INTRODUCTION
The control of nematode parasites in humans and animals requires good hygiene and the regular use of anthelmintic drugs to prevent the build-up of parasites in hosts and in the environment. The regular use of anthelmintics is expected to produce loss of potency and resistance, and is a worrying problem. Resistance is recognized in parasites of humans and animals and may be produced by four types of general mechanism: changes in drug translocation, changes in receptor numbers, receptor modification, and/or post receptor modification (Albert, 1985). Significant advances in our knowledge of anthelmintics would be to enhance potency, determine mechanisms of resistance in parasitic nematodes and to demonstrate that resistance can be countered. We have been able to make some progress with these objectives.
Several major groups of anthelmintics act therapeutically by selectively opening nematode neurotransmitter ligand-gated ion-channels (Martin, Robertson & Bjorn, 1997a; Martin et al. 2003b): macrocyclic lactones open glutamate-gated chloride channels (Martin, Robertson & Wolstenholme, 2002; Sheriff et al. 2002), piperazine opens GABA channels and levamisole opens acetylcholine-gated channels (Robertson & Martin, 1993). In this review we focus on the cholinomimetic drug levamisole.
The mode of action of levamisole has been thought to be straightforward: the drug mimics acetylcholine and acts as an agonist of the post-synaptic nicotinic acetylcholine receptors located on nematode somatic muscle and, because it is not broken down rapidly (like acetylcholine), it induces a spastic paralysis that allows expulsion of the parasite from the host. However, recent studies in parasitic nematodes and C. elegans have demonstrated a surprising complexity in the nematode receptors associated with the action of this drug. The main findings are twofold: firstly unlike their vertebrate counterparts, the nicotinic receptors on nematode muscle are not a single homogenous population; there are, in Ascaris at least, three distinct subtypes of receptor each with different properties. These receptors have different sensitivities to agonists and antagonists. Secondly, the response of an individual receptor to a drug is not fixed; molecular studies have revealed sites on the receptor protein that can be altered by processes such as phosphorylation. One consequence of phosphorylation is that it alters the receptor properties, rendering them more or less likely to open in response to ligand binding; in our case altering the response to levamisole.
This unfolding complexity has benefits for both the parasite and the parasitologist looking to control these infections. From the parasites' point of view, this complexity is permitted by the presence and expression of combinations of multiple acetylcholine receptor (AChR) subunit genes. For the parasitologists, the presence of multiple AChR subtypes offers an opportunity for developing more effective treatments without necessarily finding new groups of compounds. Understanding the effect of available anthelmintics on each receptor subtype may lead to new combinations of existing compounds. If these combinations act on a broad spectrum of the subtypes present (or contain drugs that modulate the receptor as well as activate it) then we could achieve not only enhanced efficacy but also slow the inevitable development of resistance. In this review we cover these novel concepts.
MODE OF ACTION OF LEVAMISOLE
Levamisole belongs to the nicotinic agonist group of anthelmintics that includes pyrantel and that selectively produces muscle cell depolarization and spastic paralysis in nematodes (Aceves, Erliji & Martinez-Marnon, 1970; Aubry et al. 1970). We have shown that electrophysiological responses to these anthelmintics can be observed in muscle cells of the nematode parasites Ascaris suum and Oesophagostomum dentatum. Current-clamp, voltage-clamp and patch-clamp techniques have been used (Martin, 1982; Pennington & Martin, 1990; Robertson et al. 1992; Robertson & Martin, 1993; Dale & Martin, 1995; Evans & Martin, 1996; Martin et al. 1998; Robertson, Bjorn & Martin, 1999, 2000; Trailovic et al. 2002; Trailovic et al. 2005). These studies have identified nematode AChRs over the nematode muscle cell surface that respond to these anthelmintics and have described, down to the single-channel level, the agonist action and channel-blocking action of the anthelmintics.
Levamisole opens nematode AChRs that are ligand-gated, non-selective cation-channels. The membrane channels have conductances of 15–50 pS and mean open-times of 0·5–2·0 ms. Nematode muscle AChRs have pharmacological and biophysical properties that have similarities to vertebrate neuronal receptor channels, but there are very important differences. Our studies of levamisole effects in A. suum and O. dentatum have shown that the levamisole receptors are not an homogeneous population (Martin et al. 1997b; Robertson et al. 2000). We have observed that receptors have different conductances and can have over 100-fold differences in their probability of opening when activated by levamisole. We have found that: (1) there are 3 pharmacological subtypes of receptor (N-, L- and B-subtypes: Robertson et al. 2002); (2) that there is modulation of individual receptors by protein kinase enzymes (Trailovic et al. 2002); and (3) that resistance is associated with reduction of the L-subtype (Martin et al. 2003a).
GENERAL STRUCTURE OF ACETYLCHOLINE RECEPTORS
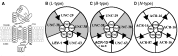
The most comprehensively studied AChR is that of Torpedo californica (Changeaux, Devillers-Thiery & Chemouilli, 1996). We assume that nematode AChRs are very similar in structure (see Fig. 1). AChRs are composed of two (or more) α-, and three (or less) non-α subunits (one β-, one γ- and one δ-subunit) that are arranged in a circle like five staves of a barrel around a central pore producing a transmembrane ion-channel. The pore of the ion-channel is formed by the second transmembrane region (TM2) of each of the 5 subunits. Negatively charged amino-acids (glutamate, aspartate) of the TM2 region are responsible for the channel conductance, cation selectivity and level of calcium permeability of the ion-channel receptor. It is expected that channels made up of UNC-38, UNC-29, UNC-63 and LEV-1 subunits or ACR-16 subunits will be more permeable to calcium than channels that include ACR-8 or ACR-13 subunits. The ligand binding sites of individual AChR receptors are composed of 6 loops of amino-acid: 3 loops (A, B & C) from the α-subunit and 3 loops (D, E & F) from the adjacent non-α subunit (Corringer et al. 1998; Brejc et al. 2001). The C loop contains two vicinal cysteines that define a subunit as an α-subunit. Since AChRs have two or more α subunits, there are 2 or more ligand binding sites on each receptor channel. Different subtypes of AChR are formed by different combinations of α- and non-α subunits. The different subtypes have a different channel conductance and calcium permeability because the lining (TM2) of the channel pores is different; and they have a different pharmacology because the binding site loops, ABC and DEF, depend on adjacent α and non-α subunits that form the AChR. Consequently, selective agonists and antagonists are expected to distinguish between receptor subtypes and to distinguish between AChRs with different channel conductances and calcium permeability. The different AChRs subtypes in nematodes are expected to have different physiological functions but, as yet, the different physiological functions remain to be determined.

Fig. 1. Putative structures of nematode muscle acetylcholine receptors. A. Diagram of an α-subunit showing the long extracellular N-terminal region with the cysteine bonds, the four trans-membrane regions: TM1, TM2, TM3, TM4, and the cytoplasmic loop between TM3 and TM4. B. Diagram of possible arrangements of five subunits forming the L-type channel (subunits include; UNC-63, UNC-38, UNC-29 and LEV-1). C. as B. for the B-type channel (note the presence of ACR-13=LEV-8 subunit). D. as B. for the N-type channel composed of ACR subunits. [ltrif ], Receptor binding sites (formed at the junctions between alpha subunits and adjacent subunits). These are heterogeneous when the adjacent subunits are not identical.
KINASE AND PHOSPHATASE MODULATION
T. californica cells also contain protein kinase activity, including cAMP-dependent kinase, which can phosphorylate the nicotinic receptor. The phosphorylation sites of α-, β-, γ- and δ- subunits differ for the kinases. Experiments have shown that cAMP-dependent phosphorylation markedly alters the channel opening in response to acetylcholine (Huganir et al. 1986). We have also observed such effects with Ascaris levamisole-activated channels. It is known that sites for kinase activity are on the intracellular loops of the subunits so that intracellular loop regions must be involved in regulating the opening of the channel. Vertebrate neuronal nicotinic receptors are assumed to be similar pentameric structures, arising from the co-expression of 2 α- and 3 non-α subunits, but different subunit arrangements around the ion-channel pore are possible (McGehee & Role, 1995). Effects of changes in phosphorylation of neuronal nicotinic receptors by protein kinases and phosphatases also lead to modulation of receptor opening (Khiroug et al. 1998). The modulation of neuronal AChRs is dependent on the balance between protein kinases and phosphatases, especially calcineurin. We have used this model of neuronal nicotinic receptors to guide us in our experiments on nematode AChRs.
NEMATODES HAVE THE MOST ACETYLCHOLINE RECEPTOR SUBUNIT GENES
Much of the information on the structure and function of nematode AChRs has advanced with the study of C. elegans, a soil nematode that has been subjected to detailed genetic analysis. We are encouraged to use C. elegans as a model for the levamisole receptor of parasitic nematodes by molecular phylogenetic analysis (Blaxter, 2001). C. elegans belongs to clade V as do many nematode parasites like O. dentatum and there are similarities between the single-channel properties of levamisole receptors of A. suum (clade III) and O. dentatum (Robertson & Martin, 1993; Robertson et al. 1999).
Initially three genes encoding AChR subunits in C. elegans, associated with strong levamisole resistance were described (Fleming et al. 1997). They were unc-38, unc-29 (both on chromosome I) and lev-1 (on chromosome IV). Unc-38 encodes an α-like subunit while unc-29 and lev-1 encode non-α subunits. The molecular structures of levamisole UNC-38 subunit homologues in the parasitic nematodes T. colubriformis and Haemonchus contortus (Hoekstra et al. 1997) are about 90% identical to C. elegans suggesting that information derived from the model nematode may be a useful guide for parasitic nematodes.
With the completion of the C. elegans genome, the size of the nematode AChR gene family grew dramatically. A genome consortium analyzed the genomic DNA to predict open reading frames and over fifty had substantial similarity to AChR subunits of other organisms. There are now a total of 27 AChR subunit genes, which is the largest number of AChR subunit genes identified in a single species (Jones & Sattelle, 2004). Homology has allowed these 27 subunits to be divided into five groups, named DEG-3-like, ACR-16-like, ACR-8-like, UNC-38-like and UNC-29-like. The UNC-29-like subunits are non-α subunits, the remainder are mostly α subunits. If all combinations of α and non-α subunit were capable of forming functional AChRs there could be over 5000 receptor subtypes, but this is not likely to occur in the organism.
PHARMACOLOGICAL SUBTYPES OFMUSCLE ACETYLCHOLINE RECEPTOR
For a variety of developmental and physiological reasons, expression of particular subunits is restricted to particular neurons and muscle cells. UNC-38, UNC-29, UNC-63, LEV-1, ACR-8, ACR 16 and ACR-13 (=LEV-8) GFP-tagged protein subunits are expressed in somatic muscle cells of C. elegans (Fleming et al. 1997; Richmond & Jorgensen, 1999; Culetto et al. 2004; Touroutine et al. 2005; Towers et al. 2005). The presence of multiple pharmacological AChR subtypes on C. elegans somatic muscle is indicated by null mutation experiments and contraction assays. Null mutants of unc-38, unc-29, unc-63 confer resistance to levamisole. A null mutant of acr-16 confers nicotine resistance with little effect on sensitivity to levamisole. While a null mutant of acr-13 (lev-8) reduces sensitivity to levamisole. Electrophysiological responses to levamisole but not nicotine are abolished in unc-38, unc-29, unc-63 null mutants and reduced (but not abolished) in lev-1 and acr-13 (lev-8) null mutants (Richmond & Jorgensen, 1999; Culetto et al. 2004; Towers et al. 2005); responses to acetylcholine but not levamisole were reduced by acr-16 null mutants. An effect of acr-8 null mutants was not detected (Touroutine et al. 2005).
These observations suggest that on C. elegans muscle there are 3 AChR subtypes (see Fig. 1). Using our descriptors, there is an N-subtype, sensitive to nicotine, and composed of ACR-16 subunits (essential) and perhaps ACR-8 (non-essential), an ACR-16 homoligomer is possible, (Raymond, Mongan & Sattelle, 2000); there is an L-subtype, sensitive to levamisole, comprised of UNC-38, UNC-29, UNC-63, LEV-1 subunits; and a third subtype (we do not know if it corresponds to our AscarisB-subtype ) that lacks the LEV-1 subunit and is comprised of UNC-38, UNC-29, UNC-63 and ACR-13. Uncertainties remain because: (1) we do not know the limitations of the C. elegans model for interpreting pharmacology and electrophysiology of parasitic nematodes; (2) if there is only one stoichiometric pentameric arrangement of the L-subtype subunits or if different stoichiometric arrangements are possible; and (3) what role the ACR-8 subunit plays. The stoichiometry of the L-subtype subunits has not been defined even in C. elegans. No single channel data from C. elegans AChRs are available so it is not possible to make comparisons between single-channel data from parasitic nematodes. If the single-channel properties in C. elegans were to be very similar to parasitic nematodes, it would strengthen the case for using the C. elegans model of levamisole receptors for parasitic nematodes. Appropriate use of the C. elegans model for pharmacological and electrophysiological investigation of levamisole receptors of parasitic nematode would offer advantages for molecular and pharmacological study.
ACETYLCHOLINE RECEPTOR ACTIVATION BY LEVAMISOLE REQUIRES G153E OF UNC-38 NOT THE YXXCC-MOTIF
Jones & Sattelle (2004) originally suggested that the selective agonist activity of levamisole is dependent upon the presence of the C-loop YxxCC motif in some nematode α-subunits (Fig. 2), rather than the usual YxCC motif that is found in most vertebrate α-subunits. Testing this prediction for mouse AChRs, it was found that insertion of proline into position 191 (yielding C-loop YPxCC) did not produce selective activation of mouse AChRs by levamisole (Rayes et al. 2004). However activation of mouse muscle AChRs by levamisole is produced by a glycine to glutamate change in the B-loop (αG153E: Rayes et al. 2004). This glutamate is only present in the B-loop in position 153 of UNC-38 (Fig. 2) of nematodes and is not present in the other nematode α-subunits ACR-8, ACR-13, or UNC-63. Thus it appears that selective activation by levamisole depends upon the presence of an E153 containing subunit like UNC-38. In parasitic nematodes UNC-38-like subunits have been found in Ascaris suum (asar-1) and Trichostrongylus colubriformis (tar-1) that have the E153 indicating activation by levamisole.

Fig. 2. C. elegans agonist binding sites on the B- and C-loops of the α-subunit. Note the YXXCC motif of ACR-8, ACR-13 and UNC-38. Only UNC-38 has the E153 in the B-loop, which is suggested to contribute to the binding of levamisole.
E238 AND E259 IN TM2 PREDICT HIGH CALCIUM PERMEABILITY FOR- BUT LOWER PERMEABILITIES FOR- AND-SUBTYPE CHANNELS
AChRs are sometimes very permeable to calcium. The calcium[ratio ]sodium permeability ratio varies between 0·02–4 and increases when glutamate is present in positions E238 (*) and E259 (!) in the channel pore region TM2, Fig. 3 (Fucile, 2004). E238 of the putative L-subtype subunits (UNC-38, UNC-63, UNC-29, LEV-1) are conserved acidic glutamate residues predicting high calcium permeability. The calcium permeabilities of L-subtypes and B-subtypes are predicted to be enhanced by E259 (!: Fig. 3) of UNC-38 & UNC-63. In contrast, position 238 (Fig. 3*) of ACR-13 and ACR-8 are unusual by having a basic histidine residue that predicts low calcium permeability for the B-subtype and for the N-type if present. A low Ca permeability in the N-type will indicate the presence of an ACR-8- like subunit.

Fig. 3. Left: TM2 regions of putative L-subtype and possible B- and N-subtype acetylcholine receptors. The L-subtype subunits show acidic amino-acid residues in bold at positions 238 (*) and 259 (!) that facilitate calcium permeability. ACR-8 and ACR-13 possess a basic amino-acid at 238 (*) that is predicted to reduce calcium permeability. Right, classical two binding site ‘hopping model’ that facilitates calcium permeability in nicotinic receptors via acidic amino acids at 238 and 239. The presence of a basic amino acid in either position would significantly reduce calcium permeability.
We predict for parasitic nematodes, on the basis of the molecular structure of C. elegans subunits and available A. suum data, that the L-subtype AChR which is sensitive to levamisole will have high calcium permeability. In contrast the N-subtype which is sensitive to nicotine is predicted to have low calcium permeability (only if an ACR-8-like subunit is present) and low sensitivity to levamisole. The B-subtype AChR is also predicted to have low calcium permeability. These predictions are therapeutically very important for parasitic nematodes, since reduction of the proportions of L-subtype AChR relative to N- and B-subtypes would reduce the entry of calcium and contraction of muscle in response to physiological release of acetylcholine as well as levamisole treatment. These observations suggest why we see a decrease in more levamisole sensitive (L-subtype) receptors with resistance (Robertson et al. 1999; Martin et al. 2003a). In the next section we present observations of the modulation of levamisole receptors and explain in more detail the evidence for multiple levamisole receptor subtypes in parasitic nematodes.
MODULATION OF ACETYLCHOLINE RECEPTORS AND PHOSPHORYLATION
Regulatory phosphorylation sites for protein kinase C, protein kinase A (PKA) and tyrosine kinase are recognized on C. elegans AChR subunits. In Onchocerca volvulus, cDNA of an AChR subunit (Ajuh et al. 1994) has identified regulatory phosphorylation sites. Analogous phosphorylation sites are also present on nAChR subunits of parasitic nematodes: (H. contortus, Hoekstra et al. 1997; T. colubriformis, Wiley et al. 1997; A. suum, Trailovic et al. 2002). Phosphorylation of the AChR subunits of the levamisole receptor is one of the most effective means the parasite could have of being able to regulate and modulate the number and activity of the receptors; so phosphorylation plays a role in adjusting the opening of the different muscle AChR subtypes and in the response to cholinergic anthelmintics. Phosphorylation enhances channel opening of levamisole receptors. We have observed that inhibitors of protein kinases decrease the muscle response to levamisole (Trailovic et al. 2002) and that kinase activity, specifically PKA, increases open-times of levamisole-activated channels.
POLY-GENETIC NATURE OF LEVAMISOLE RESISTANCE
Levamisole resistance in C. elegans has been associated with mutations of many genes (AChR subunit genes: unc-38, lev-1, unc-63, unc-29; genes of muscle proteins, calcium homeostasis and protein kinases; Jones & Satelle, 2004). Development of levamisole resistance in parasitic nematodes also appears to be polygenic (H. contortus, Sangster, Davis & Collins, 1991; O. dentatum, Varady et al. 1997; Robertson et al. 1999), but the genes involved have not yet been identified. Studies on levamisole-resistant parasitic nematodes have so far failed to identify point mutation changes in AChR subunits associated with resistance [the α-subunit tar-1 gene in T. colubriformis (Wiley et al. 1997) and the hca1 α-subunit of H. contortus (Hoekstra et al. 1997)]. However we do now know that there is a decrease in the response of L-subtype receptors in a parasitic nematode with no change in the sensitivity to N-subtype receptors (Robertson et al. 1999; Martin et al. 2003a). Although anthelmintic resistance is of concern in human ascariasis little is known about the genetics of development of resistance.

LEVAMISOLE RECEPTORS ARE MODULATED BY KINASES AND INHIBITION OF KINASES LIMITS LEVAMISOLE RESPONSES
Levamisole acts therapeutically by opening (activating) muscle AChR ion-channels in A. suum muscle (e.g. Robertson & Martin, 1993). We monitored the opening electrophysiologically using a two-micropipette current-clamp technique as we added controlled fixed pulses of acetylcholine (and levamisole in our published report) and tested the effect of selective inhibitors of protein kinase. Fig. 4 (from Trailovic et al. 2002) shows a representative experiment that demonstrates that the kinase antagonist staurosporine reversibly reduces the acetylcholine membrane potential response (notice the reduced response in the presence of staurosporine and recovery after removal). In the paper we demonstrated that levamisole receptor opening (activation) could be modulated and was dependent on the presence of kinase enzymes. We also used more selective kinase antagonists (KN93 and genistein) and demonstrated a role for calcium-calmodulin kinase II and tyrosine kinase with acetylcholine and levamisole as agonists to open the receptor channels.

Fig. 4. Staurosporine, a non-selective protein kinase antagonist, reversibly inhibits acetylcholine responses in a current-clamp recording. Note that the depolarizing membrane potential response to acetylcholine is reduced in the presence of staurosporine, and the response recovers on washing. Inhibition of protein kinases show that acetylcholine receptors (levamisole receptors) are modulated and dependent on their phosphorylation state, so that inhibition of kinases reduces receptor responses, mimicking resistance.
LEVAMISOLE MEMBRANE POTENTIAL RESPONSES ARE CALCIUM DEPENDENT
Since the opening of levamisole-activated receptor channels was dependent on kinases, including calcium-calmodulin kinase II, we tested the effect of removal of calcium on levamisole responses in current-clamp experiments. We found (Trailovic et al. 2002) that levamisole membrane potential responses are shifted to the right in the absence of external calcium showing that the responses are calcium-dependent, suggesting that entry of calcium into the muscle cell supports opening of the levamisole receptors. We reported observations that inhibition of calcium-calmodulin kinase II phosphorylation of the receptor-ion channels limits responses to levamisole.
MUSCLE CONTRACTION ASSAYS SHOW THE PRESENCE OF-, - AND -SUBTYPES OF LEVAMISOLE RECEPTOR
We have conducted Ascaris muscle strip contraction assays with the novel anthelmintic compounds, paraherquamide and 2-desoxyparaherequamide and used more recently developed quantitative pharmacological techniques (Robertson et al. 2002). We found that both of the compounds were potent competitive antagonists at cholinergic receptors of A. suum and would inhibit contractile responses to the cholinergic anthelmintics like levamisole, pyrantel, bephenium, oxantel, thenium, nicotine and methyridine to different degrees (Robertson et al. 2002; Martin et al. 2003a, 2004). Our analysis revealed the presence of the N-, B-, and L-subtypes of cholinergic receptors.
Fig. 5A illustrates a representative muscle strip cumulative dose-response experiment to the anthelmintic bephenium. Figs 5B & C illustrates greater inhibition of levamisole produced by paraherquamide compared to nicotine and show that these two drugs act on different receptors.

Fig. 5. A. Muscle strip cumulative contraction dose-response trace (bephenium was used as an agonist in this instance). B. Nicotine contraction dose-response with paraherquamide (para) as antagonist C. Levamisole contraction dose-response with paraherquamide as antagonist. Note that paraherquamide produces a significantly greater degree of antagonism when levamisole is the agonist c.f. nicotine.
Table 1 shows the values of paraherquamide pKBs (negative log dissociation constants of antagonist) and that nicotine and levamisole bind to different receptors in A. suum muscle. Similar experiments with 2-desoxyparaherquamide and methyllycaconitine separated our N-, L- and B-subtypes. Fig. 6A illustrates the selectivity dendrogram of cholinergic anthelmintics (Martin et al. 2004). In a series of papers (Robertson et al. 2002; Martin et al. 2003a, 2004), we described 3 pharmacological subtypes in greater detail. There is an N-subtype that nicotine, methyridine and oxantel are more selective for; there is the L-subtype that levamisole and pyrantel are more selective for; and there is the B-subtype that bephenium is more selective for (Fig. 6B). High concentrations (30 μM) of levamisole will activate all subtypes of AChR channels on Ascaris muscle. We consider this again under the description of our patch-clamp study results.

Fig. 6. A. Comparison of pA2 values for three antagonists using cluster analysis separates three receptor subtypes in Ascaris muscle. B. Schematic representation of acetylcholine receptor subtypes on Ascaris muscle. beph, bephenium; then, thenium; lev, levamisole; pyr, pyrantel; nic, nicotine; oxa, oxantel; meth, methyridine.

LARVAL MIGRATION ASSAYS ONLEVAMISOLE-RESISTANT ISOLATES
The question arose of how the pharmacological N-, L- and B-subtypes relate to levamisole resistance. We were influenced by the development of resistance in other parasites that can be associated with subtype changes of the target site (Suswam et al. 2001; Suswam, Ross & Martin, 2003); we had seen that there is a loss of the 35-pS AChR channels in patch-clamp experiments in levamisole-resistant isolates of O. dentatum (Robertson et al. 1999) and so anticipated that the loss of a 35-pS subtype is associated with loss of the L-subtype but not the N-subtype. We therefore tested for changes in pharmacological subtypes with resistance in O. dentatum L3 larvae. Fig. 7 shows observations using larval migration assays for levamisole-sensitive (SENS) and levamisole-resistant (LEV-R) isolates and compares the effects of levamisole, pyrantel, nicotine and methyridine (Martin et al. 2003a). Comparison of the levamisole-sensitive and levamisole-resistant isolates using EC50 concentrations shows that there is little change in the sensitivity to nicotine and methyridine but there is reduced sensitivity to levamisole and pyrantel. We developed a measure for assessing the relative selectivity to levamisole based on the change in sensitivity to levamisole and the test drug. The relative selectivities, ρL, for the L-type receptor were: levamisole ρL=1·0; pyrantel ρL=0·93; methyridine ρL=0·17; nicotine ρL=0·06. These observations confirmed an N-subtype selective action for nicotine and methyridine and show that levamisole resistance can be associated with a loss of the L-subtype but not the N-subtype receptors. The N-subtype selective pharmacology of methyridine suggests its use for treatment of levamisole-resistant parasites. These observations are important because they show that there is a reduction in the L-type of cholinergic receptor associated with levamisole resistance but that the N-type remains unaffected. Thus agents like methyridine and oxantel (Martin et al. 2003a, 2004) could remain effective in the presence of levamisole resistance.

Fig. 7. Left: Oesophagostomum dentatum used in migration assay. Plots: Percent inhibition larval migration assays by levamisole, pyrantel, nicotine and methyridine for levamisole sensitive (SENS) and levamisole resistant (LEV-R) L3 of O. dentatum. There is a significant change in the inhibition curves for levamisole and pyrantel. The LEVR isolate responds less than SENS to levamisole and pyrantel. There is no difference between LEVR and SENS when nicotine and methyridine are used. Concentrations=M.
COMPARISON OF NICOTINE AND LEVAMISOLE CHANNEL CURRENTS UNDER PATCH-CLAMP SEPARATE-, - AND- SUBTYPES IN
We saw in A. suum muscle contraction strips that we can distinguish 3 pharmacological subtypes of cholinergic receptor and knew that in levamisole resistant O. dentatum we lose 35-pS subtypes (Robertson et al. 1999) and L-subtypes (Martin et al. 2003a). The approach that we are now following is to correlate the pharmacological subtypes with the single-channel current subtypes.
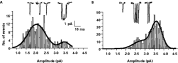
Our approach has been to compare the properties of channels activated by lower concentrations of levamisole with the properties of channels activated by lower concentrations of nicotine. Fig. 8 shows representative amplitude histograms and channel recordings from patches with 30 μM nicotine (left) as the agonist and another patch with 10 μM levamisole (right) as the agonist. It can be seen that nicotine more frequently opens a smaller channel (conductance ~26 pS) and that levamisole more often opens a larger channel (conductance ~35 pS). The significance of these observations was confirmed by applying discriminant analysis (a novel application of an established statistical technique to patch-clamp records) (Levandoski et al. 2005). These experiments show that low concentrations of nicotine preferentially activate smaller, 25-pS channels (the N-subtype) and that levamisole preferentially activates a larger conductance, 35-pS, channel. Thus we have been able to separate the single channel currents of two of the pharmacological subtypes. The separation of the remaining B-subtype at the single channel level will require a similar approach utilizing bephenium as the agonist.

Fig. 8. Examples of amplitude histograms using nicotine and levamisole as agonist in Ascaris suum single-channel measurements. Each histogram has two channels present in the membrane patch. In A. nicotine preferentially activates a low conductance channel (smaller openings), while in B. levamisole preferentially activates a higher conductance channel (larger openings).
PROTEIN KINASE A CATALYTIC SUBUNIT AND MGATP TOGETHER INCREASE THE OPENING OF-TYPE RECEPTOR CHANNELS IN ISOLATED PATCHES
We have shown (Trailovic et al. 2002) that protein kinase inhibition leads to reduction of AChR channel opening under current-clamp conditions. We sought to identify kinetic details, in patch-clamp experiments, of the effect of protein kinases and to test our hypothesis directly that opening of levamisole receptor channels can be increased and thus modulated by phosphorylation. Fig. 9 illustrates the positive modulation of an L-subtype receptor channel activated by levamisole and modulated by the PKA catalytic subunit. The effect is an increase in the channel opening rate, an increase in the mean open time and an increase in the proportion of time that the channel spends open (increased activation). These observations demonstrate that activation of the AChR ion-channels by levamisole is not fixed and can vary even with a single concentration of levamisole. Our studies demonstrate that it is possible to increase the opening of L-subtype receptor channels and this offers an approach to counter the effects of levamisole-resistance in parasitic nematodes. We are continuing these investigations to determine AChR subtype selective effects and to make a comparison to the effects of calcium/calmodulin-dependent kinase (CaMK) II.

Fig. 9. 39 pS (L-subtype) channel recorded from an inside-out patch from Ascaris suum. A. Control: top, a diagram of the control experimental set up; inset, a sample of the recording of single-channel openings (upward); histogram, the distribution of open-times (mean open time, 1·6 ms). B. Top, a diagram of the test experimental set-up and phosphorylation site following addition of protein kinase A catalytic subunit and Mg ATP-γS; inset, representative recordings of channel openings (upward) – note that they are much longer; and histogram of open times (mean open-time, 2·9 ms). The application of Mg ATP-γ-S and the catalytic subunit of protein kinase A leads to an increase in mean open time and probability of the channel being open.
AF2 PRODUCES LASTING POTENTIATION OF ACETYLCHOLINE RECEPTOR RESPONSES
We have conducted experiments on nematode AChR channels to increase the amplitude of AChR responses with the longer-term aim of countering levamisole resistance and started with the FMRFamide-related peptide (FaRP), AF2. AF2 is a neuropeptide that was first isolated from A. suum and has now been found in several nematode species including C. elegans, P. redivivus and H. contortus. AF2 is the most abundant neuropeptide so far found in nematodes. When injected into the A. suum body cavity it produces shortening and inhibits movement. AF2 was known to produce dramatic increases in cAMP concentrations in Ascaris muscle by G-protein receptor activation (Reinitz et al. 2000). Given the effect of PKA we had observed on AChRs, we tested the effect of AF2.
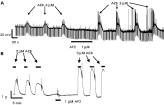
We found and reported (Trailovic et al. 2005) that short duration application of AF2 produced lasting potentiation of AChR responses. These observations are interesting and important because they show that increasing responses of nematode parasites to cholinergic agents is possible and that development of an AF2 agonist that acts synergistically with cholinergic anthelmintics could counter the development of levamisole resistance. Fig. 10A shows a current-clamp recording from A. suum demonstrating that brief application of AF2 leads to a long-lasting increase in response to acetylcholine. The potentiation outlasts the application of AF2 and is associated with an increase in calcium spike activity in muscle cells. Similar potentiation is seen in Ascaris muscle contraction experiments (see Fig. 10B). We also observed that atropine, a muscarinic antagonist, inhibited this potentiation by AF2, suggesting that muscarinic receptors are involved in the AF2 potentiation process. The long-lasting potentiation produced by AF2 has parallels to the long-term potentiations that underlie synaptic plasticity in vertebrate and invertebrate systems. In these systems multiple kinases, including calcium-calmodulin kinase II and the calcium-dependent phosphatase, calcineurin, are involved. We suggest a model that can explain these observations and our earlier observations on inhibition of kinases and calcium sensitivity of the levamisole receptor channels. Fig. 11 is a summary of this model whereby the dynamics of cytosolic calcium modulate the activity of the cholinergic ion-channels and contraction.

Fig. 10. AF2 potentiation of cholinergic responses. A. Current-clamp recording of membrane potential and conductance during short pulses of acetylcholine before and after a 2-minute application of AF2. The post-AF2 responses are increased in size. B. Ascaris muscle strip contraction responses to acetylcholine before and after AF2 application.

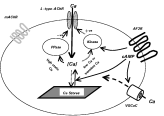
Fig. 11. Simple model of the hypothesised mechanism by which brief application of AF2 produces potentiation of acetylcholine receptor (AChR) responses. Kinases phosphorylate nicotinic AChRs (nAChRs) and increase channel opening; phosphatases have the opposite effect. AF2 activates adenyl cyclase producing cAMP that (1) activates PKA; (2) increases entry of calcium via voltage-activated calcium channels (VGCaC); (3) increases release of calcium from calcium stores via ryanodine receptors. Low levels of calcium preceded by transient high calcium (e.g. spikes) allow the kinases (PKA or CaM Kinase) to dominate over the phosphatases (PPase: including PP2B-calcineurin). Stimulation of phosphatases dominates over the kinases when there is high and static calcium. Entry of calcium through AChRs is hypothesized to be greater through the L-subtype receptor. Muscarinic AChRs (mAChRs) are shown to potentiate nAChR channel opening because atropine, a muscarinic antagonist, reduces nAChR responses.
EFFECTS OF CALCIUM, COBALT, RYANODINE AND ARECOLINE ON CONTRACTION AND AF2 POTENTIATION
Levamisole produces concentration-dependent contractions of Ascaris muscle strips (Fig. 5) in a manner that mimics its therapeutic action. We have described above, and reported, levamisole-activated depolarizations being smaller in the absence of extracellular calcium, an effect we interpreted as due to reduction in the activity of CaMK (Trailovic et al. 2002). In contraction experiments using muscle strips to test our model, we have observed that removal of extracellular calcium greatly depressed levamisole-induced contractions (see Fig. 12A), and that the addition of 5 mM cobalt, which selectively blocks voltage-activated calcium channels, depresses levamisole contractions. We have observed that 1 μM AF2 and 100 μM arecoline (a muscarinic agonist) have synergistic effects on levamisole contractions. These observations together support our model that suggests roles for voltage-activated calcium channels, AF2 and muscarinic receptors in increasing and modulating levamisole responses so that AF2 can potentiate levamisole contractions (Trailovic et al. 2005). Fig. 12B shows that a low concentration of ryanodine (0·1 μM) selectively blocks the AF2 potentiation of levamisole responses with little effect on control levamisole contractions. This latter observation indicates that the AF2 potentiation is mediated via ryanodine receptors (RyRs) of the sarcoplasmic reticulum. It is important to develop better knowledge of the details of the physiological and pharmacological properties of the voltage-activated channels, muscarinic receptors and calcium release pathways so that we can use this information to develop strategies for increasing levamisole responses and thereby overcome resistance.

Fig. 12. A. Ascaris muscle strip contraction levamisole dose-response plot in the presence (4 mM) and absence of calcium. Note that in the absence of extracellular calcium that the response is not abolished but shifted about 3-fold to the right and the maximum responses are not maintained. B. Ascaris muscle strip contraction levamisole concentration response plots: Control; +1 μM AF2; +0·1 μM ryanodine; +1 μM AF2 & 0·1 μM ryanodine. 0·1 μM ryanodine has greater inhibitory effects in the presence of AF2, than on the control levamisole concentration response.
LEVAMISOLE-ACTIVATED SINGLE-CHANNEL CURRENTS
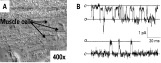
Recently we have started to compare the single-channel currents of levamisole-activated receptors in C. elegans with those present in A. suum and O. dentatum. Our approach has been to try to determine how useful is C. elegans as a model for parasitic nematodes and the limits for its use. We dissect C. elegans under the binocular microscope using a conventional sharp glass micropipette and the animal held in place with quick-setting water-sensitive glue applied from a micropipette (Richmond & Jorgensen, 1999). Single-channel recordings are made in the same manner as for A. suum. We will use cell-attached and isolated inside-out patches for most of our recordings. Fig. 13 shows a photomicrograph of the muscle cells and representative AChR channel currents from C. elegans muscle, which we are able to record with Cs to block K channels. Fig. 13 demonstrates our ability to record acetylcholine-activated channels of C. elegans muscle and shows channel currents that have a mean open-time of 1 ms and conductance of 21 pS. At this stage we can confirm that levamisole-activated channel currents can be recorded from C. elegans. We intend to continue these studies to allow a more detailed comparison of the model nematode and the parasitic nematodes.

Fig. 13. A. C. elegans muscle flap preparation for patch-clamp recording (arrows indicate individual muscle cells). B. Single-channel records using 30 μM acetylcholine as agonist at different membrane potentials; top: −50 mV, bottom: +50 mV. C and O represent channels closed and in the open state, respectively.
CONCLUSION
We have shown that the site of action of levamisole is more complex than was first imagined, with multiple genes being responsible for coding receptor ion-channels. We have also shown that the target sites are pharmacologically diverse with a separation of AChR subtypes, some of which are more and some of which are less susceptible to the effects of levamisole. We have also seen that the receptor ion-channel is modulated by phosphorylation and that the response to levamisole can be modulated through the action of other agonists. These new observations offer the possibility of developing a new approach to enhance the potency of levamisole and to counter resistance. It seems likely that the sites of action of the other anthelmintics on receptor ion-channels behave in a similar manner.

ACKNOWLEDGEMENTS
We are pleased to acknowledge the support of NIH, R01 – AI047194.