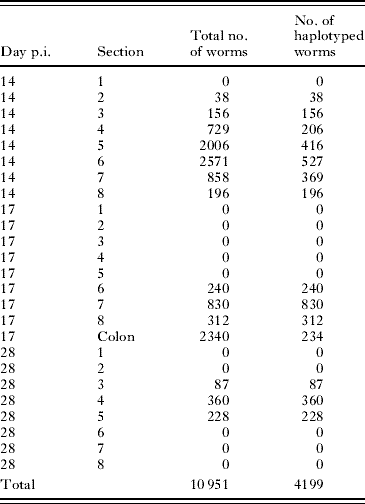INTRODUCTION
Both host and parasite and the interaction between them may determine the outcome of an infection by a parasite. The importance of host genetics is evident from a number of field studies that have revealed a substantial effect of host genetics on parasite burdens. Approximately 20–50% of the variation in egg or worm counts in both humans and animals can be explained by host genetics (e.g. Williams-Blangero et al. Reference Williams-Blangero, Subedi, Upadhayay, Manral, Rai, Jha, Robinson and Blangero1999; Gauly and Erhardt, Reference Gauly and Erhardt2001). The impact of parasite genetics has been estimated in a number of laboratory systems. For instance, 3 isolates of Trichuris muris have been shown to differ in their worm expulsion kinetics in different strains of mice (Bellaby et al. Reference Bellaby, Robinson, Wakelin and Behnke1995; Koyama and Ito, Reference Koyama and Ito1996; Johnston et al. Reference Johnston, Bradley, Behnke, Matthews and Else2005). Also, Paterson and Viney (Reference Paterson and Viney2003) showed that different genotypes of Strongyloides ratti vary in survival and fecundity in rats. For both T. muris and S. ratti, the effect of the parasite genotypes had consequences in terms of the genetic composition of eggs excreted in the faeces of the host and thus influenced the genetic composition of the next generation. Parasite genotype may also influence the immunological response(s) elicited in the host. Different isolates of T. muris influence the Th1/Th2 balance in the mouse host (Koyama and Ito, Reference Koyama and Ito1996) in an isolate-specific manner (Bellaby et al. Reference Bellaby, Robinson and Wakelin1996). The findings of these studies suggest that the interplay between host and parasite is influenced by both partners and thus influences their co-evolution. However, these studies have utilized parasite isolates which have been passaged in rodent hosts, such that it is unclear whether selection has influenced the variation in infection phenotypes observed.
Ascaris suum, a parasitic nematode of pigs, is closely related to the common roundworm of humans that infects 25% of people worldwide (Chan et al. Reference Chan, Medley, Jamison and Bundy1994). The pig model system has many advantageous attributes for experimental studies; controlled doses of infective stages can be given and the infection process can be followed closely by examining the distribution of larval worms and adults at different time-points following inoculation. Furthermore, the number of adults surviving varies among individual pigs, generating the characteristic aggregated distribution of helminth infections (e.g. Roepstorff et al. Reference Roepstorff, Eriksen, Slotved and Nansen1997). We are particularly interested in exploring the effect of different parasite genotypes (of a species) on their ability to develop to maturity within the host, determining to what degree the genetic composition of a parasite population is influenced by the host and establishing whether different hosts select specific parasite genotypes.
We have shown that host genetics explains 30–45% of the variance in egg counts and worm burdens (=intensity of infection), following controlled infections with genetically heterogeneous nematode eggs in a pig pedigree (Nejsum et al., unpublished observations). In the present study, we investigated the influence of parasite genetics on infection dynamics. To do this, we generated 4 populations of A. suum, each derived from and individual, unrelated adult female with a unique mitochondrial DNA (mtDNA) haplotype. Hence, the progeny from each of these ‘isofemale lines’ consisted of a mixture of full and half siblings marked by a unique maternally inherited haplotype (cf. Anderson et al. Reference Anderson, Komuniecki, Komuniecki and Jaenike1995a; Nejsum et al. Reference Nejsum, Thamsborg, Jørgensen, Fredholm and Roepstorff2008). We infected pigs with a mixture of embryonated eggs in equal proportions carrying these 4 haplotypes and then monitored the outcome of infection at time-points expected to coincide with the phases before, during and after the predominant immune response in the host (see Miquel et al. Reference Miquel, Roepstorff, Bailey and Eriksen2005). The results revealed (1) significant differences in abundance and size of nematodes with the 4 different mtDNA haplotypes in different pigs and (2) differential distributions of individual haplotypes and worm lengths in sections of the small intestine.
MATERIALS AND METHODS
Production of genetically marked Ascaris eggs
Eggs from 4 adult Ascaris suum, originating from different pig farms in Denmark, were isolated. The embryonation rate was 99% for eggs isolated from female A, C and D, and 92% for eggs from female B. The offspring from the 4 worms could be differentiated by polymerase chain reaction (PCR)-based restriction fragment length polymorphism (PCR-RFLP) analysis of mtDNA, as described by Nejsum et al. (Reference Nejsum, Thamsborg, Jørgensen, Fredholm and Roepstorff2008). Briefly, eggs were extracted from the lowest part of the uterus (3–4 cm) and ‘de-coated’ with 0·5% sodium hypochlorite (NaClO). The eggs were embryonated in 0·05 m H2SO4 at room temperature (22–24°C) for 12 weeks and then stored at 10°C.
Confirmation of the maternal inheritance of mtDNA
As described previously by Nejsum et al. (Reference Nejsum, Thamsborg, Jørgensen, Fredholm and Roepstorff2008), each of 4 Ascaris-naïve SPF-pigs were inoculated with 2000 embryonated eggs originating from 1 of the 4 females and the larvae were isolated by day 14 post-inoculation (p.i.) from the small intestine using an agar technique (Slotved et al. Reference Slotved, Barnes, Eriksen, Roepstorff, Nansen and Bjørn1997). Recovery rates of 26–57% were achieved, which is within the range (25–75%) usually obtained in the Danish Centre for Experimental Parasitology. DNA from the 4 A. suum females was extracted using QIAGEN tissue kit (Qiagen, Germany) and from single larvae, collected from each of the 4 pigs, genomic DNA was extracted using a worm lysis buffer, as described by Maafi et al. (Reference Maafi, Subbotin and Moens2003). A 633 bp fragment of the mtDNA, comprising 356 bp of NADH dehydrogenase subunit 4 gene (nad4), 117 bp of the non-coding spacer and 160 bp of the cytochrome c oxidase subunit 1 (cox1) gene, was amplified under the PCR conditions described by Anderson et al. (Reference Anderson, Komuniecki, Komuniecki and Jaenike1995a). The product was digested using 3 restriction endonucleases (SspI, Hpy188III and DraI) in one reaction and then separated in a 1·5% (w/v) agarose gel and stained in ethidium-bromide. The analysis of more than 400 larvae showed that each individual exhibited the same band pattern as the parental female. Therefore, the 4 haplotypes (each linked to individual females of A. suum) and their respective egg batches (designated A, B, C and D) could be unequivocally identified and distinguished based on their characteristic PCR-RFLP profiles.
Experimental infections
A single mixed-batch consisting of equal amounts of embryonated eggs (A, B, C and D) from each of the 4 A. suum females was produced. This batch was used to inoculate each of 26 Ascaris-naïve SPF-pigs (crossbred Landrace, Yorkshire and Duroc; 10 weeks of age) via a stomach tube with 2000 embryonated eggs. In this way, we ensured that all pigs were inoculated with the same proportions of eggs bearing the 4 mtDNA types. The pigs used were the outcome of mixed-semen insemination of 5 sows, which means that the piglets in a litter were either full- or half-siblings. The pigs were fed a standard diet consisting of ground barley with protein/mineral supplement and had free access to water. The pigs were randomly allocated to 3 groups after stratification for sex and sow and necropsied at day 14 (n=6), 17 (n=6) and 28 p.i. (n=14), which is expected to correspond to the phases before, during and after main immune responses, respectively (Roepstorff et al. Reference Roepstorff, Eriksen, Slotved and Nansen1997). At necropsy, the pigs were eviscerated and the small intestine was divided into 8 sections of similar lengths; the larvae were isolated using the agar technique, as described previously by Slotved et al. (Reference Slotved, Barnes, Eriksen, Roepstorff, Nansen and Bjørn1997) using a 3 h instead of an overnight incubation. At day 17 p.i., the larvae were also isolated from a 10% subsample of the colon content, in order to collect apparently ‘expelled worms’. All larvae were stored in 70% ethanol at 5°C.
Phenetic and genetic characterization of Ascaris larvae
For sections with high numbers of larvae (>240) at day 14 p.i. we subsampled in 250 ml of ethanol until at least 80 larvae were obtained for a given section. Subsequently, these worms were subjected to haplotypic analysis. We have shown that this subsampling method draws a random population of the worms with respect to length (Nejsum et al., unpublished findings). For all other sections all recovered larvae were subjected to haplotypic analysis. The length of each larva was measured employing a Leica MZ12 dissecting microscope linked with IC D Leica digital camera (for worms collected on days 14 and 17 p.i.) and using an ordinary scale at day 28 p.i. We are aware that the storage of worms in 70% ethanol can influence worm length, but expect that individual worms in this study were affected by fixation to the same degree, such that direct comparisons could be made.
After measuring the body lengths of worms on days 14 and 17 p.i., larvae were transferred to the bottom of individual wells of 96-well PCR-plates (Cat. no. 72.1978.202, Sarstedt) whereas for day 28 p.i. larvae only the anterior tip (2–3 mm) of individual worms were cut off and used for molecular analysis. Genomic DNA was extracted and the larvae were subjected to haplotypic analysis. DNA samples representing each of the females A, B, C and D were included as controls in all steps of the molecular analysis to ensure accurate identification of the progeny.
Statistical methods
Separate analyses of worms recovered on days 14, 17 and 28 p.i. were performed using SAS®, version 9.1. (SAS Institute, North Carolina, USA). We first analysed the overall distribution of haplotypes (A, B, C and D) using multinomial logistic regression, with all 4 types as response variables, and sections of the small intestine (1–8) and pigs as explanatory variables. The link-function, generalized logit, was used to integrate the discrete response data into the linear model. Subsequently, the distribution of each of the 4 haplotypes were analysed independently (e.g. A versus non-A) with generalized linear models with section (1–8) and pig included as explanatory variables. This analysis assumed a binomial distribution, and logit was used as the link function. The scale parameter (used for adjusting all statistics) was fixed to 1 to correct for overdispersion.
We also examined the distribution of haplotypes among hosts (on days 14, 17 and 28) using F-statistics. The program FSTAT v. 2.9.3.2 (Goudet, Reference Goudet1995) was used to calculate F-statistics, as described by Weir and Cockerham (Reference Weir and Cockerham1984). Here, the proportion of the total genetic variance which is subdivided between hosts is calculated and is therefore an estimate of the degree of aggregation of genetically related parasites. Each of the 4 haplotypes was coded as an allele and analysis performed for each day p.i. and among the 3 time-points. In total, 10 000 permutations of individuals were performed for statistical testing. The null hypothesis was that F ST should not differ significantly from 0, because all pigs were inoculated from a single pool of eggs. We also compared the magnitude of F ST observed in parasite populations at 14, 17 and 28 days p.i. This was done using the ‘comparison among groups of samples’ permutation option implemented by FSTAT v. 2.9.3.2. In this analysis, the haplotypes of worms from individual pigs were permuted between time-periods (days 14, 17 and 28 p.i.), keeping the number of pigs in each time-period constant. The difference in F ST values between 2 time-periods was assessed in the randomized data sets. The P-value of the test was then taken as the proportion of randomized data sets, giving a larger difference in F ST than in the observed data.
We used mixed-effect models (ANOVA) with ‘pig’ included as a random variable to analyse the response variable ‘worm length’. In this analysis, section of the small intestine (1–8) and haplotype and interaction between the two were included as explanatory variables. In these models, pairwise differences in worm length among different intestinal sections and the 4 haplotypes was analysed by comparisons of mean values. Finally, the effect of abundance of worm haplotype and pig on worm length was analysed using general linear models (ANOVA). The analysis was performed separately for each time-point (day 14, 17 or 28 p.i.). General linear models were used to test for normality and homogeneity of variation among the residuals, and the plots were visually inspected.
To further examine the contribution of parasite genetics to length, which may be an important fitness-related trait in many organisms (e.g. Stear and Bishop, Reference Stear and Bishop1999), we performed a heritability analysis using a variance components approach employing the software package SOLAR (Almasy and Blangero, Reference Almasy and Blangero1998). In this analysis, the phenotypic variance (the length of the worms) is decomposed into the effects of genetic factors, shared environmental factors (the pigs host) and individual-specific factors due to the unique environmental exposures (the different sections of the small intestine) and measurement error. The heritability analysis was performed for each of the 3 time-points (14, 17 and 28 p.i.), assuming that the progeny from each female were either all full siblings or all half-siblings expecting that the reality will be somewhere in between.
RESULTS
Dynamics of experimental infections
At necropsy on days 14, 17 and 28 p.i. 6/6 (100%), 5/6 (80%) and 5/14 (36%) pigs harboured worms, respectively. Two pairs of pigs (#61/#73 and #58/#59), sampled on days 14 and 17 p.i., respectively, were siblings, whereas the other pigs harbouring worms at each time-point were unrelated. On day 28 p.i., 2 pigs harboured only 1 and 4 worms, respectively and these were excluded from the statistical analyses. On day 14 p.i., the recovery rate was 27–75%, similar to those usually obtained (e.g. Roepstorff et al. Reference Roepstorff, Eriksen, Slotved and Nansen1997). The number of larvae analysed and total number of larvae in each section for each day p.i. is shown in Table 1. The mtDNA haplotypes of individual worms were determined for 1908 (29%), 1616 (100%), 675 (100%) worms collected on days 14, 17 and 28 p.i., respectively. On days 14 and 17 p.i., 70 and 12 larvae, respectively, failed to amplify by the PCR and, thus, the haplotypes of these worms could not be determined. On days 14, 17 and 28 p.i., 5, 3 and 35 worms were not intact and thus were not included in the length analysis but were subjected to haplotypic analysis. The number of worms recovered from each intestinal section illustrates the dynamics of the worm population of Ascaris before, during and after main immune responses (see Table 1). The distribution of Ascaris larvae in the small intestine varied substantially among the 3 sampling time-points, in agreement with previous observations by Roepstorff et al. (Reference Roepstorff, Eriksen, Slotved and Nansen1997).
Table 1. The total number of worms recovered from 8 different sections of equal length of the small intestine at 3 time periods post inoculation (p.i.)
(Also indicated are the number of individuals for which their mtDNA haplotypes were determined. The intestinal sections were of equal length and pigs inoculation with 2000 embryonated Ascaris eggs. Sections with high numbers of larvae (>240) at day 14 p.i. were subsampled until at least 80 larvae were obtained for a given section and these worms where then haplotyped. At day 17 p.i. worms were also recovered from a subsample of 10% of the intestinal content the colon.)

Abundance of the four mtDNA haplotypes in different pigs
Fig. 1 shows the percentage distribution of the 4 haplotypes in individual pigs and the average number for all pigs at each of the 3 time-points. The average percentages of haplotypes recovered from the colon of all pigs on day 17 p.i. were 23·9, 20·1, 26·5 and 29·5% for haplotypes A, B, C and D, respectively. The distribution of haplotypes among hosts was uneven at each of the 3 sampling time-points. For example on day 28, worms with haplotype A predominated in pig #51, whereas this haplotype was in the minority in pig #63. Furthermore, the degree of evenness decreased during the course of infection. The F-statistics showed an increased degree of aggregation of similar haplotypes within hosts with time: F ST was 0·006, 0·012 and 0·053 on days 14, 17 and 28 p.i., respectively, with all F ST estimates being significant different from 0 (P<0·05). The permutation of worm haplotypes from individual pigs among time-periods showed that F ST between worm populations from pigs sampled on day 28 p.i. was significantly greater (P<0·05) than for worms sampled on day 14 and 17 p.i., respectively, whereas no difference was found between the worm populations on days 14 and 17 p.i. (P=0·61).

Fig. 1. Distribution (%) of 4 haplotypes of Ascaris in the small intestine of individual pigs 14, 17 and 28 days post-inoculation (p.i.) with 500 embryonated Ascaris eggs from each of 4 female worms (A, B, C and D). ‘All’ represents the average of all worms from all pigs at the given day. The distribution of the 4 types was significantly affected by section (P<0·0001) and pig (P<0·0001) at day 14 and 28 p.i. and by ‘pig’ on day 17 p.i. (P<0·01). Numbers above columns indicate total number of worms recovered from each pig or time-point. Dotted lines represent an equal frequency (i.e. 25%) of worms of the 4 mtDNA haplotypes in each host.
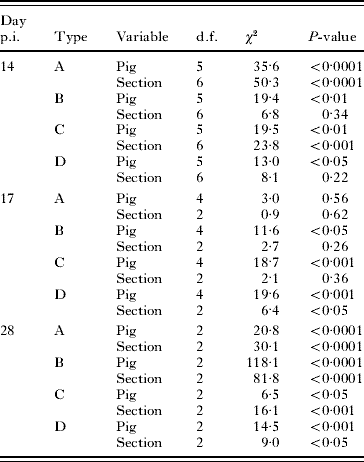
The logistic regression analysis revealed that the overall distribution of the 4 haplotypes (A, B, C and D) was associated significantly with the section of the small intestine (χ182=61·6, P<0·0001) and pig (χ152=52·6; P<0·0001) on day 14 and intestinal section (χ62=96·9, P<0·0001) and pig (χ62=80·0, P<0·0001) on day 28 p.i. On day 17 p.i., there was a significant (χ122=29·4, P<0·01) association between the pig host and the overall distribution of the 4 haplotypes in the small intestine. Table 2 shows the effect of section and pig on the distribution of each of the 4 types (i.e., A versus non-A) before- under and after the main immune responses (days 14, 17 and 28 p.i., respectively), applying generalized linear models. These results support the logistic regression analysis, with an effect of pig but no effect of section on the distribution of haplotypes on day 17 p.i., whereas the distribution of each of the 4 types was significantly affected by section and pig on days 14 and 28 p.i., except section on day 14 p.i. for haplotypes B and D.
Table 2. Effect of the pig host and location (section) on the distribution of four haplotypes (A, B, C and D) of worms
(The pigs were inoculated with an equal mixture of embryonated eggs of the 4 types and slaughtered at 3 time-points (before-, during- and after main immune response).)
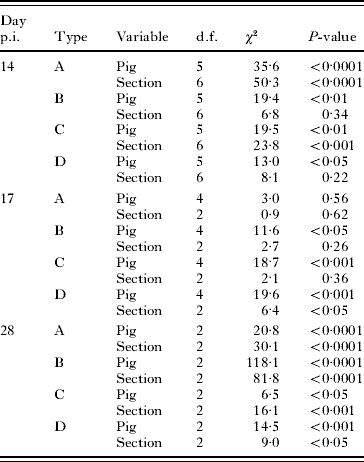
We compared the distribution of worms in the small intestine versus the distribution in the colon (i.e., worms likely to be expelled) on day 17 p.i. when the ‘immune expulsion’ is expected to take place to establish whether there was evidence for differential expulsion of larvae among the 4 haplotypes. An overall Chi-square test showed a significantly (χ32=170·5, P<0·0001) different distribution of the 4 haplotypes located in the small intestine versus those in the colon. On an individual host basis, the generalized linear models showed that ‘pig’ had a significant effect on the distribution of haplotypes A (χ12=17·0, P<0·0001) and B (χ12=31·0, P<0·0001) in the small intestine versus the colon. This was not the case for haplotypes C (χ12=0·9, P=0·34) and D (χ12=0·0, P=0·98).
Sizes and haplotypes of worms
The larvae of A. suum differed considerably in size. Fig. 2 shows the least square mean length of each of the 4 haplotypes of worms located in different regions of the intestine. At the 3 time-points p.i., the mean lengths of larvae collected from the different sections were significantly different (P<0·05), except the worms from sections 2 and 4, 2 and 5, 2 and 6, and 4 and 5 on day 14 p.i. Therefore, before and after the predominant immune responses, the longest worms were found in the proximal part of the small intestine (i.e., section 3), except for section 2 on day 14 p.i. In contrast, during the expulsion phase (day 17 p.i.), the worms in the proximal sections were the smallest and the worms which were expected to be expelled (i.e., those found in the colon) were the longest. At each of the 3 sampling time-points (14, 17 and 28 p.i.), the interactions between section and worm haplotype were significant (F(18,1875)=3·68, P<0·001; F(9,1596)=3·1, P=0·001 and F(6,661)=2·5, P=0·02, respectively) in their influence on worm length. Pairwise comparisons of the mean length on each sampling day showed that each of the 4 haplotypes were significantly (P<0·05) different from the other haplotypes, except for haplotype B and haplotype C on day 28 p.i. The ranking of the length of the worms was the same across all 3 sample periods with the following order: A>D>C>B. The effect of ‘pig’ on worm length was significant on day 14 p.i. (χ12=592, P<0·0001), day 17 p.i. (χ12=513, P<0·0001) and day 28 p.i. (χ12=79, P<0·0001), as evidenced by the likelihood ratio test.

Fig. 2. The least square means (bars: standard error of the mean, S.E.) of the length of the worms recovered from different sections of the intestine on days 14, 17 and 28 post-inoculation (p.i.) from 6, 5 and 3 pigs, respectively, inoculated with 500 embryonated Ascaris eggs from each of 4 female worms (A, B, C and D). The interactions between section and worm haplotype were significant at P<0·001, P=0·001 and P=0·02 at the 3 time-points, respectively, as well as the effect of ‘pig’ (P<0·0001). Sections without worms were not included.
In order to establish whether the most abundant worms in an intestinal section also tended to be the longest and the least frequent being the smallest, we compared the abundance of the 4 worm haplotypes with the mean worm length of each haplotype for each pig host. There was a significant positive correlation between abundance and worm length on day 28 p.i. (r2=0·91; P<0·01), whereas no correlations were found on day 14 and 17 p.i. between abundance and worm length.
We estimated the heritability of worm length, assuming that variation in length results from additive genetic effects. When we assumed that all worms with a particular mtDNA haplotype were half-siblings (i.e., the same mother but different fathers), heritability estimates were 0·40, 0·46 and 0·36 on days 14, 17 and 28 p.i., respectively. Assuming offspring to be full-siblings, the heritability estimates were halved, namely to 0·20, 0·23 and 0·18 for days 14, 17 and 28 p.i., respectively. This result suggests that 18–46% of the variation in the length of the worms may be explained by genetic factors linked to A. suum. All heritability estimates were highly significant (P<0·0001), as was the effect of host individual (P<0·0001) and section (P<0·0001) as determined using the ANOVA analysis.
DISCUSSION
In this study, all pigs were infected with the same mixture of genetically characterized Ascaris eggs. Even though this batch may not contain the exact same number of eggs of each of the 4 haplotypes (25% of each type), this procedure ensured that all pigs were infected with the same share of the 4 haplotypes. Therefore, we would expect all pigs to harbour similar proportions of the 4 mtDNA haplotypes, if we assumed that there was no impact of parasite genotype on infection dynamics. The findings of the present study show significant deviations from our expectation. (1) The proportions of Ascaris larvae with the 4 mtDNA haplotypes differed considerably among individual pigs, and these differences increased during the course of infection. (2) The spatial distribution of worms of the 4 mtDNA haplotypes differed significantly across the intestine of the host. (3) Larvae with the 4 mtDNA haplotypes showed ‘systematic’ differences in length across all 3 sampling time-points (i.e. with haplotype: A>D>C>B). The findings indicate that both parasite genetic effects and host-parasite interaction play critical roles in determining the outcome of A. suum infection. In the following, we (i) evaluate the evidence for host-parasite interactions, considering published works on other host-parasite systems and (ii) emphasize the implications of these findings in relation to understanding the biology of intestinal helminths.
Genetics of host-parasite interactions
Epidemiologists and mathematical modellers have tended to treat intestinal helminths as genetically homogenous for infection-related traits. The notable exception is Galvani (Reference Galvani2005), who explored how genotype-specific patterns of immunity against helminth infections might impact infection dynamics. Galvani's (Reference Galvani2005) analysis showed that genotype-specific immunity can help to explain patterns of parasite aggregation in human populations, and can also mimic the characteristic age infection profiles observed in human populations, which peak in school age children and are usually ascribed to age-dependent contact patterns with infective eggs. The data presented in the present study provide evidence that A. suum larvae are heterogeneous in their ability to infect different host individuals. Hence, ‘mass action’ models that seek to explain the epidemiology of Ascaris infections through contact rates between parasites and hosts may mask more complex, underlying interactions between parasites and hosts.
Co-evolution between host and parasites is expected to generate variation in infectivity among parasites and variation in resistance among hosts as well as specific interactions between host and parasite genotypes. There is direct evidence for such gene-for-gene co-evolution in plant/pathogen systems (Flor, Reference Flor1956; Thrall and Burdon, Reference Thrall and Burdon2003), whereas in free-living organisms, studies of bacterial pathogens of C. elegans (see Schulenburg and Ewbank, Reference Schulenburg and Ewbank2004), the freshwater crustacean Daphnia magna (see Carius et al. Reference Carius, Little and Ebert2001) and the rodent malaria parasite Plasmodium chabaudi in laboratory mice (Grech et al. Reference Grech, Watt and Read2006) support co-evolutionary dynamics. In contrast, in infections with macroparasites, there is no consensus. Cross-infection experiments examining infectivity of trematodes in their snail hosts in allopatric and sympatric combinations reveal a strong local adaptation (Morand et al. Reference Morand, Manning and Woolhouse1996; Lively et al. Reference Lively, Dybdahl, Jokela, Osnas and Delph2004), suggesting that trematodes ‘track’ locally and common snail genotypes. The limited work on this aspect for nematodes comes from studies of laboratory strains of T. muris or S. ratti in rodents. While extensive variation in host resistance and parasite infectivity was observed in each of these parasite systems, there was no evidence for interactions between host and parasite (Bellaby et al. Reference Bellaby, Robinson, Wakelin and Behnke1995; Paterson, Reference Paterson2005). A problem with interpretation of such laboratory-based studies is that selection during laboratory passage might alter infection phenotypes, for example due to a genetic ‘bottleneck’ (Li et al. Reference Li, Guo, Xue, Hu, Qiang, Xue, Bin, Hawdon and Xiao2004). Our study removed this potential problem, by using eggs collected directly from female Ascaris recovered from natural infections in commercial pig herds. However, the differences observed among different mtDNA haplotypes could relate to mtDNA-genomic effects or from nuclear autosomal genes associated with these mtDNA haplotypes. While variants in mtDNA are known to be linked to some genetic diseases in humans (Elliott et al. Reference Elliott, Samuels, Eden, Relton and Chinnery2008) and fitness traits in other organisms (Dowling et al. Reference Dowling, Friberg and Lindell2008), we believe that it is more likely that the mtDNA haplotypes mark Ascaris sibships bearing different nuclear encoded traits.
We have found that host genetics explains a substantial proportion of the variance in infection intensity with Ascaris (Nejsum et al., unpublished observations), while the present study shows differences in the proportion of larvae of the different mtDNA types in different pigs. Since we used commercial cross-bred production pigs and not a single breed or inbred line of pigs, it is unclear whether the interaction effects observed are associated with host genetics or non-genetic aspects of host biology (e.g., nutrition or immune status). To demonstrate that the interactions observed in this experiment have a genetic basis, we would need to show that closely related pig individuals show similar infection ‘profiles’ with the 4 groups of Ascaris larvae. We do have limited evidence for this, since 2 pairs of siblings were sampled. For pigs #61 and #73 (on day 14 p.i), there was similarity in the proportion of larvae with each haplotype, whereas this is not pronounced for the siblings (pigs #58 and #59) on day 17 p.i.
Implications for parasite biology
On day 14 p.i., we observed a heterogeneous distribution of worm haplotypes between pigs. This observation was made before the main immunological expulsion usually takes place (Roepstorff et al. Reference Roepstorff, Eriksen, Slotved and Nansen1997; Miquel et al. Reference Miquel, Roepstorff, Bailey and Eriksen2005), which might mean that innate host factors influence the survival of different parasite genotypes. However, during the apparent expulsion, we observed an increase in heterogeneity in haplotypes among hosts (increasing F ST). Hence, immune expulsion appears to provide an additional factor affecting the survival of particular parasite genotypes. A number of population genetic studies have shown that Ascaris genotypes are aggregated within the pig host (e.g., Anderson et al. Reference Anderson, Romero-Abal and Jaenike1995b; Nadler et al. Reference Nadler, Lindquist and Near1995; Nejsum et al. Reference Nejsum, Frydenberg, Roepstorff and Parker2005). This finding has been explained in two ways. First, such a pattern could suggest that hosts acquire related parasites, because related infective stages are clustered in the environment. Alternatively, Ascaris genotypes may be randomly distributed in the environment and ingested by the pig; however, within hosts, different Ascaris genotypes have different fitness and, thus, establishment success. The present findings lend support to this latter explanation, although both epidemiology and selection may contribute to the patterns observed.
Also the proportion of larvae of the 4 mtDNA haplotypes varied across the intestine of the host. This finding suggests that within a single host there may be intricate parasite-parasite or host-parasite interactions. In the latter case, for example, worms with haplotype A could be fitter (reflected in abundance and length) in section 2 and worms with haplotype B fitter in section 7 of a given host genotype. If this were the case for adult Ascaris and other parasites, this would have important implications for sampling and population genetics studies. When parasites are abundant, only a fraction of the infrapopulation is subjected to genetic analysis. Subsampling from a single region of the intestine is likely to result in a biased parasite sample. Therefore, in order to collect a representative subsample from a host, all parasites need to be isolated, after which specimens can be isolated for subsequent genetic analysis. If a non-random distribution of genotypes exists, as we have shown is the case for Ascaris in the pig host, this might lead to an overestimation of F ST.
We have found that parasite length was highly dependent on the female from which eggs were isolated. It is notable that this size difference is consistent before, during and after the predominant immune responses. For example, the offspring from female ‘A’ were usually the longest, whereas those from female ‘B’ were the smallest. The location in the intestine also had an effect on the size of the worms: on day 14 p.i., the worms in section 3 (proximal part) were ∼60% longer than those in section 8. In contrast, during the expulsion, the opposite was the case. The longest worms were located distal in the small intestine or in the colon. The worms in the colon were expected to be destined for ‘immune expulsion’. After the main immune response (day 28 p.i.), when a small residual and stable population has been established (cf. Roepstorff et al. Reference Roepstorff, Eriksen, Slotved and Nansen1997), the longest worms are again found in the proximal part of the small intestine.
Worm size is an important fitness trait for parasitic nematodes (e.g., Skørping et al. Reference Skørping, Read and Keymer1991; Stear and Bishop, Reference Stear and Bishop1999). This is certainly likely to be the case for Ascaris for which worm size and fecundity are strongly linked, because large females can produce more offspring than small females. However, in the early stages of infection during establishment, when immune expulsion of the majority of larvae occurs, fitness advantages of size are unclear. We observed that the longest larvae were found in the colon and were therefore likely to be expelled. Hence, there appears to be trade-off between fast growth/length and survival at different stages in the life cycle. It might be an advantage for the worm to be small during expulsion (day 17 p.i.) (less risk of being expelled) but long after this phase, since fecundity is higher for large worms (e.g., Stear and Bishop, Reference Stear and Bishop1999; Rowe et al. Reference Rowe, McMaster, Emery and Sangster2008). However, we acknowledge that there might be alternative scenarios, in which, for example, worms can be small but reach maturity and egg production more rapidly than large worms, but this proposal has not yet been tested.
Another aspect of worm size and the related fecundity is the abundance of different haplotypes of worm within the host. We found a strong positive correlation between abundance and worm length on day 28 p.i., which means that not only are some genotypes the predominant ones but also have the potential to produce more eggs. These two factors may act together to have a profound effect on the genetic composition of the excreted eggs among different host individuals.
Variation in the length of A. suum larvae might be due to maternal effects or to additive genetic effects of the worm. If we assume a simple ‘additive’ model and that the larvae from a single female worm are full siblings (i.e. sired by a single male) or half-siblings (sired by multiple males), then this trait shows 18–46% heritability. Maternal cytoplasmic effects could also directly influence larval size. One possibility is that large larvae originate from large eggs, and small larvae from small eggs. Such a maternal effect in the parasite could have a genetic basis, but equally, might reflect difference in age/maturity of the females.
In conclusion, we have shown that controlled inoculations using mixtures of Ascaris eggs derived from 4 female worms and with different mtDNA haplotypes result in markedly uneven distributions of infection in different porcine individuals. These results challenge the hypothesis that Ascaris infection intensity is a simple product of ‘contact rates’ with infective stages (e.g., Anderson and May, Reference Anderson and May1985) and suggests that specific interactions between host and parasite genotypes determine the outcome of infection as occurs in microparasite infections. While the focus of the present study was on A. suum in pigs, the findings and the approach taken might have implications in relation to other macroparasite systems.
The assistance of Frederik Andersen and Niels Peter K. Hansen, is greatly appreciated. The Danish National Research Foundation is acknowledged for the financial support of this project under the auspices of the Danish Centre for Experimental Parasitology. All animals were treated according to Danish legislation (13 October 2000, Allan Roepstorff: 2000/561-321).