mouse macrophages. RAW 264.7 cells (A) and mouse peritoneal macrophages (B) were treated with EgCF and LPS for the indicated time points. Activation status of NF-κB p65, p38 MAPK and ERK1/2 signalling was detected via western blotting. PM, peritoneal macrophages. (C) Mouse peritoneal macrophages were incubated with LPS in the presence or absence of EgCF for 4 h. Cells were subjected to immunofluorescence assay for NF-κB p65 nuclear translocation, and the rates of peritoneal macrophages with nuclear p65 staining were quantified.
As previously reported, TRAF6 could mediate signal transduction from the TLR family, and it is a key adaptor for the activation of the NF-κB signalling pathway. Therefore, we reasoned that EgCF might modulate the NF-κB signalling through modulation of TRAF6. To verify the hypothesis, we further measured the TRAF6 expression level upon EgCF treatment by western blotting. The results showed that the expression of TRAF6 protein markedly decreased in both EgCF-treated RAW 264.7 cells and mouse peritoneal macrophages when compared to the LPS alone group (Fig. 4A and B), in concordance with the reduced NF-κB p65 phosphorylation. To explore whether EgCF might affect the degradation of TRAF6 protein, we treated RAW 264.7 cells with the protein synthesis inhibitor cycloheximide (CHX), an inhibitor of protein synthesis. Indeed, results showed that EgCF could accelerate TRAF6 protein degradation (Fig. 4C), indicating the post-translational modification of TRAF6 by EgCF. To examine whether the shortened TRAF6 half-life was mediated through the ubiquitin–proteasome pathway, we treated RAW 264.7 cells with the proteasome inhibitor MG132. MG132 treatment rescued TRAF6 protein levels in EgCF-exposed mouse macrophages (Fig. 4D), suggesting that EgCF promoted TRAF6 degradation in a proteasome-dependent manner.

Fig. 4. EgCF promotes the proteasomal degradation of TRAF6 in mouse macrophages. RAW 264.7 cells (A) and mouse peritoneal macrophages (B) were treated with EgCF and LPS for the periods indicated. TRAF6 protein levels were evaluated by western blotting. PM, peritoneal macrophages. (C) The protein expression of TRAF6 was checked by western blotting in RAW 264.7 cells treated with EgCF and LPS in the presence of CHX (50 μg mL−1) for 2 or 4 h. (D) RAW 264.7 cells were treated with EgCF and LPS in the presence of MG132 (20 μ m) to block the proteasomal degradation for 4 h. Western blotting was used to analyse TRAF6 protein levels.
EgCF inhibits the LPS-induced inflammatory response by promoting the proteasomal degradation of TRAF6 in human macrophages
To determine whether the EgCF's anti-inflammatory effect observed in mouse macrophages also exists in human, we measured the inflammatory response in human THP-1 cells. As shown in Fig. 5A and B, a marked inhibitory effect of EgCF treatment on the mRNA expression of LPS-induced pro-inflammatory cytokines IL-6 and TNF-α was observed in THP-1 macrophages. We also examined the phosphorylation of critical components in the NF-κB and MAPK signalling pathways in THP-1 cells. Compared to the LPS group, the phosphorylation of p65 in NF-κB signalling pathway and p38, ERK1/2 in MAPK signalling pathway was decreased in a time-dependent manner in the EgCF-treated group (Fig. 5C), consistent with the phenomenon observed in mouse RAW 264.7 cells and primary peritoneal macrophages. Western blotting showed that EgCF treatment decreased the protein stability of TRAF6 in the presence of CHX (Fig. 5D), whereas MG132 could rescue the expression of TRAF6 in THP-1 cells (Fig. 5E), suggesting that the EgCF-promoted anti-inflammatory response and TRAF6 proteasomal degradation were conserved in human macrophages.

Fig. 5. EgCF inhibits LPS-induced inflammatory response by promoting proteasomal degradation of TRAF6 in human macrophages. Relative mRNA expression levels of IL-6 (A) and TNF-α (B) in THP-1 cells treated with EgCF and LPS for the indicated time points were measured by real-time PCR. (C) The protein levels TRAF6 and the activation status of NF-κB p65, p38 MAPK and ERK1/2 signalling in THP-1 cells stimulated with LPS and EgCF for the periods indicated were checked by western blotting. (D) THP-1 cells were treated with EgCF and LPS in the presence of CHX (50 μg mL−1) for 2 or 4 h. Western blotting was used to analyse TRAF6 protein expression. (E) The protein levels of TRAF6 were evaluated by western blotting in THP-1 cells treated with EgCF and LPS in the presence of MG132 (20 μ m) to block the proteasomal degradation for 4 h.
Discussion
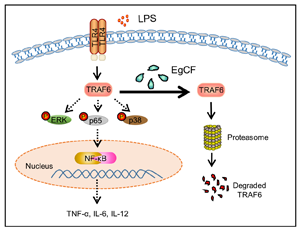
Cystic echinococcosis is a chronic, complex and neglected zoonotic infection. As a globally distributed parasitic disease, the infection of E. granulosus sensu lato poses a considerable threat to human life (OudniM'rad et al., Reference OudniM'rad, M'rad, Ksia, Lamiri, Mekki, Nouri, Mezhoud and Babba2015). Echinococcus granulosus sensu lato has elaborate defence mechanisms, protecting it from the anti-parasite immune responses (Siracusano et al., Reference Siracusano, Delunardo, Teggi and Ortona2012). In this study, it was demonstrated that EgCF inhibited the activation of NF-κB and MAPK signalling pathways and the pro-inflammatory responses by promoting the proteasomal degradation of TRAF6 in both human and mouse macrophages (Fig. 6).

Fig. 6. Working model for EgCF suppressing LPS-induced inflammatory response in macrophages. EgCF promotes the proteasomal degradation of TRAF6 and subsequently impairs the LPS-induced activation of NF-κB and MAPKs signalling pathways and thus the inflammatory response.
Macrophages are essential cells for inflammatory processes involved in regulating acute inflammatory response and chronic pathological changes (Hasturk et al., Reference Hasturk, Kantarci and Van Dyke2012). Bacterial LPS was a typical ligand for membrane protein TLR4, which can induce the host's innate immune response (Peri et al., Reference Peri, Piazza, Calabrese, Damore and Cighetti2010). In this study, we utilized three different macrophages to investigate the roles of EgCF in the inflammation process. Pro-inflammatory cytokines TNF-α, IL-12 and IL-6, which are regarded as biomarkers of inflammatory processes (Rossol et al., Reference Rossol, Heine, Meusch, Quandt, Klein, Sweet and Hauschildt2011), increased dramatically over the following several hours following LPS stimulation. We provided sufficient and systemic evidence that EgCF reduced the LPS-induced production of pro-inflammatory mediators and cytokines (TNF-α, IL-6 and IL-12) at mRNA and protein levels not only in the mouse RAW 264.7 cell line and primary mouse peritoneal macrophages but also in human THP-1 cells. In line with our findings, E. multilocularis vesicles are also able to suppress the LPS-induced release of IL-12 and TNF-α by PBMC (Hübner et al., Reference Hübner, Manfras, Margos, Eiffler, Hoffmann, Schulz-Key, Kern and Soboslay2006).
NF-κB and MAPK pathways are two critical pathways that participated in the LPS/TLR4 signalling cascades. Here, we detected multiple key components in these signalling pathways and found that phosphorylation of NF-κB p65 was significantly downregulated in all three macrophages after EgCF treatment. Consistently, the inactivation of NF-κB signalling has been reported to be an immune evasion approach shared by other parasites. For instance, Giardia lamblia decreases NF-κB p65 protein levels and impairs LPS-evoked pro-inflammatory response in mouse RAW 264.7 cells and human monocyte-derived macrophages (Faria et al., Reference Faria, Neves, Lourenço, Cruz, Martins, Silva, Pereira and Sousa2020). Additionally, EgCF suppressed the LPS-induced phosphorylation of p38 MAPK and ERK1/2 in macrophages. In contrast, p38 MAPK and ERK1/2 are activated in rat hepatocytes upon exposure to E. multilocularis vesicle fluid (Lin et al., Reference Lin, Wang, Lu, Zhou, Mantion, Wen, Vuitton and Richert2009), suggesting context-dependent effects of these two metacestodes on the host environment.
A recent study shows that resveratrol could suppress NF-κB activation by attenuating TRAF6 expression and ubiquitination (Jakus et al., Reference Jakus, Kalman, Antus, Radnai, Tucsek, Gallyas, Sumegi and Veres2013; Wang et al., Reference Wang, Hu, Fu, Song, Cui, Jia, Zou, He, Li and Yin2017). As an essential molecule in the TLR-induced NF-κB and MAPK signalling, TRAF6 has been demonstrated to be tightly regulated. TRAF6 can be ubiquitinated with both K48-linked degradative and K63-linked non-degradative chains at multiple locations. K63-linked autoubiquitination of TRAF6 is essential for the formation and activation of the TAK1 complex and NF-κB signalling (Yanbao et al., Reference Yanbao, Fu, Wenji, Chang, Wahl, Medvedev and Jbc2011). TRAF6 can also be modified by K48-linked ubiquitination, which promotes TRAF6 degradation through proteasome and blocks the inflammatory signal transduction in the TLR4 pathway (Zhou et al., Reference Zhou, Ma, Shi and Huo2010; Zhao et al., Reference Zhao, Wang, Zhang, Yuan and Gao2012). We assumed that EgCF negatively regulated inflammation by targeting TRAF6. Our study verified that EgCF could promote the degradation of TRAF6 in a proteasome-dependent manner, confirming that EgCF could inhibit the activation of NF-κB and MAPK signalling pathways by promoting proteasomal degradation of TRAF6.
In conclusion, we demonstrate that EgCF could promote the proteasomal degradation of TRAF6 and then inhibit the activation of NF-κB and MAPK signalling pathways and negatively regulate the inflammatory response. To better understand the E. granulosus-induced immune evasion, how E. granulosus cyst fluid makes contact with the immune system, and which component functions to suppress macrophages warrant further investigation.
Data
All data generated or analysed during this study are included in this published article.
Author contributions
JH, XC and KL conceived and designed the study. KL, DZ, ML, JM, FH, XY, DD, XW and XW conducted data gathering. KL performed statistical analyses. KL, XC and JH wrote the article.
Financial support
This study was supported by the National Natural Science Foundation of China (Nos. 82060579 and 82060297).
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Animal study was performed with approval from the Animal Experimental Ethical Inspection of First Affiliated Hospital, Shihezi University School of Medicine.