INTRODUCTION
In Europe the hard-bodied tick Ixodes ricinus (L.) (Acari: Ixodidae) is the main vector of Borrelia burgdorferi sensu lato (s.l.), the causative agent of the most commonly reported tick-borne disease of the northern hemisphere, Lyme borreliosis. Eleven Borrelia genospecies have been found associated with this tick species in Europe: B. afzelii, B. bavariensis, B. bissettii, B. burgdorferi sensu stricto (s.s.), B. carolinensis, B. finlandensis, B. garinii, B. lusitaniae, B. spielmanii, B. valaisiana, and a Borrelia genospecies related to relapsing fever spirochetes, B. miyamotoi (Rauter and Hartung, Reference Rauter and Hartung2005; Margos et al. Reference Margos, Vollmer, Cornet, Garnier, Fingerle, Wilske, Bormane, Vitorino, Collares-Pereira, Drancourt and Kurtenbach2009; Gern et al. Reference Gern, Douet, Lopez, Rais and Morán Cadenas2010; Cotté et al. Reference Cotté, Bonnet, Cote and Vayssier-Taussat2010; Casjens et al. Reference Casjens, Fraser-Liggett, Mongodin, Qiu, Dunn, Luft and Schutzer2011).
The biggest survival challenge for ticks is to maintain water balance whilst living in a relatively dry environment. This is even more essential to I. ricinus since this tick is extremely sensitive to temperature and humidity compared with other tick species (MacLeod, Reference MacLeod1935; Lees, Reference Lees1946; Aeschlimann, Reference Aeschlimann1972; Knülle and Rudolph, Reference Knülle, Rudolph, Obenchain and Galun1982; Sonenshine, Reference Sonenshine1991). Hence, saturation deficit, a measure of the drying power of the atmosphere depending on both temperature and relative humidity (Randolph and Storey, Reference Randolph and Storey1999), limits I. ricinus duration of questing (Perret et al. Reference Perret, Guerin, Diehl, Vlimant and Gern2003, Reference Perret, Rais and Gern2004) and survival in nature (Perret, Reference Perret2002; Perret et al. Reference Perret, Guigoz, Rais and Gern2000, Reference Perret, Rais and Gern2004; Burri et al. Reference Burri, Morán Cadenas, Douet, Moret and Gern2007; Morán Cadenas et al. Reference Morán Cadenas, Rais, Jouda, Douet, Humair, Moret and Gern2007) and in laboratory settings (Herrmann and Gern, Reference Herrmann and Gern2010).
Whilst questing on vegetation for their vertebrate hosts, I. ricinus ticks increase water loss so that they must return periodically to moister surroundings, such as the litter layer. There, they can extract water vapour from the atmosphere above a certain humidity level (Knülle and Rudolph, Reference Knülle, Rudolph, Obenchain and Galun1982). Hence, ticks are considered to move primarily in the vertical rather than the horizontal plane in order to seek the two resources that are necessary to their survival, i.e. water vapour and/or hosts (Goddard, Reference Goddard1993). However, Perret et al. (Reference Perret, Guerin, Diehl, Vlimant and Gern2003) observed that nymphs walked great distances, up to 9·56 m in a laboratory setting. In these experiments, ticks were constrained within vertical channels and, although it was difficult to evaluate the part that would represent horizontal movements, Perret et al. (Reference Perret, Guerin, Diehl, Vlimant and Gern2003) assumed that some of the displacements could represent horizontal movements. Later, Crooks and Randolph (Reference Crooks and Randolph2006) showed that nymphs with high-energy resources walked horizontally but over short distances only. Furthermore, they also reported that ticks that were slightly dehydrated were more likely to walk towards fully saturated than drier air.
Recently, we observed that field-collected I. ricinus ticks infected by B. burgdorferi s.l. survived better under unfavourable conditions of temperature and humidity (saturation deficit), suggesting that infected ticks might be affected differently by surrounding humidity (Herrmann and Gern, Reference Herrmann and Gern2010). In that context, it was interesting to determine whether field-collected I. ricinus nymphs of varying hydration status and level of energy reserves displayed a different walking activity and direction within a humidity gradient in the horizontal plane when infected by B. burgdorferi s.l. spirochetes.
MATERIALS AND METHODS
Tick collection and maintenance
The sampling site was a mixed forest (deciduous dominant) situated at 600 m above sea level (47°00′N and 6°57′E) on the south-facing slope of Chaumont Mountain (Neuchâtel, Switzerland) (Herrmann and Gern, Reference Herrmann and Gern2010). Host-seeking I. ricinus nymphs were sampled by flagging the low vegetation using a 1-m2 terry flag on 3 consecutive days in May 2010. In the laboratory, collected nymphs were divided into 3 groups exposed to different conditions. To obtain high-fat and fully hydrated ticks (H&F, Group 1), ticks were held over water in a box with a tight-fitting lid (98% relative humidity; RH) in the dark within a cold chamber at 4°C for at least 4 weeks as described by Crooks and Randolph (Reference Crooks and Randolph2006). Group 2 contained low-fat and moderately hydrated (L&M) ticks. These ticks were maintained over water in a transparent plastic box with small holes allowing airflow (87% RH) at room temperature (∼23°C); while group 3 ticks, low-fat and fully hydrated (L&F), were held over water in a transparent plastic box with a tight-fitting lid (98% RH) at room temperature (∼23°C). L&M and L&F ticks were exposed to the natural daylight conditions in May–June for 4 weeks before being returned to the cold chamber until use (Crooks and Randolph, Reference Crooks and Randolph2006).
Fat content analysis
Fat content was quantified in order to verify whether fat content of high-fat and low-fat groups of ticks differed. Samples of 40 nymphs from each group (H&F, L&F, and L&M) were analysed for their fat content as described by Randolph and Storey (Reference Randolph and Storey1999) and as calculated by Crooks and Randolph (Reference Crooks and Randolph2006). Briefly, ticks were dried at 70°C for 24 h, weighed individually to the nearest 1 μg, washed in 3 changes of chloroform for 24 h each, re-dried at 70°C for 24 h and re-weighed using a MX5 microbalance (Mettler Toledo, Greifensee, Switzerland).
Attraction tests
Choice arenas were slightly modified from those described by Crooks and Randolph (Reference Crooks and Randolph2006). They were made of 2 transparent polystyrene boxes (19 cm×9 cm×9 cm) with tight-fitting lids, joined by a transparent polystyrene tunnel (3 cm diameter, 28 cm long) sealed into holes cut in one side of each box. Humidity was monitored using an EE07-PFT5 hygrometer (E+E Elektronik, Engerwitzdorf, Austria) with a probe within different parts of the arena. A small dish of water was placed in one box whereas the other box remained empty, producing a humidity gradient of 90–45% RH within 30 min. The humidity gradient did not evolve much after 3 h (end time of test, see details below), only reaching 95–45% RH. In the experimental room at ambient temperature (∼23°C), this corresponds to saturation deficits (SD) ranging from 1·10 mmHg to 10·98 mmHg (1 mmHg=133·3 Pa), as calculated by Randolph and Storey (Reference Randolph and Storey1999).
Each replicate consisted of a set of 20 nymphs placed into a 1 cm×5 cm plastic culture tube (Milian, Meyrin, Switzerland) that was closed at both ends by pieces of cotton wool. Each piece of cotton wool was tied to a length of cotton thread. Experimental runs were conducted as described by Crooks and Randolph (Reference Crooks and Randolph2006). Briefly, 1 tick-laden tube was positioned in the centre of the arena tunnel with the cotton threads passing out through small holes cut into the box side that were closed by laboratory film (Parafilm M, Pechiney Plastic Packaging, Menasha, WI, USA). The arena was left undisturbed for 1 h with the lights on. Then the threads were gently pulled to remove the cotton wool from each end of the tube. The cotton wool pieces were left very close to the tube. Lights were left on for 1 h and switched off for 1 additional hour since, according to Perret et al. (Reference Perret, Guerin, Diehl, Vlimant and Gern2003), darkness induces I. ricinus ticks to move. Ticks were collected 2 h post-release and their position was recorded. Testing time was determined so that half of the ticks would walk out of the tube, based on the protocol of Crooks and Randolph (Reference Crooks and Randolph2006). Twenty-five replicates were performed for each tick group (H&F, L&F, and L&M). Humidity side and tick group were changed for every new experimental run.
Borrelia infection in ticks
Borrelia infection in ticks was detected using real-time PCR. Before DNA isolation, ticks were soaked in 70% ethanol and air-dried. Extraction of DNA from ticks was achieved using ammonium hydroxide as previously described (Guy and Stanek, Reference Guy and Stanek1991; Rijpkema et al. Reference Rijpkema, Golubic, Molkenboer, Verbeek-De Kruif and Schellekens1996; Herrmann and Gern, Reference Herrmann and Gern2010). Negative controls were included during DNA isolation, which consisted of reagents without template DNA.
A real-time PCR amplifying a fragment of the flagellin gene (Schwaiger et al. Reference Schwaiger, Péter and Cassinotti2001; Herrmann and Gern, Reference Herrmann and Gern2010) was used to detect and quantify Borrelia DNA in all field-collected ticks that were subjected to the humidity gradient tests. The strain B. afzelii NE1817 was used as quantification standard. Spirochete concentration in culture was evaluated using the Helber chamber. To extract DNA, the culture was washed twice with phosphate-buffered saline/MgCl2, and the pellet was resuspended in 30 μl of water and heated for 15 min at 100 °C (Postic et al. Reference Postic, Assous, Grimont and Baranton1994). The Borrelia DNA stock was aliquoted at 105 spirochetes per μl and stored at −20°C. Serial dilutions were made from stored spirochete DNA in order to obtain 5 standard solutions with concentrations of Borrelia DNA ranging from 102 to 105 copies per μl.
The 50 μl real-time PCR mixture (Schwaiger et al. Reference Schwaiger, Péter and Cassinotti2001) consisted of 10 μl of 5×buffer, 5 μl of 25 mm MgCl2, 1 μl of 10 mm dNTPs, 1 μl of 20 μ m FlaF1A forward primer, 1 μl of 20 μ m FlaR1 reverse primer, 1 μl of 10 μ m FlaProbe1 probe, 0·25 μl of HotStart Taq Polymerase (Kapa Biosystems, Woburn, MA, USA), 20·75 μl of water and 10 μl of the extracted DNA. In each run, 1 extraction negative control (10 μl, see above), 1 PCR negative control (10 μl of water instead of 10 μl of the extracted DNA) and 3 series of the 5 standards were included.
Following an incubation step at 95°C for 10 min, the samples were submitted to 45 repeated amplification cycles (95°C for 15 s, 60°C for 1 min) (Schwaiger et al. Reference Schwaiger, Péter and Cassinotti2001) in an iCycler Optical Module (Bio-Rad, Reinach, Switzerland) using strip PCR tubes and flat caps (Scientific Specialties Inc, Lodi, CA, USA).
PCR and RLB were used to identify the Borrelia species in the field-collected ticks that were detected positive by real-time PCR as described in Herrmann and Gern (Reference Herrmann and Gern2010). The variable spacer region between 2 repeated copies of the 23S and 5S ribosomal genes was amplified with primers 23S-Bor and B-5S-Bor (Alekseev et al. Reference Alekseev, Dubinina, Van de Pol and Schouls2001). PCR amplifications were run in a Tgradient Thermocycler 96 (Whatman Biometra, Göttingen, Germany) by using a touchdown PCR program (Burri et al. Reference Burri, Morán Cadenas, Douet, Moret and Gern2007; Herrmann and Gern, Reference Herrmann and Gern2010). Positive and negative controls were included in each PCR. In positive controls, isolates of B. valaisiana (VS116), B. lusitaniae (PotiB1), B. burgdorferi s.s. (B31) or B. garinii (NE11) replaced DNA samples, whereas water substituted them in negative controls.
For Borrelia identification by RLB, PCR products were hybridized to 15 oligonucleotide probes (Rijpkema et al. Reference Rijpkema, Molkenboer, Schouls, Jongejan and Schellekens1995; Poupon et al. Reference Poupon, Lommano, Humair, Douet, Rais, Schaad, Jenni and Gern2006; Gern et al. Reference Gern, Douet, Lopez, Rais and Morán Cadenas2010) blotted in lines on an activated Biodyne C membrane (Pall Europe Ltd, Portsmouth, UK) using a Miniblotter 45 (Immunetic, Cambridge, MA, USA). Hybridization was visualized by incubating the membrane with enhanced chemiluminescence detection liquid (Amersham Biosciences Europe, Switzerland) and by exposing the membrane to X-ray film (Hyperfilm, GE Healthcare, UK).
Statistical analysis
The Mann-Whitney test was performed in order to determine whether there was a difference in fat content between tick groups, and whether spirochete load differed among Borrelia genospecies. Simple χ 2 tests were used to compare walking activity, direction of movement between tick groups. All statistics were calculated with R for Mac OS X (R Development Core Team, 2011).
RESULTS
Fat content analysis
The fat content of the low-fat moderately hydrated (L&M) ticks (4·15±3·98 μg) was not statistically different from that of the low-fat fully hydrated (L&F) ticks (4·28±3·38 μg) (Mann-Whitney test; P=0·51). In contrast, the fat content of high-fat fully hydrated (H&F) ticks (9·25±6·64 μg) was significantly higher than that of the L&M and L&F ticks (Mann-Whitney test; H&F-L&M P=0, H&F-L&F P=0·0001). According to Crooks and Randolph (Reference Crooks and Randolph2006), fat content is positively correlated with tick size, measured by tick fat-free (reduced) dry mass. Therefore a correction for size was calculated by dividing fat content by tick reduced dry mass (Crooks and Randolph, Reference Crooks and Randolph2006). After correction for size, fat content of the H&F ticks (0·137±0·099) remained significantly higher than that of the L&M (0·073±0·076) and L&F (0·074±0·059) ticks (Mann-Whitney test; H&F-L&M P=0·0003, H&F-L&F P=0·001) and it did not differ between the L&M and L&F ticks (Mann-Whitney test; P=0·78).
Borrelia infection in ticks
Among the 1500 questing nymphs that were tested for B. burgdorferi s.l. by real-time PCR, 29·85% (n=448) were infected. Globally, infection load in questing nymphs ranged from 2 to 896 000 spirochetes per tick. Mean spirochete number was 33 971 spirochetes per nymph while median spirochete number was 4300.
Identification of Borrelia genospecies by RLB was possible in 413/448 nymphs. Nymphs were mainly infected by 1 Borrelia genospecies (86·4%) (Table 1). Six Borrelia genospecies were identified: B. afzelii, B. bavariensis, B. burgdorferi s.s, B. garinii, B. miyamotoi, and B. valaisiana (Table 1). B. bissettii, B. lusitaniae, and B. spielmanii were not observed.
Table 1. Distribution of Borrelia genospecies and mean spirochete number in questing Ixodes ricinus nymphs in Neuchâtel, Switzerland

a af, B. afzelii; bav, B. bavariensis; ga, B. garinii; miy, B. miyamotoi; ss, B. burgdorferi sensu stricto; vs, B. valaisiana.
b n=413.
c Mean spirochete number was not calculated when frequency was below 10.
Spirochete load varied between Borrelia genospecies (Table 1). Infections by B. bavariensis and by B. garinii consisted of significantly more spirochetes per tick (89 419 and 69 660, respectively) than infections by B. afzelii (15 531) (Mann-Whitney test; P=0 and P=0·008, respectively), by B. valaisiana (12 981) (Mann-Whitney test; P=0 and P=0·005, respectively), and B. burgdorferi s.s. (8985) (Mann-Whitney test; P=0·001 and P=0, respectively). Infections by B. miyamotoi (n=2) were excluded from the statistical analyses due to their low frequency.
Walking activity
Within 2 h, a significantly higher number of low-fat ticks walked out of the tube than stayed inside (χ 2 test; L&M: P=0; L&F: P=0). Hence, 65% (323/500) L&M and 60% (298/500) L&F nymphs crawled out of the tube. In contrast, high-fat ticks had a higher tendency to stay inside the introduction tube (54%, 270/500) than walk out (χ 2 test; P=0·07). Thus, more low-fat nymphs (L&M: 323/500 and L&F: 298/500) walked out of the tube than high-fat (H&F: 230/500) individuals (Fig. 1). The difference was highly significant for moderately hydrated ticks (χ 2 test; P=0) and for fully hydrated ticks (χ 2 test; P=0). Among low-fat individuals, there was no difference in walking activity between moderately (323/500 out of tube) and fully hydrated ticks (298/500 out of tube) (χ 2 test; P=0·12).

Fig. 1. The percentage of high-fat fully hydrated (H&F), low-fat fully hydrated (L&F), and low-fat moderately hydrated (L&M) Ixodes ricinus ticks that stayed inside (light grey bars) or walked (white bars) out of the introduction tube after 2 h within a humidity gradient.
Among low-fat (L&M and L&F) ticks, walking activity was not different between uninfected and Borrelia-infected individuals. In fact, among L&M ticks 64% (222/347) uninfected and 66% (101/153) infected individuals crawled out of the tube (χ 2 test; P=0·74), and among L&F nymphs 61% (221/363) uninfected and 56% (77/137) infected ticks walked out of the tube (χ 2 test; P=0·4). In contrast, among H&F individuals, significantly more uninfected ticks (51%, 173/342) walked out of the tube than infected ticks (36%, 57/158) (χ 2 test; P=0·003) (Fig. 2). Hence, H&F infected ticks rather stayed inside the introduction tube (64%, 101/158) than walked out of it (36%, 57/158).
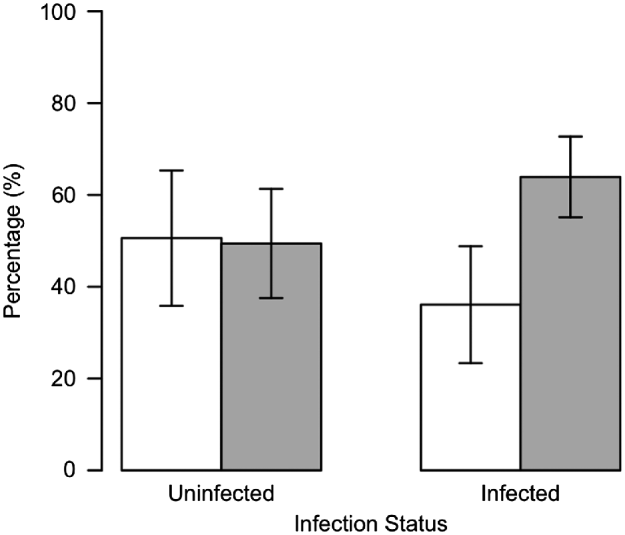
Fig. 2. The effect of Borrelia infection on walking activity of high-fat fully hydrated Ixodes ricinus nymphs after 2 h within a humidity gradient. Light grey bars represent ticks that stayed within the introduction tube while white bars represent those that walked out.
Direction of movement (dry versus wet end of the arena)
To evaluate direction of movement, 3 categories of ticks were considered: (1) those that reached the empty box, i.e. moved to the dry end, (2) those that reached the box containing the dish of water, i.e. moved to the wet end, and (3) those that stayed in the tunnel, i.e. did not move. Although some ticks might have moved 1–2 cm inside the tunnel and have been categorized as ‘did not move’, such cases were rare (less than 1%). There was a clear distinction between ticks that moved to the boxes or stayed close to the plastic culture tube. A higher proportion of low-fat individuals moved towards the wet than the dry end of the arena (χ 2 test; L&M: P=0; L&F: P=0), whereas a lower proportion of high-fat nymphs (H&F) walked towards the wet than the dry end of the arena (χ 2 test; P=0·01) (Fig. 3). Hence, 42% (210/500) L&M and 38% (191/500) L&F ticks moved towards the wet end, and 23% (113/500) L&M and 21% (107/500) L&F nymphs walked to the dry end of the arena. In contrast, 19% (96/500) H&F ticks walked towards the wet end and 27% (134/500) H&F nymphs moved towards the dry end of the arena. The difference in walking direction between groups of ticks with different fat contents was statistically significant (χ 2 test; L&M-H&F: P=0, L&F-H&F: P=0). However, tick hydration status (only tested among low-fat ticks) (moderate, L&M: 87% RH, or fully, L&F: 98% RH) had no effect on the direction of movement (χ 2 test; P=0·88). Similarly, Borrelia-infection had no significant effect on walking direction towards the dry or the wet end of the arena (χ 2 test; H&F: P=0·09, L&F: P=0·16, L&M: P=0·29).

Fig. 3. The percentage of high-fat fully hydrated (H&F), low-fat fully hydrated (L&F), and low-fat moderately hydrated (L&M) Ixodes ricinus ticks that walked towards the wet (light grey bars) or the dry end (white bars) of the arena after 2 h.
DISCUSSION
Fat content analysis
Fat content is a source of energy derived from each bloodmeal (Uspensky, Reference Uspensky1995). Here it was quantified in order to determine available energy reserves in I. ricinus nymphs among the different groups (high-fat and low-fat). Nymphs treated to conserve their fat did indeed contain higher lipid content (9·3 μg) than those treated to lose some of their fat (4·2–4·3 μg). However, it appeared that low-fat nymphs from the present study had a fat content that was in between that of high-fat and low-fat nymphs (4·7 μg and 3·9 μg, respectively) from the work by Crooks and Randolph (Reference Crooks and Randolph2006). In fact, nymphs collected in the spring in Neuchâtel did not only have a higher mean fat content (9·3 μg, this study; 7·12 μg; Herrmann and Gern, Reference Herrmann and Gern2010) but also a higher fat range (1–25 μg, this study; 1–29 μg; Herrmann and Gern, Reference Herrmann and Gern2010) than those sampled in southern England all over the year (0·2–15 μg; Randolph et al. Reference Randolph, Green, Hoodless and Peacey2002; 1·6–18·2 μg; Randolph and Storey, Reference Randolph and Storey1999). Moreover, when fat content of spring ticks from Neuchâtel was corrected for size and converted into fat index (Randolph et al. Reference Randolph, Green, Hoodless and Peacey2002) (mean=0·035), it was strikingly higher than that of spring ticks from southern England (approximately 0·015), and even slightly higher than that of fall ticks from southern England (approximately 0·032). This suggests that I. ricinus ticks from central Europe and southern England are more distinct than previously thought. Hence, they seem to distinguish themselves not only by different host-seeking behaviour and activity as suggested by Kurtenbach et al. (Reference Kurtenbach, Hanincova, Tsao, Margos, Fish and Ogden2006), i.e. in southern England, ticks might quest higher in the vegetation and host-seeking activity is present in spring-summer while in central Europe questing activity is present in spring and occasionally in autumn, but also by overall fat content.
Borrelia infection in ticks
Prevalence of infection by Borrelia in I. ricinus nymphs (29·85%) was similar to that reported by Casati et al. (Reference Casati, Bernasconi, Gern and Piffaretti2004) (29%) and slightly higher than that observed by Herrmann and Gern (Reference Herrmann and Gern2010) (25·5%). All these studies were conducted in the same suburban forest of Neuchâtel, Switzerland. Such slight fluctuations of B. burgdorferi s.l. prevalence over the years have already been observed in a nearby area on Chaumont Mountain (Jouda et al. Reference Jouda, Perret and Gern2004; Morán Cadenas et al. Reference Morán Cadenas, Rais, Jouda, Douet, Humair, Moret and Gern2007).
Borrelia genospecies frequency was in line with what was previously observed in this area: the most abundant species was B. afzelii (42·9%), followed by B. garinii (31%), B. valaisiana (25·4%), B. burgdorferi s.s. (5·6%), while B. miyamotoi (2·9%) was the least common one (Casati et al. Reference Casati, Bernasconi, Gern and Piffaretti2004; Jouda et al. Reference Jouda, Perret and Gern2004; Morán Cadenas et al. Reference Morán Cadenas, Rais, Jouda, Douet, Humair, Moret and Gern2007; Gern et al. Reference Gern, Douet, Lopez, Rais and Morán Cadenas2010; Herrmann and Gern, Reference Herrmann and Gern2010). B. bavariensis (6·8%), formerly B. garinii OspA serotype 4, a newly described genospecies (Margos et al. Reference Margos, Vollmer, Cornet, Garnier, Fingerle, Wilske, Bormane, Vitorino, Collares-Pereira, Drancourt and Kurtenbach2009), is reported for the first time in the area, although it was already described in Switzerland (Staatswald) (Hu et al. Reference Hu, Wilske, Fingerle, Lobet and Gern2001; Huegli et al. Reference Huegli, Hu, Humair, Wilske and Gern2002; Pérez et al. manuscript submitted).
The spirochete load in questing ticks observed in the present study reached a mean of 33 971 and a median of 4300 spirochetes per tick, showing a higher load of spirochetes than that reported in a previous study conducted in the same area (mean=18 640; median=2760 spirochetes per tick) (Herrmann and Gern, Reference Herrmann and Gern2010). Since Borrelia genospecies influence infection load (Herrmann and Gern, Reference Herrmann and Gern2010), these differences in spirochete load may be due to different genospecies distribution in the two studies. For example, infections by B. garinii, which consisted of high spirochete numbers (78 000 per tick), represented 23·4% of infections in the study by Herrmann and Gern (Reference Herrmann and Gern2010), while infection by B. garinii and by B. bavariensis (previously included in infections by B. garinii) (69 660 and 89 419, respectively) accounted for 37·8% of infections in this study.
Influence of Borrelia infection on walking activity
To date, numerous vector-borne parasites have been shown to alter phenotypic traits of their arthropod vectors, most likely in a way that enhances their probability of transmission (Hurd, Reference Hurd2003; Lefèvre and Thomas, Reference Lefèvre and Thomas2008). For example, Trypanosoma cruzi increases biting rate in bug Mepraia spinolai (Botto-Mahan et al. Reference Botto-Mahan, Cattan and Medel2006), Plasmodium mexicanum alters temperature preference in sand fly Lutzomyia vexator (Fiahlo and Schall, Reference Fiahlo and Schall1995), P. falciparum alters immune response in mosquito Anopheles gambiae (Lambrechts et al. Reference Lambrechts, Morlais, Awono-Ambene, Cohuet, Simard, Jacques, Bourgouin and Koella2007), Babesia microti increases lifespan in the tick I. trianguliceps (Randolph, Reference Randolph1991), or B. burgdorferi increases survival in I. ricinus under hot and dry conditions (Herrmann and Gern, Reference Herrmann and Gern2010). Another example of behavioural/physiological alteration in the arthropod vector due to parasite presence is brought to light in the present study. Although walking activity was similar between low-fat uninfected and Borrelia-infected nymphs, it seems that among high-fat ticks, Borrelia infection influenced I. ricinus walking activity. Thus, more high-fat uninfected nymphs walked out of the introduction tube than high-fat Borrelia-infected individuals, which rather stayed inside the tube. Higher locomotion activity in uninfected ticks than in individuals infected by B. burgdorferi s.l. has been previously reported. In fact, Alekseev et al. (Reference Alekseev, Jensen, Dubinina, Smirnova, Makrouchina and Zharkov2000) observed that infection by B. burgdorferi s.l. in I. persulcatus and I. ricinus ticks suppressed walking activity of both adult and immature individuals compared to uninfected individuals. Hence, B. burgdorferi s.l. spirochetes seem to reduce tick motor activity in high-fat individuals (ticks used by Alekseev et al. in 2000 were field-collected and stored, similarly to high-fat individuals in this study), apparently manipulating ticks to behave in a beneficial way to them. It may be hypothesized that Borrelia spirochetes only affect the behaviour of ticks with high-energy reserves because they may have access to their host vector energy resources without threatening to make the tick run out of energy and die, which would be a disaster for the spirochetes. Thus, energy-using borreliae may manipulate the tick to reduce its motor activity when the arthropod is placed in favourable surroundings for the spirochete, for example when the tick is on the vegetation, increasing the tick chances to find a passing host, in turn allowing the spread of Borrelia spirochetes to a new host. In contrast, spirochetes in ticks with reduced energy reserves may not have access to their vector energy resources, forcing them to reduce their metabolism and preventing the spirochetes from manipulating tick behaviour in any way.
Influence of energy reserves and hydration status on walking activity
A 2-h period was chosen as testing time based on Crooks and Randolph (Reference Crooks and Randolph2006) so that half of the ticks would walk out of the tube. The estimate was quite correct since 46% of the high-fat and 62% of the low-fat ticks moved out of the tube during that period.
Interestingly, high-fat nymphs walked less than low-fat ones in the present study while the opposite phenomenon (about 70% and 40%, respectively) was observed by Crooks and Randolph (Reference Crooks and Randolph2006). Differing levels of energy reserves between the nymphs in the two studies seem to be one of the factors explaining this differential walking behaviour. The proportion of low-fat nymphs that walked (62%), which proved not to be affected by Borrelia infection, was in between that of high-fat (70%) and low-fat (40%) individuals that walked in the study by Crooks and Randolph (Reference Crooks and Randolph2006). Similarly, fat content in low-fat ticks in our study (4·2–4·3 μg) was in between that of high-fat (4·7 μg) and low-fat (3·9 μg) ticks, in Crooks and Randolph (Reference Crooks and Randolph2006). Thus, low-fat nymphs in our study did not correspond to either low-fat or high-fat individuals in the work by Crooks and Randolph (Reference Crooks and Randolph2006). They had more energy reserves than the first and less than the second, and behaved as such, i.e. walked more than low-fat ticks and less than high-fat ticks.
Another factor that may explain the difference between the two studies is Borrelia infection. Although Crooks and Randolph (Reference Crooks and Randolph2006) did not provide information about Borrelia infection, it may be considered that infection prevalence was much lower among the nymphs they tested. In fact, tested ticks were collected in southern England where infection prevalence is below 12·4% (Rauter and Hartung, Reference Rauter and Hartung2005; Vollmer et al. Reference Vollmer, Borman, Dinnis, Seelig, Dobson, Aanensen, James, Donaghy, Randolph, Feil, Kurtenbach and Margos2011) whereas infection prevalence reached 30% in the present study. Hence, this difference in infection prevalence may explain the lower proportion of high-fat nymphs walking outside the introduction tube (since Borrelia reduced walking activity of high-fat individuals) in the present study (36% of infected ticks moved versus 51% of uninfected ticks).
Hydration status (only tested among low-fat nymphs) had no significant effect on tick walking activity. However, it appeared that moderately hydrated individuals had a higher tendency to walk than fully hydrated ones. This is in line with what Lees (Reference Lees1948) described. He reported that dehydrated ticks were intensely active in dry air (about 70% RH in this study) while hydrated individuals were not. This seems to indicate that ticks with higher deficiencies in water reserves are more prone to walk, probably in order to seek for what they lack.
Direction of movement (dry versus wet end of the arena)
Hydration status, which only differed among low-fat individuals, appeared not to influence direction of movement. This may probably be explained by the fact that their hydration status was very similar (they were maintained at 98% and 87% RH, respectively). Moderately hydrated ticks would probably have needed to be maintained under drier conditions in order to be differentiated from fully hydrated individuals.
The level of energy reserves played a role in the direction of movement of I. ricinus nymphs within a humidity gradient. Low-fat individuals (fully and moderately hydrated) tended to walk up the humidity gradient, i.e. towards the area saturated in water vapour, whereas high-fat individuals tended to move down the humidity gradient, i.e. towards an area containing less water vapour than where they were released. Walking of high-fat fully hydrated ticks towards the dry end of the arena is in line with what has been described by Lees (Reference Lees1948). He reported that nymphal and female I. ricinus ticks that had been previously exposed to saturated air avoided the wet side (95% RH) of the arena, preferring to walk towards the dry side (34% RH). On the contrary, ticks that were kept under unsaturated air conditions moved towards the opposite direction. This behaviour was observed on the horizontal and the vertical plane (Lees, Reference Lees1948). It appears in the present study that when I. ricinus nymphs are treated so that they become low-fat, they behave similarly to ticks exposed to unsaturated air in the study of Lees (Reference Lees1948), suggesting that not only state of hydration but also the level of energy reserves plays a role in triggering the locomotion response.
These results seem to indicate that I. ricinus nymphs that are not in immediate need for energy or water move towards unfavourable dry surroundings, supposedly representing in nature the walk up the vegetation to quest for a host where humidity decreases. In contrast, ticks with lower energy reserves, despite high water reserves, tend to walk towards a wet comfortable zone where they remain fully hydrated, such as the litter layer in the field. Thus, it is as though nymphs with sufficient energy reserves may take the risk to wander randomly outside their comfort zone in order to find a potential host, while leaner counterparts need to be cautious about their energy-costly movements and move towards favourable (wet) conditions before waiting until a positive stimulus (such as host odour) trigger them to walk again.
Nymphs infected by Borrelia spirochetes did not walk preferentially towards the wet or the dry end within a humidity gradient. This seems to indicate that borreliae do not affect the need of I. ricinus nymphs for moist conditions as might have been expected because of higher survival of ticks infected by B. burgdorferi s.l. under hot and dry conditions (Herrmann and Gern, Reference Herrmann and Gern2010).
In conclusion, the results described here point out that I. ricinus nymphs with lower energy reserves are more likely to walk horizontally than their counterparts with higher energy reserves. When I. ricinus nymphs walk, those with lower energy reserves are more likely to move towards fully saturated air than drier air, while those with higher energy reserves are more likely to move to the opposite direction. Moreover, it seems that I. ricinus immature ticks that are infected by Borrelia spirochetes are less likely to move on the horizontal plane than uninfected specimens, suggesting a manipulation by spirochetes in ticks with sufficient available energy reserves. Thus, it appears that I. ricinus nymphs will crawl horizontally over short distances within a humidity gradient depending both on their energy reserves and on the presence of B. burgdorferi spirochetes.
ACKNOWLEDGEMENTS
We would like to thank C. Dupasquier and O. Rais for their help in collecting ticks, as well as P. Guerin for providing useful material. This study is part of the Ph.D. thesis of C. Herrmann and has been supported by the Swiss National Science Foundation grant (FN 310030_127064).






