INTRODUCTION
Changes in the community composition of free-living biodiversity can greatly influence parasite transmission intensity in positive and negative ways (Randolph and Dobson, Reference Randolph and Dobson2012; Johnson et al. Reference Johnson, Preston, Hoverman and Richgels2013). A mechanistic understanding of the observed patterns between free-living and parasitic diversity is a prerequisite to explorations of the consistency of diversity-transmission relationships across parasites with diverse ecologies.
Macroparasite-based model systems with which to explore these relationships remain relatively scarce (Thieltges et al. Reference Thieltges, Jensen and Poulin2008; Johnson and Thieltges, Reference Johnson and Thieltges2010). From a basic ecology perspective, this paucity of macroparasite systems precludes a wider view of the complex ecological networks that link parasitic life stages and free-living biodiversity. From an applied perspective, macroparasitic diseases (e.g. helminthiases) present a tremendous global disease burden to both domestic (Over et al. Reference Over, Jansen and van Olm1992) and wild animals (Albon et al. Reference Albon, Stien, Irvine, Langvatn, Ropstad and Halvorsen2002), and represent the most common infectious agents of humans in developing countries (Lustigman et al. Reference Lustigman, Prichard, Gazzinelli, Grant, Boatin, McCarthy and Basáñez2012). The expansion of drug resistance across several helminth families (Prichard et al. Reference Prichard, Basáñez, Boatin, McCarthy, García, Yang, Sripa and Lustigman2012) has further led to recent calls to consider complementary preventative approaches, turning research on the biological regulation of macroparasites into a frontier applied concern (Lustigman et al. Reference Lustigman, Prichard, Gazzinelli, Grant, Boatin, McCarthy and Basáñez2012). In addition, as free-living infectious stages (e.g. eggs, larvae or oncospheres) and complex life-cycles that involve multiple hosts are both common features of helminth natural histories (Johnson and Thieltges, Reference Johnson and Thieltges2010; Johnson et al. Reference Johnson, Preston, Hoverman, Henderson, Paull, Richgels and Redmond2012a ), macroparasite transmission success is strongly influenced by interactions with free-living biodiversity and by external environmental conditions. Understanding how these interactions suppress, maintain or amplify transmission requires a mechanistic understanding of the ecological context of parasite transmission.
Here we review the current knowledge of the mechanisms underlying interactions between the fecal helminths of vertebrates and coprophagous dung beetles. Dung beetles are a diverse and cosmopolitan group of detrivorous insects that use vertebrate feces for both adult feeding and reproduction, an association dating back to the Cenozoic (Davis, Reference Davis, Scholtz, Davis and Kryger2009). As a consequence of this resource use, many coprophagous species in families Scarabaeidae (subfamilies Scarabaeinae and Aphodiinae) and Geotrupidae (subfamily Geotrupinae) play roles in the transmission of vertebrate parasites. Previous studies have shown that some species of dung beetles reduce the number of emergent nematode larvae in livestock pastures (Mfitilodze and Hutchinson, Reference Mfitilodze and Hutchinson1988; Hutchinson et al. Reference Hutchinson, Abba and Mfitilodze1989) and contribute to lower parasite loads in vertebrate hosts (Fincher, Reference Fincher1973), while others are also involved in the maintenance of helminth transmission cycles, through their roles as intermediate hosts (Gottlieb et al. Reference Gottlieb, Markovics, lKlementa, Naora, Samish, Arocha and Lavya2011). As these interactions with helminths consequently result in both positive and negative parasite transmission outcomes, the net epidemiological effect of these interactions may ultimately be context-dependent. Understanding whether dung beetle communities buffer, maintain, or amplify parasite transmission, and how these outcomes depend on local environmental conditions is a key basic and applied ecology question.
Here we synthesize over five decades of study on dung beetle-helminth relationships. We propose a series of five underlying mechanisms by which dung beetles may influence helminth survival and transmission, review the observational evidence that links dung beetles to parasite survival and transmission outcomes, and highlight areas for future research. While we focus on interactions between dung beetles and mammal macroparasites, other coprophagous invertebrate species also influence parasite transmission cycles, and dung beetles also interact with other parasites of vertebrates.
IMPLICATIONS OF DUNG BEETLE-HELMINTH INTERACTIONS FOR HELMINTH SURVIVAL AND TRANSMISSION
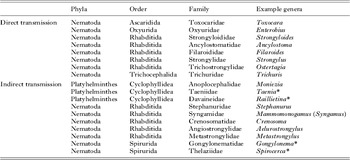
The frequent interaction with fecal material puts dung beetles in contact with at least 19 families of parasitic helminths with a fecal component in their life-cycle, predominantly within the phyla Platyhelminthes (flatworms) and Nematoda (roundworms) (Table 1; Fig. 1). Dung beetles may influence helminth survival and transmission success through both direct and indirect effects on the viability, survivorship or transport of parasite eggs or larvae, and/or by directly participating in transmission cycles (Table S1).
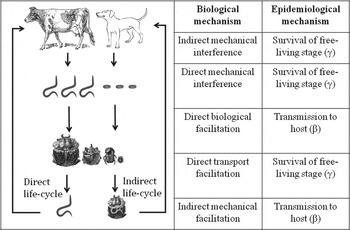
Fig. 1. Biological and epidemiological mechanisms by which dung beetles influence the transmission outcomes of direct and indirect life-cycle helminths.
Table 1. Common fecal parasitic helminths of vertebrates expected to interact with coprophagous beetle fauna during their life-cycle. Asterisks denote helminth genera known to use dung beetles as obligate intermediate hosts. Compiled from Bowman (Reference Bowman2008) and Schmidt et al. (Reference Schmidt, Roberts and Janovy2000)
Direct mechanical interference
Adult dung beetles feed on the microorganism-rich liquid in feces by first collecting fecal particles using their maxillary galeae and then removing larger particles before ingestion by passing fecal material through a set of filtering setae (Madle, Reference Madle1934; Holter, Reference Holter2000). The remaining small particles are then further squeezed between the beetle's molar ridges, removing excess liquid and concentrating the ingestible microorganisms and dead vertebrate epithelial cell components that together represent the adult beetle's primary diet. Together, these feeding activities can restrict food ingestion to particles with diameters in the range of 2–150 μm (Holter et al. Reference Holter, Scholtz and Wardhaugh2002; Holter, Reference Holter2004; Holter and Scholtz, Reference Holter and Scholtz2005, Reference Holter and Scholtz2007), and significantly reduce the likelihood of successful passage of helminth eggs (Miller, Reference Miller1954; Bílý and Prokopic, Reference Bílý and Prokopic1977; Bílý et al. Reference Bílý, Stĕrba and Dyková1978; Holter, Reference Holter2000). The strength of this reduction varies depending on the identity of both the helminth and beetle species. For example, Miller et al. (Reference Miller, Chi-Rodriquez and Nichols1961) observed that the feeding actions of four species in the genera Canthon and Phanaeus reduced the passage of hook and roundworm eggs by nearly 100%, while Dichotomius carolinus had little effect. Similarly, although Ascaris sp., Trichuris sp., and Taenia sp. eggs ingested by Canthon species showed varying degrees of external and internal damage, a significant fraction of those ingested by beetles in the genus Phanaeus were still recognizable after passage (Miller et al. Reference Miller, Chi-Rodriquez and Nichols1961). A recent experiment in Australia found beetle feces burial significantly reduced the number of emergent helminth larvae compared with human hand-burial of infected sheep feces – a difference the authors attributed to the mechanical impacts of beetles on larval survival (Coldham, Reference Coldham2011). While such direct mechanical interference may result both from the feeding actions of beetle adults and larvae as well as subsequent digestive processes within the beetles’ gastrointestinal tract, we are not aware of published reports that separate out these effects, nor explicitly examine the viability of helminth eggs after passage.
Indirect mechanical interference
Most adult dung beetles craft brood balls from the fibrous components of feces, oviposit directly within these balls, and store brood balls in excavated chambers under the soil surface to provision developing larvae (Halffter and Edmonds, Reference Halffter and Edmonds1982; Edwards and Aschenborn, Reference Edwards and Aschenborn1987). Such extensive manipulation of fecal material during these activities may interfere with helminth survival, through inducing microclimate changes to the fecal deposit itself (Bryan, Reference Bryan1973), and through underground burial of feces containing helminth free-living infectious stages (Bornemissza, Reference Bornemissza1960).
As adult beetles tunnel through vertebrate fecal deposits during feeding and nesting activities, they contribute to pat-fracturing and eventual breakdown (Bryan, Reference Bryan1973). The subsequent increase in pat desiccation rates can have strong effects on parasite development and survival (Durie, Reference Durie1961; Williams and Bilkovich, Reference Williams and Bilkovich1971; Mfitilodze and Hutchinson, Reference Mfitilodze and Hutchinson1988). These impacts may be particularly important for direct life-cycle parasites, where definitive hosts are (re)infected by free-living infectious larvae released during or immediately following defecation (Fig. 1). For example, Bryan (Reference Bryan1973) found that under relatively dry seasonal conditions, fecal pat aeration by small dung beetles led to the complete desiccation of the fecal pat and the rapid death of parasitic nematode larvae. The burial of parasitic larvae or eggs as a consequence of beetle brood ball relocation may also reduce the number of emerging larvae (Bryan, Reference Bryan1976; Bryan and Kerr, Reference Bryan and Kerr1989), and therefore overall contact rates with the final host. This negative influence of dung beetle feces burial should be most pronounced when brood balls are buried at depths that exceed each helminth species’ maximum vertical migration distance within a given soil type (Lucker, Reference Lucker1936, Reference Lucker1938).
Finally, beetle burial activities also reduce the likelihood of splash dispersal – a passive parasite dispersal mechanism that occurs with contact between rain droplets and infective stage larvae (Grønvold et al. Reference Grønvold, Sommer, Holter and Nansen1992, Reference Grønvold, Henriksen, Larsen, Nansen and Wolstrup1996). For example, Grønvold et al. (Reference Grønvold, Sommer, Holter and Nansen1992) reported a 70–90% reduction in splash dispersal of infective (L3) Cooperia spp. from cattle feces experimentally exposed to a single dung beetle species (Diastellopalpus quinquedens), compared with beetle-free controls.
Indirect mechanical facilitation
Beetle-mediated changes in the abiotic conditions of feces may alternatively enhance parasite survival by fostering a temperature-buffered and oxygenated environment, although empirical evidence for this role is lacking (Bryan, Reference Bryan1976; Houston et al. Reference Houston, Craig and Fincher1984; Chirico et al. Reference Chirico, Wiktelius and Waller2003; Coldham, Reference Coldham2011). Waghorn et al. (Reference Waghorn, Leathwick, Chen, Gray and Skipp2002) reported an increase in parasite abundance in experimental soil columns in treatments where dung was experimentally hand-buried at a distance of 5 cm, relative to an unburied control. The same shallow experimental burial had no clear influence on final parasite emergence above ground (i.e. where contact with definitive hosts occurs). Shallow hand-burial trials may be an ecologically unrealistic proxy for beetle-mediated facilitation of parasite survival, as the maximum feces burial depth for many dung beetle species can much deeper: e.g. 8 cm Vulinec (Reference Vulinec2002), 12 m (Estrada and Coates-Estrada, Reference Estrada and Coates-Estrada1991), 27 cm (Shepherd and Chapman, Reference Shepherd and Chapman1998), 102 cm (Lindquist, Reference Lindquist1933), and 130 cm (Edwards and Aschenborn, Reference Edwards and Aschenborn1987). However, as burial depth is positively associated with beetle body size (Fig. 2b), dung beetle communities dominated by small-bodied beetle species may have a neutral or positive community-level influence on helminth transmission. While these ideas require further exploration, if dung beetles indeed demonstrate size-ordered sensitivity to environmental change as has been suggested (Larsen et al. Reference Larsen, Williams and Kremen2005; Gardner et al. Reference Gardner, Hernandez, Barlow and Peres2008), this inverse relationship between body size and fecal helminth survival may contribute to enhanced transmission risk in degraded landscapes.

Fig. 2. Relationship between dung beetle body mass and two mechanisms of beetle-macroparasite interactions. (A) Intermediate host competence for a given parasite with an indirect life-cycle is likely to be in part a function of the maximum ingestible food particles (MDIP), and therefore related to beetle body size. Parasite eggs that exceed a given dung beetle's maximum MDIP value are less likely to be ingested by that beetle, potentially reducing potential host competency. Data fromHolter (Reference Holter2000, Reference Holter2004), Holter et al. (Reference Holter, Scholtz and Wardhaugh2002), Holter and Scholtz (Reference Holter and Scholtz2005) and du Toit et al. (Reference du Toit, Holter, Lutermann and Scholtz2012). (B) Indirect mechanical interference as a function of dung burial depth is positively related to beetle body mass. For macroparasites with direct life-cycles, beetle feces burial depth is likely negatively related to helminth survival. Different symbols represent different dung beetle species: Canthon aequinoctialis, C. triangularis, Dichotomius batesi, D. lucasi, Eurysternus caribaeus, Oxysternon conspicillatum, Phanaeus cambeforti, P. chalcomelas, Scybalocanthon pygidialis, burial depth (Vulinec, Reference Vulinec2002), body mass (Vulinec, Reference Vulinec2000); D. carolinus, burial depth (Lindquist, Reference Lindquist1933), body mass (Estrada and Coates-Estrada, Reference Estrada and Coates-Estrada2002); Onitis alexis, O. fulgidus, O. unicatus, O. viridulus, burial depth (Edwards and Aschenborn, Reference Edwards and Aschenborn1987), body mass (Davis et al. Reference Davis, Scholtz and Swemmer2012).
Direct biological facilitation
Beetles are obligate intermediate hosts for a diverse group of helminths with indirect life-cycles (i.e. those involving a definitive and one or more intermediate hosts) (Table 1; Fig. 1). Here, beetles ingest eggs from infected feces, parasites develop into an infective larval stage within the dung beetle's body, and successful transmission occurs upon beetle consumption by a definitive host. Species from least 18 dung beetle genera (Anomiopsoides, Ateuchus, Canthon, Copris Catharsius, Dichotomius, Epirinus, Eucranium, Euonthophagus, Geotrupes, Gymnopleurus Megathopa, Onthophagus, Onitis, Phanaeus, Sarophorus, Scarabaeus and Sisyphus) have been reported as likely or confirmed intermediate hosts of parasites of omnivores and carnivores, including Ascarops strongylina, Physocephalus sexalatus, Macracanthorhynchus hirudinaceus, Gongylonema verrucosum and Spirocerca lupi (Alicata, Reference Alicata1935; Martínez, Reference Martínez1959; Bailey et al. Reference Bailey, Cabrera and Diamond1963; Stewart and Kent, Reference Stewart and Kent1963; Bailey, Reference Bailey1972; Fincher and Marti, Reference Fincher and Marti1982; Stumpf, Reference Stumpf1986; Mukaratirwa et al. Reference Mukaratirwa, Pillay and Munsammy2010; Gottlieb et al. Reference Gottlieb, Markovics, lKlementa, Naora, Samish, Arocha and Lavya2011; du Toit et al. Reference du Toit, Holter, Lutermann and Scholtz2012). For a given helminth species, prevalence can range widely across dung beetle hosts. For example, Bílý and Prokopic (Reference Bílý and Prokopic1977) reported post experimental infection prevalence of Ascaris suum in dung beetles to range from 90% (Geotrupes stercorosus), 66·7% (Aphodius fimetarius), 27% (Onthophagus fracticornis) to 5% (Onthophagus verticornis). To our knowledge, the mechanisms of this variability in prevalence such as dung beetle exposure to infection, and infection susceptibility remain uncharacterized for even a single parasite species to date.
Direct transport facilitation
Dung beetles can play a role in helminth transmission and dispersal when a fraction of ingested eggs survive passage through the beetles’ masticatory and gastrointestinal systems. For example, Trichuris trichuria eggs have been found in the excrement of Phanaeus vindex and D. carolinus (Miller et al. Reference Miller, Chi-Rodriquez and Nichols1961). Taenia saginata ova have been reported as viable in Onitis sp. and Heliocopris sp. feces for at least 4 days, with some unfragmented ova recovered up to 10 days after ingestion (Mutinga and Madel, Reference Mutinga and Madel1981). In contrast, Bergstrom et al. (Reference Bergstrom, Maki and Werner1976) found no trichostrongylid eggs (Trichostrongylus colubriformis, Nematodirus sp., Ostertagia sp., or Marshallagia marshalli) in the intestinal tract of four different species of Aphodius and Canthon beetles following parasite egg consumption.
Finally, dung beetles may theoretically act as transport hosts for parasite eggs or larvae that adhere to beetle exoskeletons, although empirical evidence for this role is lacking. For example, Bergstrom et al. (Reference Bergstrom, Maki and Werner1976) found no trichostrongylid eggs (T. colubriformis, Nematodirus sp., Ostertagia sp. or M. marshalli) either within the intestinal tract or on the exoskeleton of four different species of Aphodius and Canthon. Other coprophagous invertebrates (e.g. earthworms) have been investigated for their role as transport or paratenic hosts (i.e. intermediate hosts that contribute to parasite life-cycles, but are not required for development), also with generally inconclusive results (Roepstorff et al. Reference Roepstorff, Grønvold, Larsen, Kraglund and Fagerholm2002).
LINKING BIOLOGICAL AND EPIDEMIOLOGICAL MECHANISMS
Predicting the overall impact of dung beetles on parasitic helminth transmission risk will ultimately require information on the per-capita impact of each dung beetle species in a given community on parasite transmission success. A simplified view of helminth transmission can be given as:
(modified from Dobson and Hudson, Reference Dobson and Hudson1992), where the impact on transmission of free-living helminths (W) by a dung beetle community (H) depends upon the production of infectious parasitic stages (λ) by infected definitive hosts (P), subsequent survival of infectious stages in the environment (γ), the probability of an infectious stage encountering the beetle community (WH), and the proportion of helminths that successfully produce an infective unit following an interaction with the beetle community (β) – a parameter directly linked to the likelihood of transmission to the definitive host, following a parasite encounter with the dung beetle community. For direct life-cycle helminths, infective units are free-living infective larvae that directly infect final hosts (Fig. 1). Dung beetles interact with direct life-cycle helminths during nesting, where the burial of brood balls containing feces and helminths can interfere with helminth vertical migration to the soil surface. An estimate of the proportion of free-living infectious units (helminths) that survive an encounter with the entire beetle community could be given as:
where helminth survival depends on the per-capita proportion of a fecal mass buried by beetle species i (B i ), the proportion of helminths that survive direct mechanical interference during the feeding activities of beetle species i (S i ), and the proportion of helminths able to successfully emerge from the average burial depth of beetle species i (E i ). The proportion of beetle diet represented by definitive host feces (P i ) will also be an important parameter, as beetle-helminth interactions cannot occur if a given beetle is not attracted to infected feces of the appropriate mammal host. For those indirect life-cycle helminths for which dung beetles act as intermediate hosts (Fig. 1), the infective unit of interest is the proportion of infected beetles within the community:
which depends upon the capacity of a given beetle species i to become successfully infected and transmit that infection (i.e. competence, C i ), and the same S i and P i terms as above.
FUTURE RESEARCH PRIORITIES
Community-level influences on parasite transmission
Understanding the influence of biological communities on parasitic disease transmission requires an integrative view of the ecological mechanisms by which overall community structure influences variation in transmission success (Johnson et al. Reference Johnson, Preston, Hoverman, Henderson, Paull, Richgels and Redmond2012a ; Randolph and Dobson, Reference Randolph and Dobson2012; Wood and Lafferty, Reference Wood and Lafferty2012). For example, host species diversity may inversely correlate with transmission success, either when additional host species have low or zero competence and therefore act as epidemiological dead ends, or when increased host diversity is associated with a decrease in the density of competent hosts (Keesing et al. Reference Keesing, Holt and Ostfeld2006; Suzán et al. Reference Suzán, Marcé, Giermakowski, Mills, Ceballos, Ostfeld, Armién, Pascale and Yates2009). We found no published work that quantified the relevance of different dung beetle community compositions on the survival or transmission of parasitic helminths. Future studies that link such observational data with experimental manipulation will be critical to investigations of the influence of beetle community structure on parasite transmission.
Species traits
Species’ traits are hereditary morphological, physiological or phenological characteristics that influence individual fitness through impacts on organism growth, reproduction or survival, which can be measured without reference to the external environment (Arnold, Reference Arnold1983; Violle et al. Reference Violle, Navas, Vile, Kazakou, Fortunel, Hummel and Garnier2007). Traits interact with contemporary environmental conditions and historical biogeographic conditions to influence species’ patterns of abundance and distribution (Nichols et al. Reference Nichols, Uriarte, Bunker, Favila, Slade, Vulinec, Larsen, Mello, Louzada, Naeem and Spector2013), including those of hosts and parasites. The application of trait-based models in disease ecology is remarkably recent (Johnson et al. Reference Johnson, Rohr, Hoverman, Kellermanns, Bowerman and Lunde2012b ) and have been used to predict intermediate host competency (du Toit et al. Reference du Toit, Holter, Lutermann and Scholtz2012) as well as which hosts may function as pathogen reservoirs (Cronin et al. Reference Cronin, Welsh, Dekkers, Abercrombie and Mitchell2010; Hawley and Altizer, Reference Hawley and Altizer2010). Given the diversity of mechanisms that link free-living biodiversity to infection outcomes in macroparasite systems (Orlofske et al. Reference Orlofske, Jadin, Preston and Johnson2012) and the strong influence of individual variation in host susceptibility on host-parasite interactions, trait-based disease ecology models are likely to be extremely useful in efforts to understand the role of complex community structure on infection risk.
Beetle body mass may be a useful predictor of the impacts of dung beetles on indirect life-cycle helminths (via interspecific variation in host competence; Fig. 2a), as well as on direct life-cycle helminths (via per-capita influences on burial depth; Fig 2b). Given the positive relationship between beetle body mass and burial depth (e.g. Vulinec, Reference Vulinec2002), indirect mechanical interference between larger-bodied beetles and direct life-cycle helminths may reduce overall helminth survivorship and transmission risk. However, the relationship between beetle body size and other mechanisms anticipated to influence the survivorship of direct life-cycle helminths (e.g. direct mechanical interference) remains unexplored to date.
Dung beetle influence on indirect life-cycle helminths is also likely to be related to beetle body mass, as well as other physiological traits. For example, beetle body mass and the intensity of Spirocerca lupi infections of beetles appear to be positively related (Mukaratirwa et al. Reference Mukaratirwa, Pillay and Munsammy2010). This may be driven by the positive correlation between beetle body size and the maximum diameter of ingestible particles (Fig. 2a) (du Toit et al. Reference du Toit, Holter, Lutermann and Scholtz2012). Large beetles also appear less choosy about particle size than smaller beetles, potentially due to evolutionary trade-offs between high food quality (ingestion of very small particles only) and quantity (ingestion of larger particles too) that contributes to reduced ‘pickiness’ about particle size by large beetles (Holter et al. Reference Holter, Scholtz and Wardhaugh2002; Holter and Scholtz, Reference Holter and Scholtz2005). These mechanisms are likely to interact, and suggest that beetle body size may be a key morphological trait determining beetle exposure to infection, an important component of competence.
Beetle nesting strategy may also play a role in determining maximum ingestible particle size (Holter and Scholtz, Reference Holter and Scholtz2005). Roller species tend to accept larger particles than tunnellers of similar body mass, potentially as a consequence of reduced feces selectivity by tunnellers due to reduced exploitative competition pressures at the feces deposition site (Holter and Scholtz, Reference Holter and Scholtz2005). Finally, while host immunology clearly plays a key role in the probability of infection and survivorship (and therefore competence), these parameters remain unexplored for dung beetles.
Diet breadth
A key attribute that shapes dung beetle–vertebrate–parasite ecological networks is beetle diet breadth. For this diverse group as a whole, we lack basic data about feeding ecology, including diet breadth, plasticity and their ecological correlates (Nichols et al. Reference Nichols, Gardner, Peres and Spector2009). As attraction to infected feces is a prerequisite of beetle interaction with fecal helminths, there is a dire need for basic investigation into these aspects of dung beetle natural history. The few existing published cafeteria studies demonstrate that dung beetle species range from extreme dietary specialism (e.g. obligate on single species) to extreme generalists (e.g. capable of feeding across multiple vertebrate guilds) (Whipple and Hoback, Reference Whipple and Hoback2012). Given the ephemeral nature of dung beetle-vertebrate interactions, both traditional field observations and cafeteria experiments are limited in their ability to cost-effectively evaluate dietary preferences in the wild (Nichols et al. Reference Nichols, Gardner, Peres and Spector2009; García-Robledo et al. Reference García-Robledo, Erickson, Staines, Erwin and Kress2013). Recently, the use of molecular methods has helped expand our knowledge about animal diets in the wild (Pompanon et al. Reference Pompanon, Deagle, Symondson, Brown, Jarman and Taberlet2012) and may prove to be particularly useful in studying dung beetle feeding patterns.
Influence of seasonality and climate events
Environmental conditions (i.e. moisture and temperature) have a major impact on the development, survival and migratory behaviour of parasitic nematode larvae with direct life-cycles (Durie, Reference Durie1961; Stromberg, Reference Stromberg1997). Particularly in their ensheathed infective stage, the free-living larvae of many nematode species may survive for months after deposition, depending on environmental conditions, raising concern that the beetle-mediated burial of infected feces in arid regions or dry seasons may result in an infection ‘time bomb’, although no empirical evidence currently supports this concern (Coldham, Reference Coldham2011). For indirect life-cycle parasites, seasonal changes in the abundance of competent intermediate hosts will influence seasonal transmission dynamics. For example, the seasonal variation in S. lupi prevalence in Israel is correlated with the seasonal abundance variation of its principal intermediate host Onthophagus sellatus (Mazaki-Tovi et al. Reference Mazaki-Tovi, Baneth, Aroch, Harrus, Kass, Ben-Ari, Zur, Aizenberg, Bark and Lavy2002).
Interactions with other pathogens: microparasites and fungi
The mechanisms that interfere with or facilitate macroparasite transmission by dung beetles may also modulate the transmission of fecal microparasites. Saitoh and Itagaki (Reference Saitoh and Itagaki1990) concluded that two species of Onthophagus beetles that emerged from cat feces infected with Toxoplasma gondii carried infective oocysts, both in their feces and on their bodies. These individuals subsequently transmitted toxoplasmosis to mice, and onwards to kittens that consumed them (1990). The same authors additionally detected two additional strains of feline coccidian (Isopora felis and Isopora rivolta) on dung beetles collected from urban dog feces. These dung beetles were also able to successfully transmit feline coccidia to kittens via dung beetle–mouse consumption, suggesting a paratenic or intermediate host role for some beetle species in feline coccidia (Saitoh and Itagaki, Reference Saitoh and Itagaki1990).
In contrast, in an investigation of the fate of Cryptosporidium parvum oocysts ingested by three beetle species (Anoplotrupes stercorosus, Aphodius rufus and O. fracticornis), Mathison and Ditrich (Reference Mathison and Ditrich1999) reported that the majority of oocysts were destroyed following passage through dung beetle mouthparts and gastrointestinal tract, suggesting a potentially negative influence of beetles on C. parvum transmission. A similarly negative impact of beetle activity on Cryptosporidium oocysts’ viability was reported by Ryan et al. (Reference Ryan, Yang, Gordon and Doube2011), who found that oocysts’ viability in feces burial by seven pairs of Bubas bison declined from 58% (control) to 10% (burial treatment). In a study of the ability of the dung beetle species Catharsius molossus to act as transport host for the pathogenic Escherichia coli strain O157:H7, only 5% of dung beetles tested positive for its presence in their gut contents, leading the authors to conclude that dung beetles appeared to play no epidemiological role in its transmission (Xu et al. 2003). Dung beetles have also been implicated in the reduction in abundance of the exploding fungus Pilobolus sporangia, which forcefully disperses nematodes in pasture systems along with its own spores (Gormally, Reference Gormally1993; Biggane and Gormally, Reference Biggane and Gormally1994).
A background regulatory role for dung beetles in public health?
Given that open defecation is practiced by nearly 1 in 5 people in developing countries (c. 1·1 billion people worldwide (WHO and UNICEF, 2012) and that dung beetles readily bury human feces (Miller, Reference Miller1954; Nichols and Gardner, Reference Nichols, Gardner, Simmons and Ridsdill-Smith2011), it should be expected that dung beetles interact with the transmission of helminths of public health concern. Human helminth infections (also known as soil-transmitted helminths, or STHs) are associated with approximately 10 000–135 000 deaths annually, severe annual morbidity for an estimated 300 million people and the extensive impairment of physical and mental development in children (Lustigman et al. Reference Lustigman, Prichard, Gazzinelli, Grant, Boatin, McCarthy and Basáñez2012). The principal STH agents (i.e. Ascaris lumbricoides, Trichuris trichiura, Necator americanus and Ancylostoma duodenale) that disproportionately represent the morbidity burdens of the neglected tropical diseases recognized by the World Health Organization (WHO, 2004; Lopez and Mathers, Reference Lopez and Mathers2006) are all direct life-cycle helminths for which dung beetles are expected to play a strong regulatory role. Although chemotherapeutic intervention is clearly effective in reducing the prevalence, intensity and morbidity of STH infection (Hotez, Reference Hotez2009), mass chemotherapy has its own challenges, including drug resistance risk (Vercruysse et al. Reference Vercruysse, Albonico, Behnke, Kotze, Prichard, McCarthy, Montresor and Levecke2011) and barriers to the optimal treatment coverage required for acceptable reductions in the probability of reinfection (Prichard et al. Reference Prichard, Basáñez, Boatin, McCarthy, García, Yang, Sripa and Lustigman2012). Given these constraints, it is generally accepted that anthelmintic treatment must be complemented by improvements in environmental sanitation, housing, health education and access, and vector control where relevant (Gazzinelli et al. Reference Gazzinelli, Correa-Oliveira, Yang, Boatin and Kloos2012). Dung beetle-mediated transmission suppression may be especially important in reducing environmental reservoirs of viable STH eggs or larvae (i.e. infected feces or soil), and therefore likely plays a positive role in the reduction of re-infection risk.
CONCLUSIONS
Two important factors emerge from consideration of the mechanisms that link dung beetle community composition to fecal helminth survival and transmission. First, beetle-parasite interactions may have divergent effects on transmission intensity within a given transmission cycle. For example, beetles above a body mass threshold may have a neutral or positive influence on transmission of indirect life-cycle parasites, while smaller species are likely to suppress the quantity of available infecting stages, by being fully incompetent hosts (Fig 2a). For direct life-cycle helminths, small beetles may exert relatively weak indirect mechanical interference as a consequence of their shallow feces burial, yet continue to reduce helminth viability through direct mechanical interference effects on parasite larvae and eggs.
Second, beetle-parasite interactions may have divergent effects on transmission intensity across transmission cycle types, given the divergent relationships between beetle body mass and parasite transmission and survival for direct and indirect-life-cycle parasites. Ultimately, the net epidemiological consequences of dung beetles on the parasitic helminths of vertebrates will be a function of the community-wide distribution and redundancy of beetle traits such as body size, diet breadth and feeding strategy. The dung beetle-fecal helminth system is a potentially ideal model system to understand the epidemiological consequences of community disassembly under environmental change, given the cosmopolitan distribution of both beetles and helminths, as well as their amenability to experimental manipulation in both laboratory and field settings. Here we have sought to draw attention to the diverse ways in which coprophagous beetles may contribute towards the maintenance, amplification or dilution of parasitic helminth transmission. Enhanced understanding of such mechanistic links will be an important step in future efforts to understand how environmental change may influence the interactions between free-living and parasitic species that ultimately alter infection risk (Randolph and Dobson, Reference Randolph and Dobson2012; Johnson et al. Reference Johnson, Preston, Hoverman and Richgels2013).
ACKNOWLEDGEMENTS
The editor, three anonymous reviewers and Nichar Gregory provided thoughtful comments that greatly improved an earlier version of this manuscript.
FINANCIAL SUPPORT
EN is supported by a postdoctoral fellowship (award # 1158817) from the National Science Foundation's International Research Fellowship Program (IRFP).
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/S0031182013002011.





