Introduction
Compared to other wildlife, the relationship between parasites and their environment is much more complex. Parasites are not uniformly distributed across their range, but rather they cluster on hosts as if like the individual ‘islands’ their host represents (Krasnov, Reference Krasnov2008). Furthermore, interactions with other parasite species (Krasnov et al., Reference Krasnov, Stanko, Miklisova and Morand2006) and the level of parasite specialization may restrict distribution and abundance due to resource availability (Combes, Reference Combes2001). Small mammals are a frequently analysed group of flea hosts, attractive both ecologically and epidemiologically (Bonnefoy et al., Reference Bonnefoy, Kampen and Sweeney2008; Stanko, Reference Stanko2014; Kraljik et al., Reference Kraljik, Paziewska-Harris, Miklisová and Blaňarová2016; Kurucz et al., Reference Kurucz, Madai, Bali, Hedericz, Horváth, Kemenesi and Jacab2018). They also accommodate the highest diversity of flea species (Krasnov et al., Reference Krasnov, Shenbrot, Khokhlova and Poulin2007; Krasnov, Reference Krasnov2008) but, unlike endoparasites and permanent ectoparasites, fleas spend much of their lives outside the body of these small mammals. Therefore, they are not only affected by conditions associated with their host, but also by abiotic factors in the environment where the host lives (Krasnov, Reference Krasnov2008), creating a causal chain of flea–host–environment interactions; in other words a ‘dual parasite environment’. Although the host mammal is the parasite's immediate, first-order environment, the outside environment where it lives becomes its second-order environment. Comprehending the pattern of spatial changes on the structure of parasite communities contributes towards mapping, predicting and preventing the risks parasites pose as pathogen vectors (Caminade et al., Reference Caminade, Kovats, Rocklov, Tompkins, Morse, Colón-González, Stenlund, Martens and Lloyd2014; Maestri et al., Reference Maestri, Shenbrot and Krasnov2017).
Elevation gradients have great potential for increasing the level of knowledge about the mechanisms that affect the diversity, distribution and species richness of organisms (Grytnes and McCain, Reference Grytnes and McCain2007; McCain and Grytnes, Reference McCain, Grytnes and Chichester.2010). There are changes in climatic, biotic, spatial, historical and even anthropogenic factors at higher and lower elevations (Rahbek, Reference Rahbek1995; Grytnes and McCain, Reference Grytnes and McCain2007; Barry, Reference Barry2008; Fischer et al., Reference Fischer, Blaschke and Bässler2011). Currently, four main, altitude-related biodiversity patterns are recognized: (i) declining biodiversity with increasing elevation; (ii) unimodal pattern with a mid-elevation peak; (iii) plateaus with consecutively high richness across lower elevations in the diversity gradient and (iv) plateaus in richness across low elevations with a mid-elevation peak (McCain and Grytnes, Reference McCain, Grytnes and Chichester.2010). Understanding the biodiversity patterns of parasites is considered extremely important because they are an essential part of the communities found on animals (Poulin, Reference Poulin2007; Krasnov, Reference Krasnov2008).
Mahnert (Reference Mahnert1972) observed hosts in subalpine and alpine zones of the Alps with higher flea infestations than those at lower elevations. Maher and Timm (Reference Maher and Timm2014) examined in Colorado the impact of a short elevational gradient (approximately 900 m) on small mammals and their fleas. Although flea species richness was related to the number of host species, there was no evidence of a significant relationship between richness and elevation. Instead, diversity increased with elevation and changes in flea communities were independent of those in host communities. Unimodal patterns of peak flea diversity were recorded in both the mountains of East Asia (Gong et al., Reference Gong, Wu, Duan, Feng, Zhang and Liu2005) and western Siberia (Sapegina, Reference Sapegina2000) with a mid-peak, whereas Gong et al. (Reference Gong, Wu, Duan, Feng, Zhang and Liu2005) adds that species richness was influenced at varying elevations by factors such as precipitation, climate, habitat and human activity. Conversely, increased diversity among fleas has been recorded at higher elevations in East Africa (Eisen et al., Reference Eisen, Borchert, Mpanga, Atiku, MacMillan, Boegler, Montenieri, Monaghan and Gage2012; Meliyo et al., Reference Meliyo, Kimaro, Msanya, Mulungu, Hieronimo, Kihupi, Gulinck and Deckers2014). In addition to altitude, other factors such as relief, landscape, human impact on the soil and therewith its effect on vegetation formations have all had a significant effect on the incidence of fleas. Similarly, Beer et al. (Reference Beer, Cook and Schwab1959) observed a higher affinity of fleas in North America for the physical properties of the environment than for the host species. Benton and Altmann (Reference Benton and Altmann1964) also considered elevation and a set of factors (humidity, air temperature and vegetation cover) associated with changing altitudes to have influenced significantly the occurrence of certain flea species, whereas Sapegina (Reference Sapegina2000) viewed the distribution of fleas to be dependent equally on host and climatic factors in the external environment. Critical discrepancies between the results from flea research might be caused by different evolution of flea communities in a region affected by local environmental conditions, such as the climate or the landscape itself (Krasnov, Reference Krasnov2008).
Flea activity, reproduction and development are influenced by a combination of factors, where environmental conditions such as temperature and humidity play the most important roles. Their diversity is expressed in seasonal fluctuations in the number of fleas. These fluctuations in abundance are less pronounced where the climate is stable and more evident in areas with significant seasonal changes (Krasnov, Reference Krasnov2008). The seasonal abundance of fleas is generally associated with climatic fluctuations (Marshall, Reference Marshall1981), which has been noted by Theodor and Costa (Reference Theodor and Costa1967). In Central Europe, conditions cause some species to appear at the beginning of the season and others only in the second half of the year, leading to structural changes in the flea community during each season (Krasnov et al., Reference Krasnov, Shenbrot, Mouillot, Khokhlova and Poulin2005).
The impact on effect elevation on flea communities was only observed indirectly in the natural conditions found in the Pannonian Plain and the Carpathian Mountains. Dudich (Reference Dudich1992) evaluated the quantitative structures of communities that were composed of several groups of ectoparasites colonizing small terrestrial mammals, whereas Krasnov et al. (Reference Krasnov, Stanko, Miklisova and Morand2006) analysed the composition of flea communities in different habitats throughout Slovakia. Both studies looked at their variability among populations of the same host species inhabiting different lowland and mountain habitats.
Host specialization is another basic characteristic of any parasitic organism (Krasnov, Reference Krasnov2008). Although some flea species prefer specific hosts, others are opportunistic and infest a wider range. Rosicky (Reference Rosicky1957) divides fleas into three groups, based on their degree of host specialization. Two are the monozoic and stenozoic associations between host and parasite, whereas the third covers more opportunistic parasites. On the other hand, Rosicky (Reference Rosicky1957) distinguishes four types of hosts – main, secondary, random and temporary – according to the flea species' own preferences. A preference index that measures how a particular flea associates with a certain host (Dudich, Reference Dudich1987) has been calculated from data on species frequency and dominance.
All of these studies monitored changes in the distribution and diversity of fleas along specifically outlined elevational gradients. Our study seeks from extensive research to analyse the effect of elevation on the distribution, diversity and change in flea communities from the Pannonian Plain to summits of the Carpathian Mountains, with a view towards seasonality.
Materials and methods
Study area
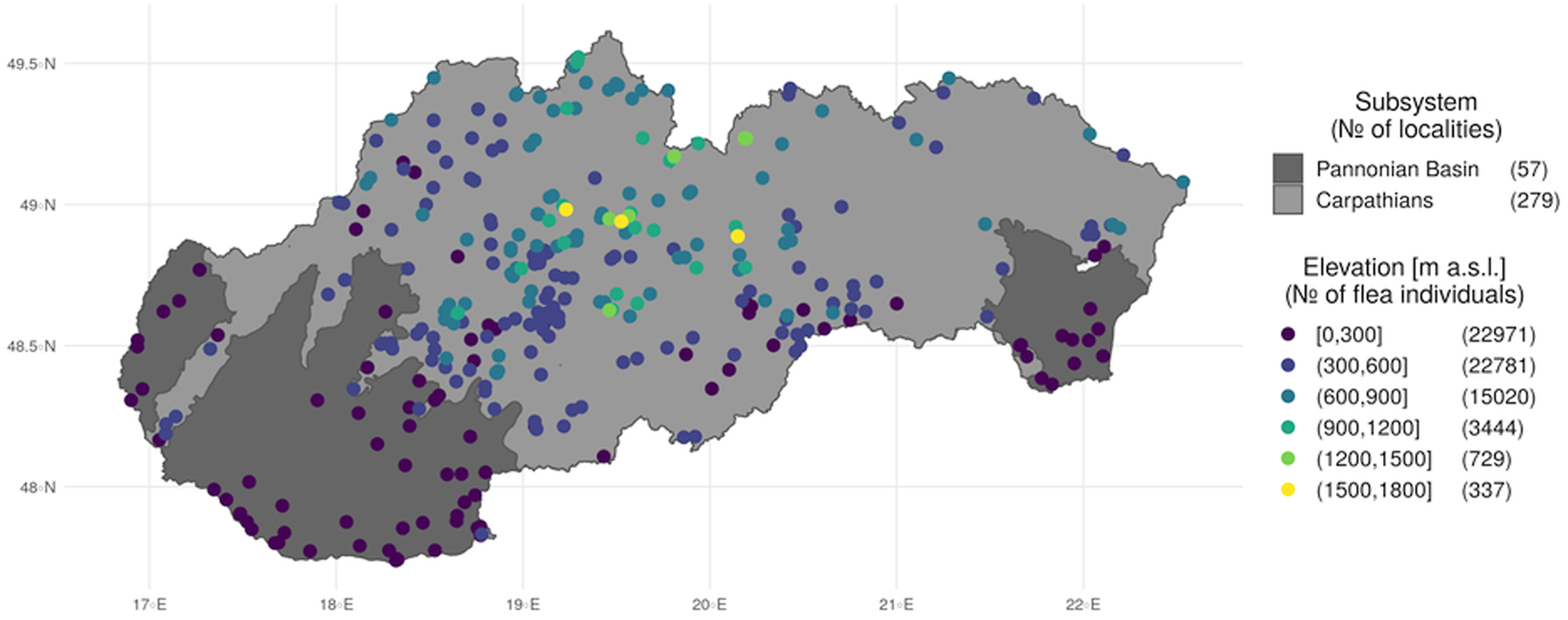
Small mammals were captured at 736 locations, in 82 orographic units predominantly composed of woodland habitats and at ecotones along watercourses. They were scattered throughout Slovakia from the lowlands of the Pannonian Plain to river valleys and the base of the mountain peaks in the Carpathians.
The Danubian Lowland was the warmest of the areas where the animals were trapped. Here in this section of the Pannonian Plain in south-western Slovakia, mean air temperature in January has been measured at −1 to −2°C, in July at 18–21°C and over the year it averages between 9 and 11°C. In comparison, the Eastern Slovak Lowland, located in south-eastern Slovakia within the north-eastern portion of the Great Hungarian Plain, experiences a slightly lower air temperature on average. The annual mean air temperature at river basins and valleys crossing the lowlands range from 6 to 8°C, and less than 6°C at the highest altitudes of the rivers near their sources, while falling further at higher elevations. Average temperatures are 4–5°C at 1000 m above sea level, around −1°C at 2000 m and less than −3°C at the ridges of the Carpathians in Slovakia.
Mean annual total precipitation varies from less than 500 mm on the Pannonian Plain to 2000 mm at the highest peaks of the Carpathians. In Slovakia, precipitation generally increases with elevation at a rate of approximately 50–60 mm per 100 m altitude. The rainiest month of the year is either June or July, with the least precipitation occurring between January and March. The high rainfall variability causes frequent droughts, especially in the lowlands, which sometimes last for long periods. At mid-peak and peak elevations in mountainous regions, a large percentage of precipitation falls during the winter as snow. It has been recorded in lowland regions between October and April and at elevations above 1500–2000 m throughout the year, including during the summer months. The average duration of snow cover at lower elevations in Western Slovakia is less than 40 days, whereas in the Eastern Slovak Lowland, with its more continental climate, snow remains on the ground more than 50 days out of the year. In drainage basins, the figure is 60–80 days, rising in the mountains to 80–120 days. The peaks of the Carpathian Mountains have snow cover for more than 200 days out of the year, the largest number of days in Slovakia. At elevations higher than 1300 m above sea level, snow cover is common and it can be more than 100 cm thick (Fig. 1).
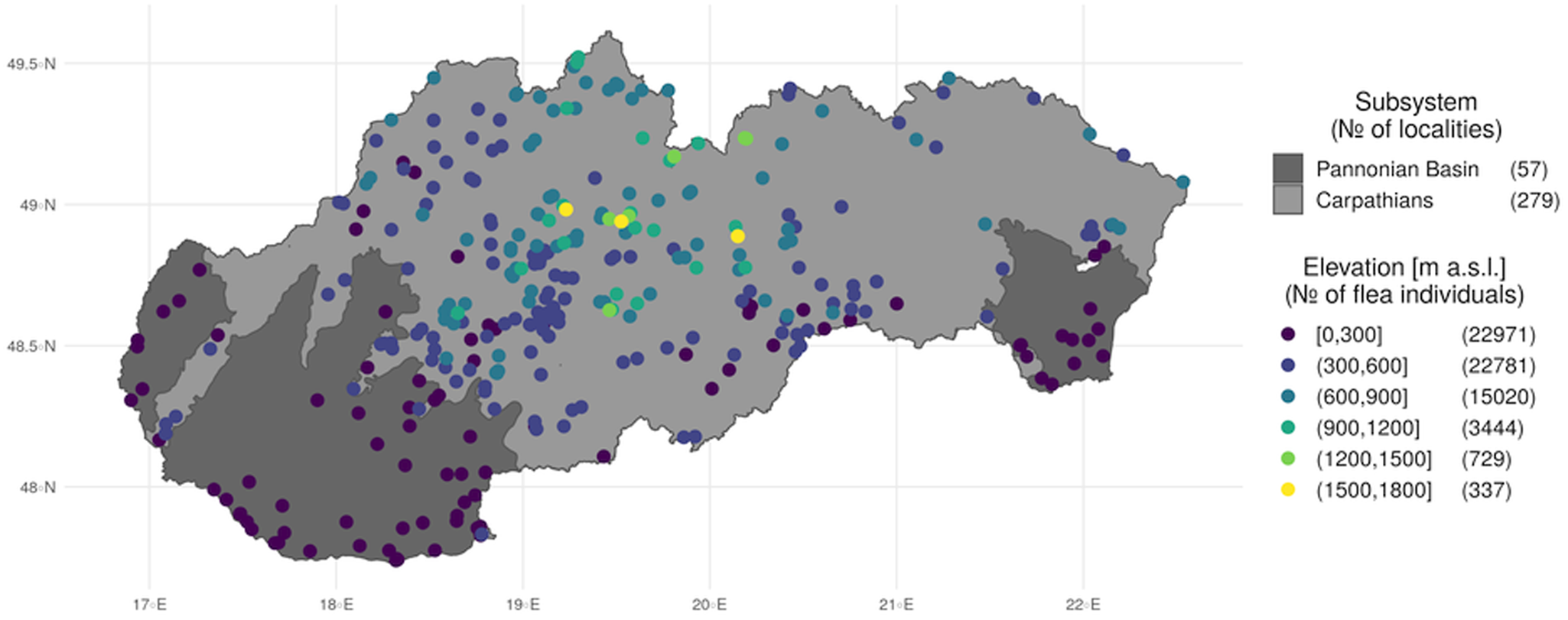
Fig. 1. Distribution of sample locations categorized by geomorphological subsystem and elevation.
Small mammals sampling and flea collection
Small animals were captured in Snap traps (break-back, Nipper type traps) placed linearly in 1976–83 and later in 1998 and 2001. A single line comprised of 50 traps placed at 10-m intervals was left exposed over two or three nights (Anděra and Horáček, Reference Anděra and Horáček2005). Fried wick impregnated with a mixture of fat (oil) and nuts was used as bait. These types of traps were commonly used in the Czech Republic and Slovakia after the end of World War II, until the end of the 20th century (Stanko, Reference Stanko2014). The traps were checked early in the morning when the temperature is the coldest and fleas had remained on the host's corpse for a longer period of time (Rosicky, Reference Rosicky1957). Ectoparasites were collected manually, combing the hair of the host against a white background. This method has been traditionally used, is sufficiently efficient and allows a flea community from any environment to be studied (e.g. Rosicky, Reference Rosicky1957; Dudich, Reference Dudich1989, Reference Dudich1992, Reference Dudich1995). On the other hand, possible losses of fleas from dead host individuals [in comparison with amount of fleas collected from live animals (Krasnov et al., Reference Krasnov, Shenbrot, Khokhlova, Medvedev and Vatschenok1998; de Oliveira et al., Reference De Oliveira, da Silva, Evaristo, Serpa, Campos, Dutra, Nakazato, de Aguiar, Labruna and Horta2020 a.o.)] are compensated by a large amount of evaluated material (see below). The ectoparasite material thereby collected was then fixed in 70% ethyl alcohol.
Data preparation
There were 104 983 fleas documented from 36 species which were originally found as parasites on 30 host insectivore and rodent species. Whenever a single series of traps yielded less than 10 hosts, the fleas collected from them would not be included in the study. This was done to avoid skewing flea abundance and to cover for any possible inaccuracies in smaller host samples. Host species, where the total number of recorded catches was small (n < 25), were also excluded from further consideration. Habitat-specific hosts spread over less than 500 m in elevation, such as the Tatra pine vole (Microtus tatricus, Kratochvíl, 1952) and the snow vole (Chionomys nivalis, Martins, 1842) were likewise not included in any assessment of the impact of altitudinal changes. These exclusions reduced the range of hosts analysed to nine species of small terrestrial mammals: pygmy shrew (Sorex minutus, Linnaeus, 1766); common shrew (Sorex araneus, Linnaeus, 1758), common pine vole (Microtus subterraneus, de Selys-Longchamps, 1836), common vole (Microtus arvalis, Pallas, 1778); field vole (Microtus agrestis, Linnaeus, 1761), bank vole (Myodes glareolus, Schreber, 1780), wood mouse (Apodemus sylvaticus, Linnaeus, 1758), yellow-necked mouse (Apodemus flavicollis, Melchior, 1834) and striped field mouse (Apodemus agrarius, Pallas, 1771). Thus, 65 282 fleas collected from 31 species at 336 locations were analysed.
Estimates of parasite abundance may be biased if some hosts are studied more intensively than others (Stanko, Reference Stanko2014). Consequently, unequal study effort among host species may result in confounding variation in estimates of number of flea (Magurran, Reference Magurran2004). To overcome the effect of sampling effort, the number of flea species was regressed against the number of hosts examined in logarithmic space (Krasnov et al., Reference Krasnov, Shenbrot, Khokhlova and Degen2004). Flea species richness was affected by sampling effort ($r_{{\rm adj}}^2 = 0.015$![]() , F 1 = 21.97, P < 0.001). The original values for flea species richness were then substituted with their residual deviations from log–log regression to become corrected flea species richness (dependent variable). Due to a similar effect of different sampling effort ($r_{{\rm adj}}^2 = 0.014$
, F 1 = 21.97, P < 0.001). The original values for flea species richness were then substituted with their residual deviations from log–log regression to become corrected flea species richness (dependent variable). Due to a similar effect of different sampling effort ($r_{{\rm adj}}^2 = 0.014$![]() , F 1 = 20.99, P < 0.001), was the same procedure used also for Shannon index. Species richness and Shannon's index of diversity for each host species were calculated for sampling site pooled data i.e. diversity of fleas per each host species in a given trapping session. Shannon's indices were calculated using the ‘vegan’ R package (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2019) using a natural logarithm.
, F 1 = 20.99, P < 0.001), was the same procedure used also for Shannon index. Species richness and Shannon's index of diversity for each host species were calculated for sampling site pooled data i.e. diversity of fleas per each host species in a given trapping session. Shannon's indices were calculated using the ‘vegan’ R package (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2019) using a natural logarithm.
Data analysis
The effect of elevation on corrected flea species richness was examined with the aid of a linear-mixed model (LMM) with normal (Gaussian) error distribution. To capture the possible effect of seasonality and the nonlinear relationship of elevation, data from spring (20 March to 20 June), summer (21 June to 22 September), autumn (22 September to 20 December) and winter (21 December to 19 March) along with the interaction between the seasons and elevation were used as the fixed effect with a second degree polynomial. The model also incorporated the random effects of different years, orographic units (at the area level) and host species in order to account for possible influences of time, space and phylogeny in the individual records. Although the years and orography were defined as effects with random intercept, random slopes due to the variability of altitude were also permitted in the random effect of host species. The same model was also used to determine the effect of altitude on changes in the Shannon diversity index. This was analysed in the R environment (R Core Team, 2020) with the lme4 package (Bates et al., Reference Bates, Maechler, Bolker and Walker2015) and augmented with functions from the lmerTest package (Kuznetsova et al., Reference Kuznetsova, Brockhoff and Christensen2017).
The classification and grouping of individual flea species, based on their distribution and frequency along an elevational diversity gradient, were analysed by hierarchical agglomerative clustering using Euclidian distance among all (104 983) records in the common logarithm scale, and complete-linkage method. To further investigate the effect of elevation on the composition of flea communities, multivariate general linear models with a negative-binomial error distribution and log link function for flea species abundance data were used for selected host species, using mvabund, another R package (Wang et al., Reference Wang, Naumann, Eddelbuettel, Wilshire and Warton2020). As with the univariate LLM analysis mentioned earlier, data from years and orographic units were used in addition to second degree polynomial interactions of altitude and seasons. Because of the high number of categories and low proportion of explained overall variability (from LMM results), however, these variables were reclassified into a lower number of categories, namely four 5-year periods (1976–1980; 1981–1985; 1986–1990; 1991–1993) plus a fifth period comprising 1998 and 2001 and six orographic unit categories according to sub-provinces. To accommodate different host numbers, logarithms of the standardized host numbers were used as an offset. The significance of the individual factors was obtained from applying the Monte Carlo sampling method at 999 permutations. To visualize the multivariate relationship, non-metric multidimensional scaling (NMDS) was used with the Bray Curtis dissimilarity index for collections where the abundance of fleas ‘n’ was equal to or greater than 10 (Cox and Cox, Reference Cox and Cox2000) and the scaling performed by using the R package vegan. The only four host species included in the multivariate analysis were the common shrew, common pine vole, yellow-necked mouse and bank vole. These four species were selected (i) because the largest number of records had been kept of these species; (ii) due to their wide environmental tolerance and (iii) since the species simultaneously represent different trophic and topic groups of small mammals. The common shrew is a carnivorous and insectivorous species living in woodlands and also open habitats. The common pine vole is an herbivore predominately active in subterranean environments, while the yellow-necked mouse is a mainly granivorous forest species active in a horizontal environment at the tops of trees and the bank vole is a predominately herbivorous woodland species living among forest undergrowth (Baláž et al., Reference Baláž, Ambros, Tulis, Veselovský, Klimant and Augustiničová2013).
Results
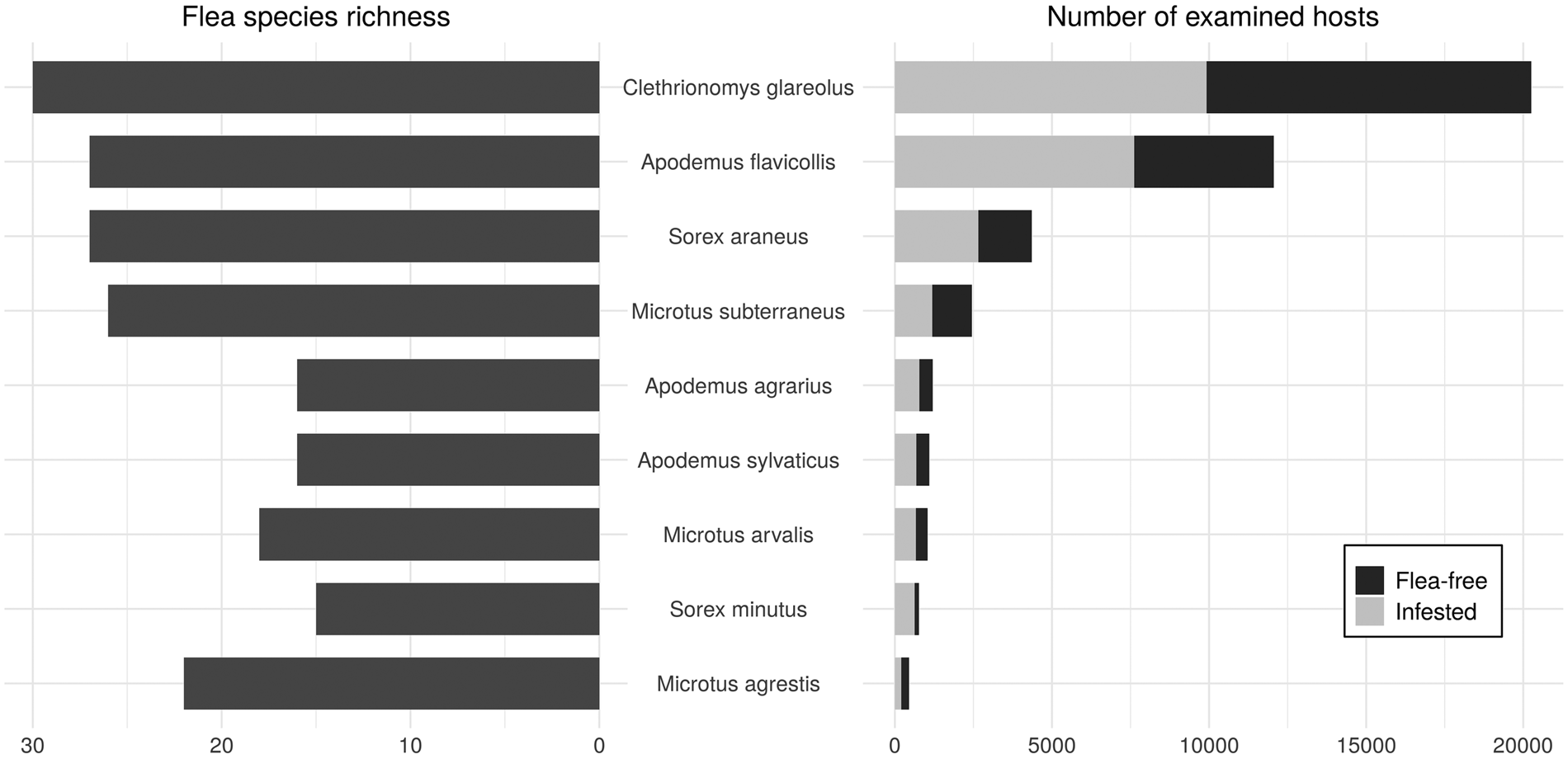
A total of 43 682 captured mammals were examined. Flea parasitism was confirmed in 19 571 of them, or 44.8% of the total population collected. Both flea infestation and species richness varied depending on the host species (see Fig. 2).

Fig. 2. Flea species richness (left) and number of examined fleas (right) for different host species.
Impact of elevation on species richness and diversity of flea communities
The LMM results showed altitude (F 2.11 = 5.57, P = 0.021), the season (F 3.1347 = 15.25, P < 0.001) and the interaction between them (F 6.13 = 5.54, P < 0.001) to be statistically significant for flea species richness.
Similarly, the Shannon diversity index recorded the statistically significant effect of altitude (F 2.12 = 4.49, P = 0.034), the season (F 3.1344 = 17.59, P < 0.001) and the interaction between them (F 6.1290 = 4.08, P < 0.001) for the species richness of fleas.
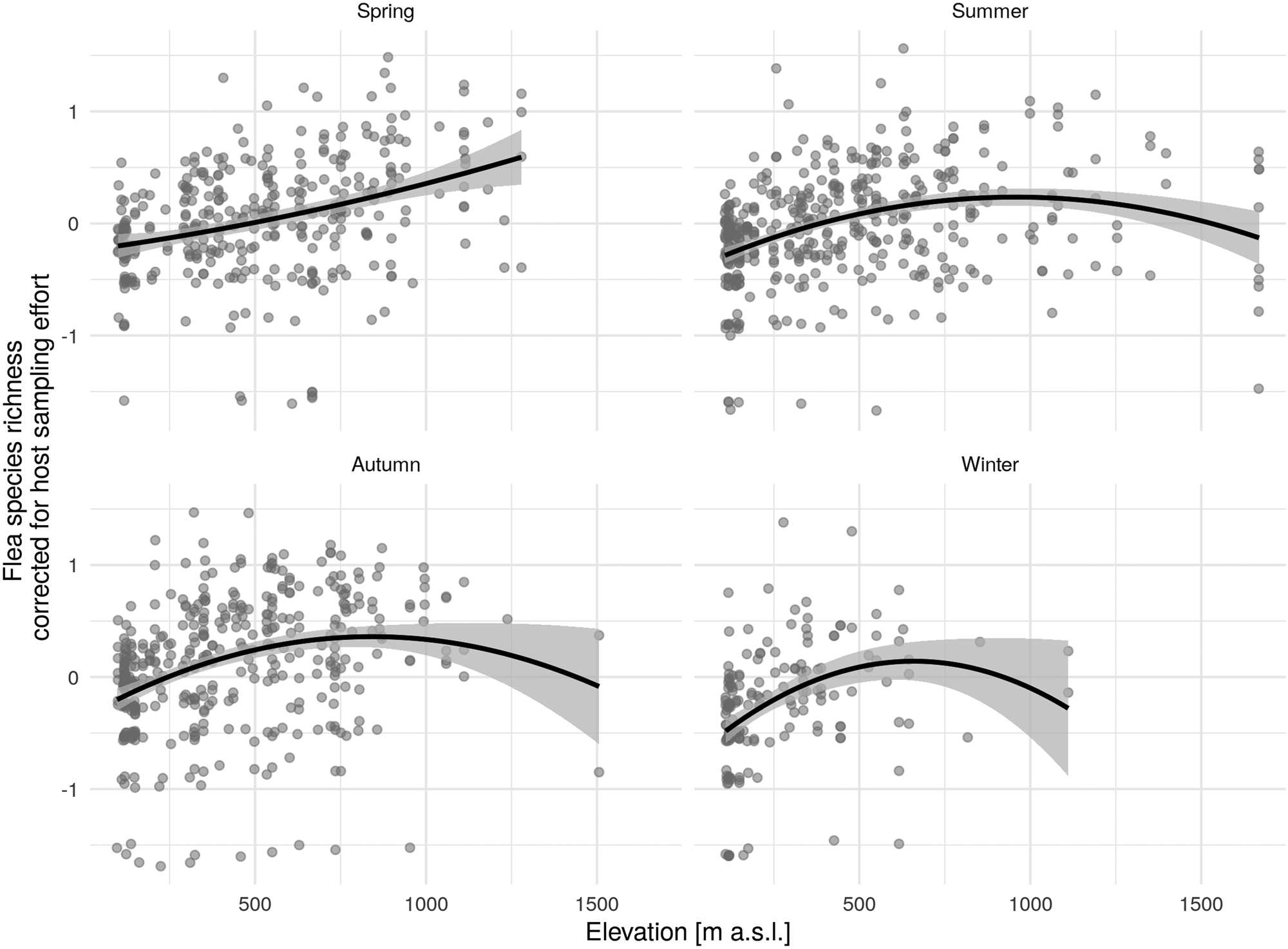
Figure 3 shows a relatively linear increase in species richness as elevation rises, starting at about 900–1000 m above sea level (in spring up to 1250 m), and a subsequent drop at higher altitudes during the remaining seasons.
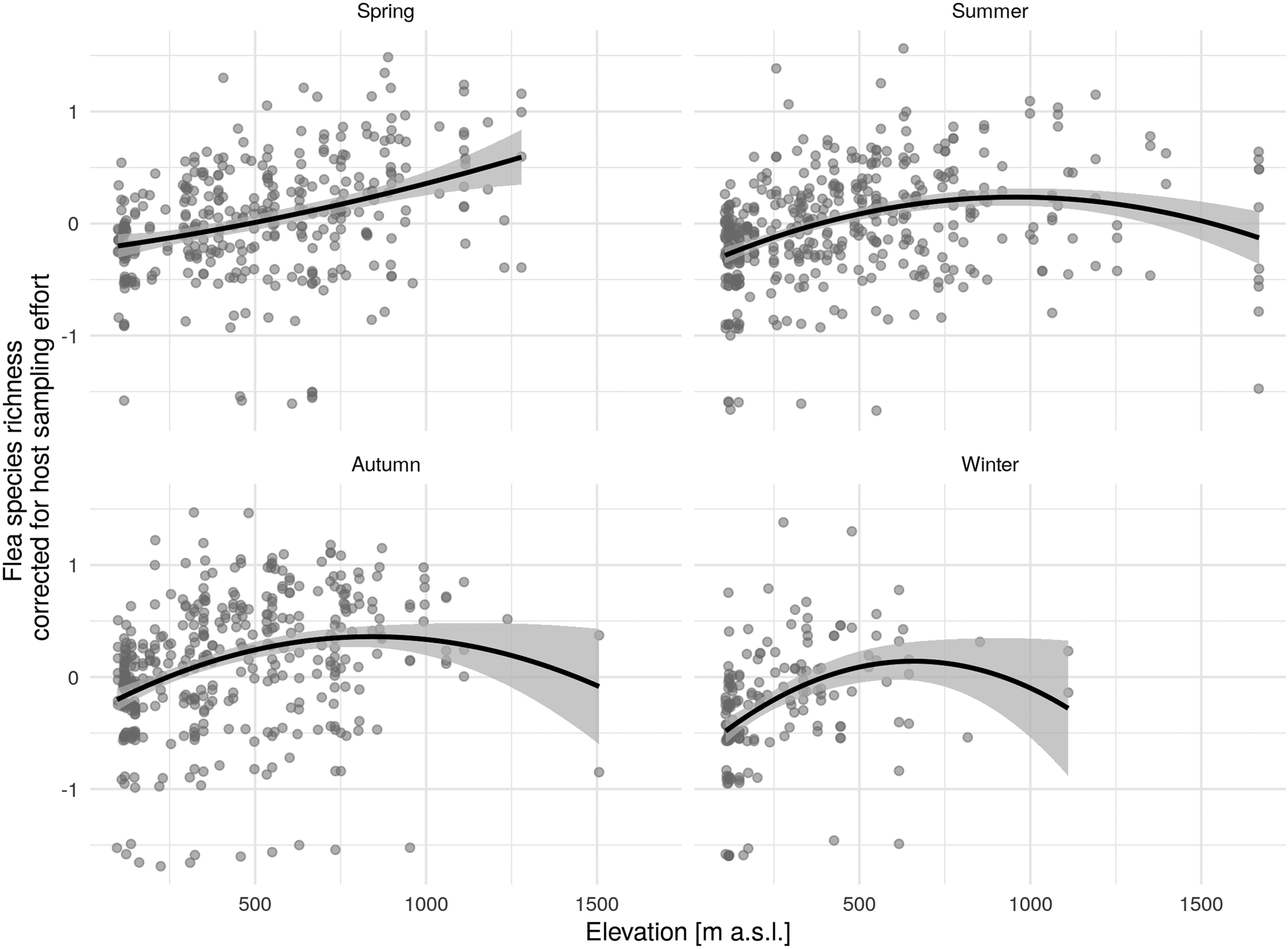
Fig. 3. Quadratic relationship of elevation and corrected flea species richness (regression residuals of original flea species richness and different sampling effort) by season (solid black line) with 95% confidence interval (CI).
The variability in species richness and Shannon diversity index was explained for the most part by the host (25.87% for species richness and 17.63% for Shannon diversity index, respectively), followed by the orographic unit (8.56 and 7.22%) and year of capture (2.65 and 2.04%, respectively).
Taking a more detailed look at the different host species analysed, unimodal flea species richness patterns can be observed in yellow-necked mice, bank voles, common pine voles, common shrews and pygmy shrews along the elevational diversity gradient (Fig. 4), as was the case in all the hosts. A unimodal relationship was observed in most species. The exception was species with a smaller range. Data from elevations above 1000 m were also insufficient, specifically among field and common voles where it was evident that the species richness of fleas was slightly increasing at higher altitudes. On the other hand, the species richness of fleas fell even at lower elevations as altitude rose. The same outcome was registered in the Shannon diversity index, so no graphs outlining the results are presented.
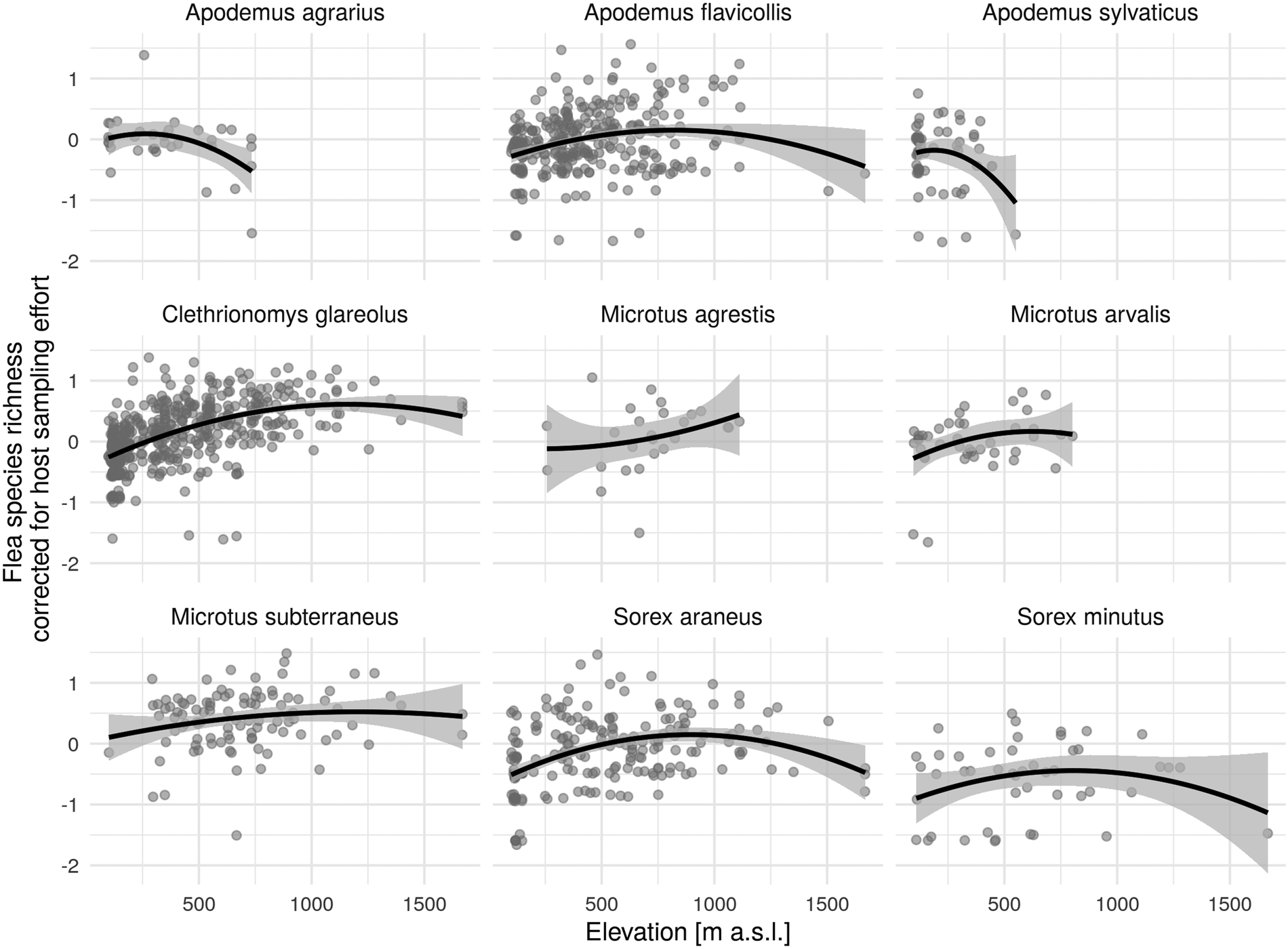
Fig. 4. Quadratic relationship of elevation and corrected flea species richness (regression residuals of original flea species richness and different sampling effort) by season (solid black line) with 95% CI.
Impact of elevation on the distribution of fleas
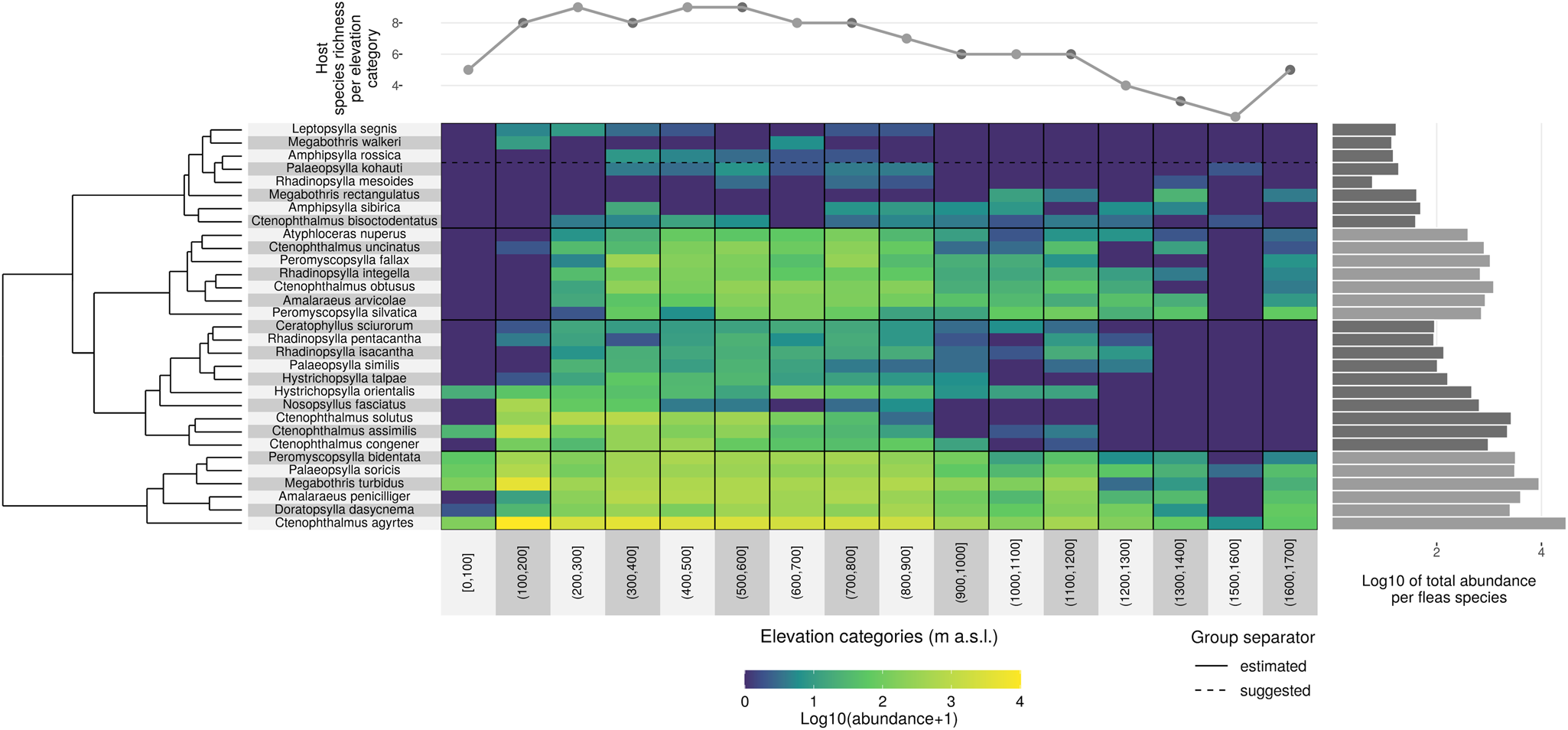
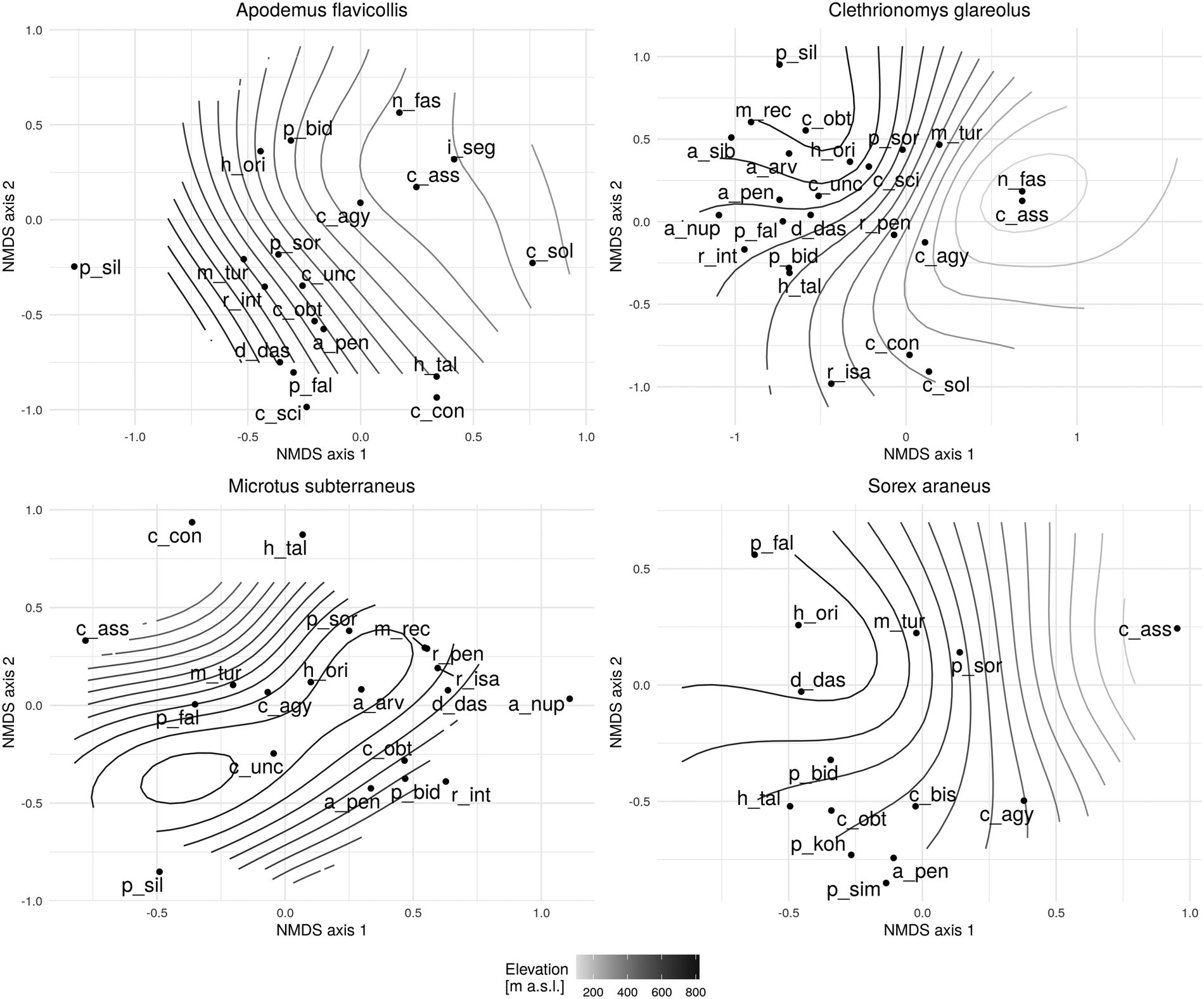
Using cluster analyses, fleas were segregated into four groups based on the changes in abundance and occurrence along the gradient (Fig. 5).
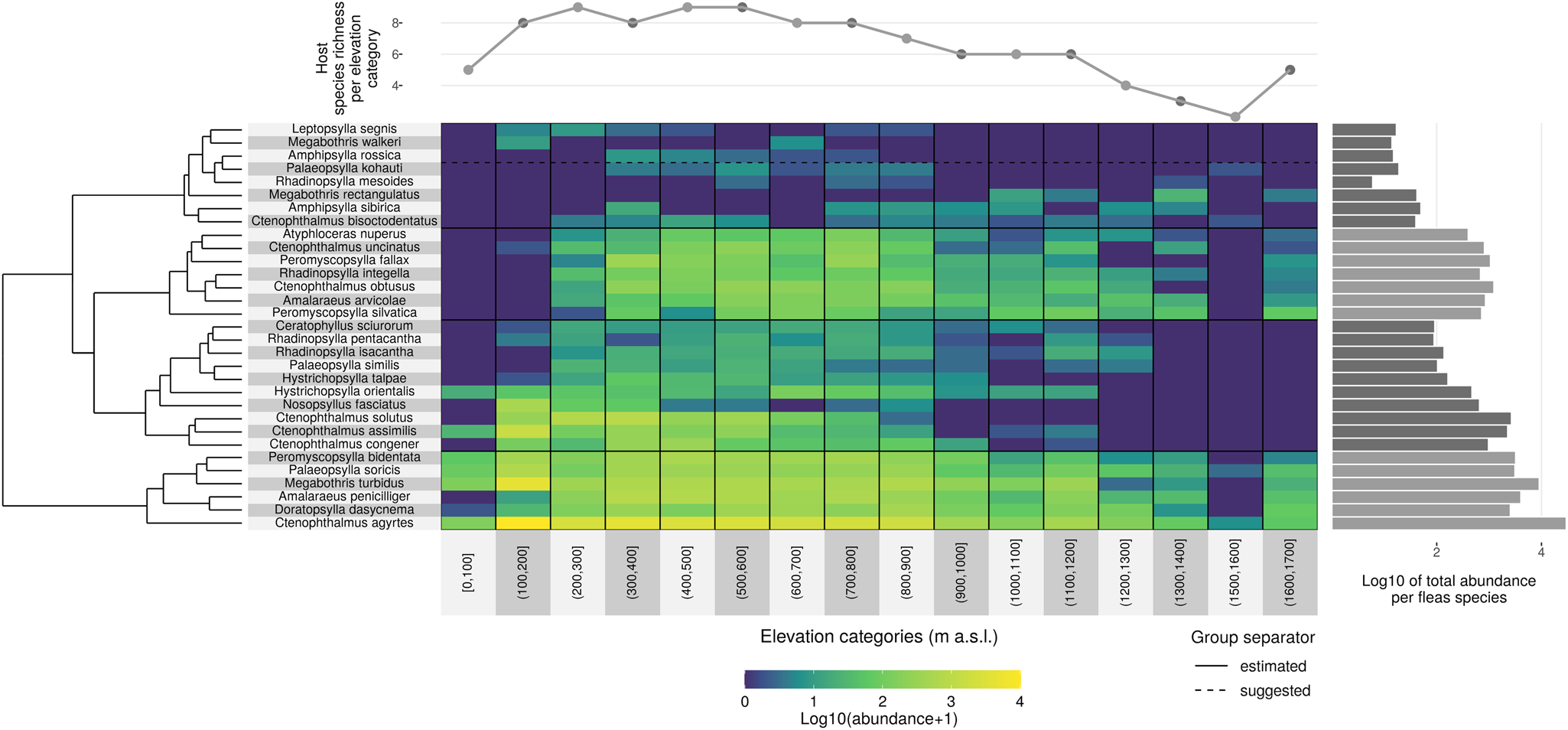
Fig. 5. Changes in flea species distribution and abundance along the elevational diversity gradient.
Flea communities that changed with elevation were assigned to first group. These species are characterized by a wide ecological tolerance to environmental conditions and were documented along the entire length of the gradient from the lowlands to the subalpine zone. These are euryvalent flea species with a low host specialization, but also include host-specific species whose host is characterized by broad ecological tolerance which enables them to range across a wide range of elevations. This group consists of Peromyscopsylla bidentata, Palaeopsylla soricis, Megabothris turbidus, Doratopsylla dasycnema, Ctenophthalmus agyrtes and Amalaraeus penicilliger, which normally avoid lowlands.
Second group covers species that occur along a relatively wide range of elevations (above 1000 m), but either avoid higher altitudes or prefer lower altitudes. It contains Hystrichopsylla orientalis, Ctenophthalmus congener, Ctenophthalmus assimilis, Nosopsyllus fasciatus and Ctenophthalmus solutus.
Third group is composed of communities whose flea species were not documented in lowland areas, but nonetheless could be found in the highest positions on the elevational diversity gradient, ranging above 1000 m. Although these species were algorithmically (from cluster analysis) included in a single group, they can be divided from both our own knowledge and other references into (i) fleas that specifically colonize common mole species (Palaeopsylla similis, Palaeopsylla kohauti and Ctenophthalmus bisoctodentatus), mammals that live in trees (Ceratophyllus sciurorum), bank voles (Atyphloceras nuperus) and wide-ranging species (Rhadinopsylla isacantha, Rhadinopsylla pentacantha and Hystrichopsylla talpae); and (ii) mountain species (Rhadinopsylla integella, Ctenophthalmus obtusus, Amalaraeus arvicolae, Ctenophthalmus uncinatus, Peromyscopsylla fallax and Peromyscopsylla silvatica).
Species whose range covers elevations below 1000 m comprise fourth group. It can also be divided into two ecologically subgroups, composed of species flourishing most at higher altitudes: Rhadinopsylla mesoides, Megabothris rectangulatus and Amphipsylla sibirica; and species preferring lower to moderate altitudes: Amphipsylla rossica, Megabothris walkeri and Leptopsylla segnis.
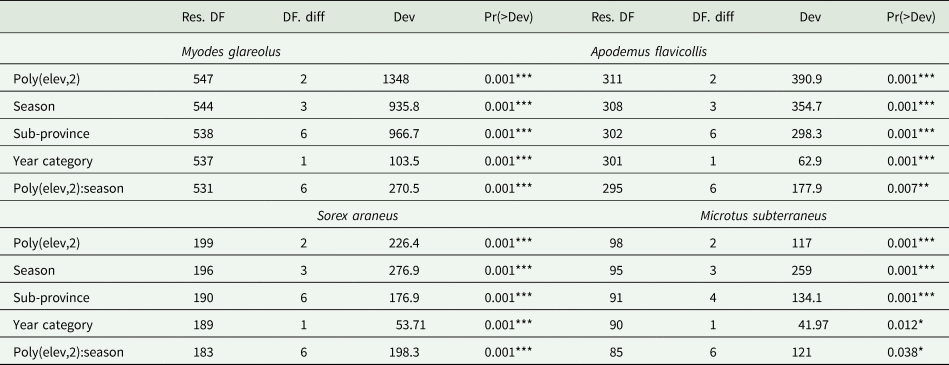
Multivariate analysis of all four selected host species similarly confirmed a conclusive relationship between the composition of the flea community and the second-degree altitude polynomial, the season and also the interaction between them (Table 1).
Table 1. Deviance table and multivariate models for selected host species
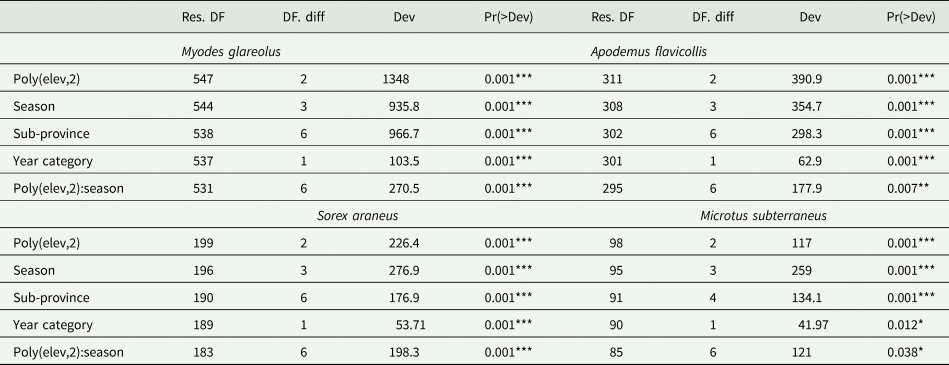
Significance codes: 0 ‘***’; 0.001 ‘**’; 0.01 ‘*’; 0.05 ‘.’; 0.1 ‘’ 1.
At lower elevations along the gradient, yellow-necked mice were colonized by flea species such as N. fasciatus, L. segnis, C. solutus and C. assimilis, while more opportunistic species infested them at moderate elevations. Typical species found at higher elevations were D. dasycnema, P. fallax and P. silvatica. Species typically found in M. glareolus communities at lower elevations were N. fasciatus, C. assimilis and C. agyrtes, and at moderate elevations M. turbidus, R. pentacantha and R. isacantha. The majority of flea communities were found at higher elevations, mainly mountain species such as M. rectangulatus, P. silvatica and A. sibirica, as well as species that prefer hosts with an optimum also at higher elevations, such as C. uncinatus, P. fallax and R. integella. There was only a single species of C. assimilis found on the host species M. subterraneus. Other species collected are typical of mid-peak and peak elevations. Opportunistic species such as C. agyrtes and M. turbidus were also evident at mid-peak elevations, while species that specifically seek European water voles (Arvicolae) as hosts were particularly found at the highest elevations, with occurrences of C. uncinatus and H. orientalis also noticed. As with the previously mentioned host, S. araneus, there was just one species – C. assimilis – also colonizing at lower elevations. A characteristic shrew community is D. dasycnema, which appears to be the case in higher elevations along the gradient. In addition, cases of P. fallax and H. orientalis were also found. Palaeopsylla soricis is a species typical at mid-peak elevations, as are the opportunistic species M. turbidus and C. agyrtes (Fig. 6).
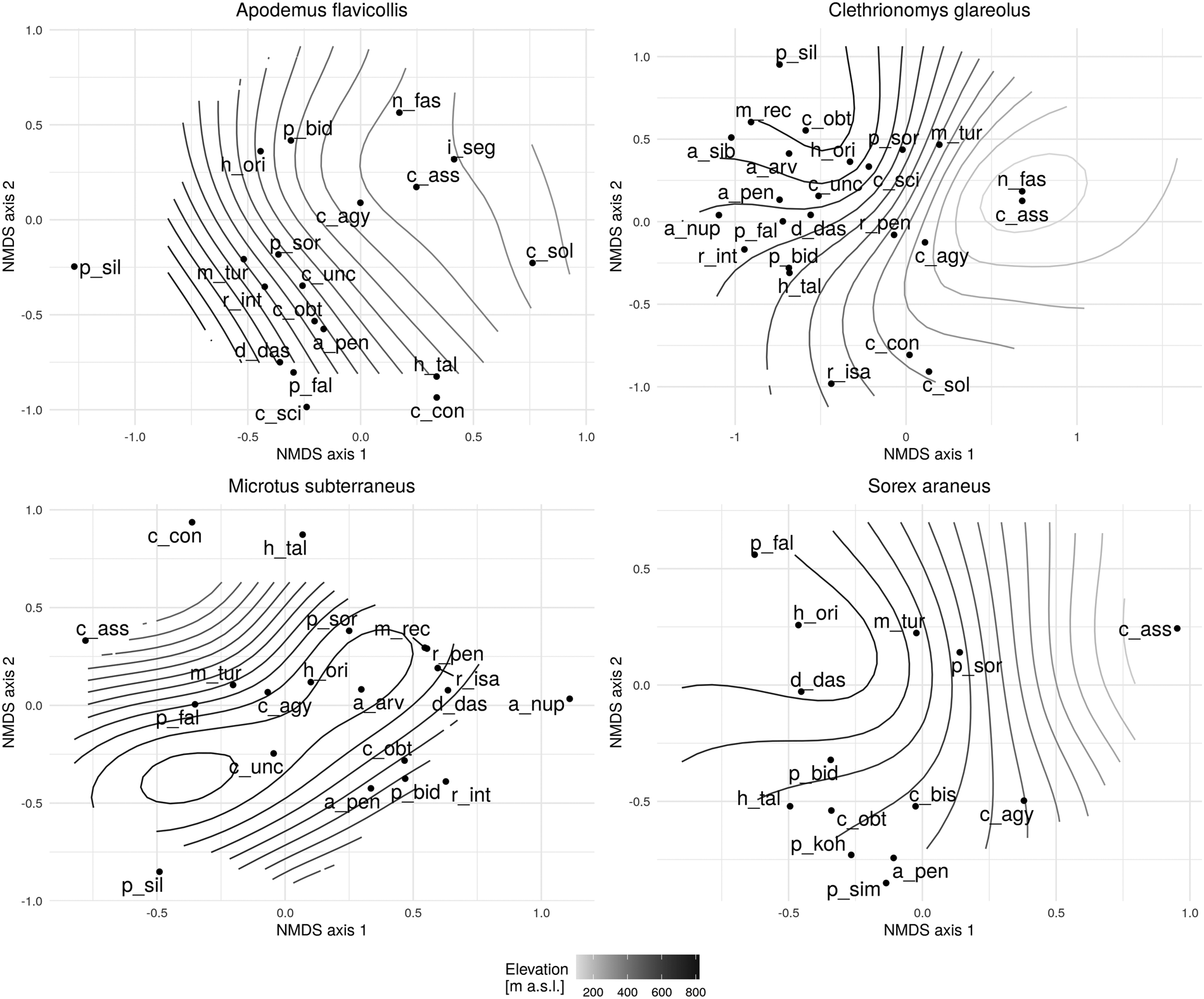
Fig. 6. NMDS flea community responses (frequency >10) for selected host species on the elevational diversity gradient [Apodemus flavicollis (stress = 0.24); Myodes glareolus (stress = 0.23); Microtus subterraneus (stress = 0.26); Sorex araneus (stress = 0.21)] a_arv – Amalaraeus arvicolae, a_nup – Atyphloceras nuperus, a_pen – Amalaraeus penicilliger, a_sib – Amphipsylla sibirica, c_agy – Ctenophthalmus agyrtes, c_ass – Ctenophthalmus assimilis, c_con – Ctenophthalmus congener, c_obt – Ctenophthalmus obtusus, c_sci – Ceratophyllus sciurorum, c_sol – Ctenophthalmus solutus, c_unc – Ctenophthalmus uncinatus, d_das – Doratopsylla dasycnema, h_ori – Hystrichopsylla orientalis, h_tal – Hystrichopsylla talpae, l_seg – Leptopsylla segnis, m_tur – Megabothris turbidus, m_rec – Megabothris rectangulatus, n_fas – Nosopsyllus fasciatus, p_bid – Peromyscopsylla bidentata, p_fal – Peromyscopsylla fallax, p_sil – Peromyscopsylla silvatica, p_sim – Palaeopsylla similis, p_sor – Palaeopsylla soricis, r_int – Rhadinopsylla integella, r_isa – Rhadinopsylla isacantha, r_pen – Rhadinopsylla pentacantha.
Discussion
Changes in community characteristics such as diversity and composition in the elevational gradient are generally associated with common climatic influences (local abiotic conditions), topography (terrain relief), history (occurrence of species across evolutionary timescales), heterogeneity and habitat complexity (vertical strata within habitat) or with the influence of other biotic factors (Kelt et al., Reference Kelt, Meserve and Lang1994; Lomolino, Reference Lomolino2001; Rahbek and Graves, Reference Rahbek and Graves2001; Jetz and Rahbek, Reference Jetz and Rahbek2002; McCain and Grytnes, Reference McCain, Grytnes and Chichester.2010; Mena and Medellín, Reference Mena and Medellín2017). Our study noted changes in flea community diversity due to changing altitude, season and the interaction between them. These changes varied depending on the host species. Altitude also affected flea distribution and diversity along an elevational gradient between 96 and 1669 m above sea level. Although fleas are affected by environmental conditions, nonetheless the dual environment (see the Introduction to this paper) lets their hosts also play an important role (Krasnov, Reference Krasnov2008), as they constitute for the parasites their primary environment, food and a necessary condition for their reproduction (Price, Reference Price, Esch, Bush and Aho1990; Krasnov et al., Reference Krasnov, Stanko, Miklisova and Morand2006; Maestri et al., Reference Maestri, Shenbrot and Krasnov2017).
The results indicated unimodal behaviour coming from mid-peak pattern changes in flea diversity, with the pinnacle at moderate elevations of about 800–1000 m during the summer. The same trend was evident during autumn and winter but, due to the challenge of obtaining material during those seasons of the year, there was a smaller amount of data collected from higher elevations that could be analysed. During the winter, no data at all were collected from above 1100 m. In general, the mid-peak pattern of diversity changes is one of the most widespread found (Lomolino, Reference Lomolino2001; Fischer et al., Reference Fischer, Blaschke and Bässler2011) and it is also seen frequently among insect taxa (McCoy, Reference McCoy1990). The most common explanation is the mid-domain effect, which presupposes increased overlapping of well-adaptable species at moderate elevations. Although individual environmental factors may be less optimal at mid-gradient ranges, their combined effect and the associated processes and interactions have a positive effect on the cohabitation of several species.
Another reason why the unimodal pattern is often evident is the onset of endemic species, which increases species diversity at the middle of the elevation gradient (Grytnes and McCain, Reference Grytnes and McCain2007). The optimum for some taxonomic or ecological groupings also ends above this bound, with diversity starting to decline further along the gradient (Lomolino, Reference Lomolino2001). In the case of the mid-peak pattern, the peak of the curve depends on the size of the mountain range and maximum diversity occurs in higher mountains at their higher elevations (McCain, Reference McCain2005). The mid-peak pattern has been documented in fleas both in East Asia (Gong et al., Reference Gong, Wu, Duan, Feng, Zhang and Liu2005) and western Siberia (Sapegina, Reference Sapegina2000). Gong et al. (Reference Gong, Wu, Duan, Feng, Zhang and Liu2005) explain it by citing the ecotone effect and relative humidity at mid-peak, while also admitting to other altitude-influenced factors. The presence of endemic species in the middle of the gradient also influences the outcome. Maestri et al. (Reference Maestri, Shenbrot and Krasnov2017) identified the temperature gradient and changes in the host communities as the most serious factors affecting flea diversity. Changes in precipitation were again indicated as having the greatest impact on the hosts. The mid-peak pattern is also common non-flying small mammals (McCain, Reference McCain2004, Reference McCain2005). In the Western Carpathians, species richness declined among small mammals between 944 and 1755 m elevation (Kamenišťák et al., Reference Kamenišťák, Baláž, Tulis, Jakab, Ševčík, Poláčiková, Klimant, Ambros and Rychlik2019). Analysing on a long elevational diversity gradient, Maher and Timm (Reference Maher and Timm2014) found the richness of flea species to decline as the number of small mammal host species fell, yet no elevation-connected patterns in richness were evident. Our analysed data show a drop in host species richness starting at a height of approximately 1000 m elevation. The decreasing diversity of fleas we noticed may be partially mirroring the reduced host diversity. Sapegina (Reference Sapegina2000) views the distribution of fleas to be generally dependent to an equal degree on both the host and climatic factors in the external environment. Benton and Altmann (Reference Benton and Altmann1964) adds that some flea species occur only at certain elevational gradient intervals where their host ranges, suggesting them to be also affected by another ecological factor besides the presence of a suitable host.
On the other hand, our study found flea diversity growing during the spring as elevation increased, but no data were available to us for elevations above 1250 m. Nonetheless, a continual rise in the higher elevations along the gradient is unlikely from what is known in studies conducted at mountains in Africa (Eisen et al., Reference Eisen, Borchert, Mpanga, Atiku, MacMillan, Boegler, Montenieri, Monaghan and Gage2012; Meliyo et al., Reference Meliyo, Kimaro, Msanya, Mulungu, Hieronimo, Kihupi, Gulinck and Deckers2014). This is especially due to the growing diversity of both the fleas and the hosts (Eisen et al., Reference Eisen, Borchert, Mpanga, Atiku, MacMillan, Boegler, Montenieri, Monaghan and Gage2012; Meliyo et al., Reference Meliyo, Kimaro, Msanya, Mulungu, Hieronimo, Kihupi, Gulinck and Deckers2014).
The mid-peak pattern was documented in five of the hosts we compared. These were the yellow-necked mouse, bank vole, common pine vole, common shrew and pygmy shrew. On the other hand, a decline in flea diversity was recorded among striped field mice and wood mice as elevation rose, while it grew among field voles and common voles. Even though there was a smaller amount of field vole and common vole data analysed, the results nevertheless pointed towards interspecies differences in flea diversity along the elevational gradient.
Most of the studies generally identified elevation to be the main factor influencing flea diversity, with an impact on factors such as rainfall, climate, habitat and environmental productivity, alongside anthropogenic influences (Benton and Altmann, Reference Benton and Altmann1964; Gong et al., Reference Gong, Wu, Duan, Feng, Zhang and Liu2005; Eisen et al., Reference Eisen, Borchert, Mpanga, Atiku, MacMillan, Boegler, Montenieri, Monaghan and Gage2012; Meliyo et al., Reference Meliyo, Kimaro, Msanya, Mulungu, Hieronimo, Kihupi, Gulinck and Deckers2014; Maestri et al., Reference Maestri, Shenbrot and Krasnov2017). Between the Pannonian Plain and the base of the Carpathian Mountains, air temperature falls and precipitation rises like it does in other increasing elevational diversity gradients. In Slovakia, there is usually more than 100 cm of snow cover at elevations above 1300 m (Slovak Hydrometeorological Institute, 2020), causing diverse vertical fragmentation (Futák, Reference Futák1972) and varying land use. At lower elevations, the landscape is used mainly for agriculture and only small refuges remain from the original forests (Petluš and Vanková, Reference Petluš and Vanková2009). Meanwhile, farmland is receding from moderate elevations and woodlands are growing (Hreško et al., Reference Hreško, Petrovič and Mišovičová2015; Súľovský et al., Reference Súľovský, Falťan, Skokanová, Havlíček and Petrovič2017; Izakovičová et al., Reference Izakovičová, Špulerová and Petrovič2018).
Temperature is often considered to be the most important factor changing elevational diversity (Lomolino, Reference Lomolino2001; Fischer et al., Reference Fischer, Blaschke and Bässler2011). But if temperature were the single factor affecting species diversity, then the diversity would be declining. Other abiotic components affecting fleas were host burrow structure and microclimate (especially temperature and humidity), where soil type plays a major role (Krasnov et al., Reference Krasnov, Shenbrot, Medvedev, Vatschenok and Khokhlova1997). Changing factors due to variances in elevation also affect developmental stages in fleas and these changes are expected to be more sensitive to environmental factors than after the fleas reach adulthood (Eisen et al., Reference Eisen, Borchert, Mpanga, Atiku, MacMillan, Boegler, Montenieri, Monaghan and Gage2012). Diversity patterns also depend largely on geographical location (Fischer et al., Reference Fischer, Blaschke and Bässler2011). Krasnov (Reference Krasnov2008) also identified the cause of discrepancies between the results from flea research to have been the different evolution of flea communities in a particular region due to the impact of local environmental conditions, such as climate or the landscape itself. Although the species richness of fleas in the Palaeo-Arctic Region correlates with the mean elevation of the mountains and the number of available host species, no such dependencies were found in the Neo-Arctic Region (Krasnov et al., Reference Krasnov, Shenbrot, Khokhlova and Poulin2007).
The results imply the significant impact of seasonality on flea diversity among the small mammals that had been collected in the observed elevational gradient. Seasonal changes in environmental conditions alter flea abundance, reproduction and parasitism (Darskaya, Reference Darskaya1970; Vatschenok, Reference Vatschenok1988; Krasnov, Reference Krasnov2008). These fluctuations are most pronounced where significant seasonal changes in temperature and precipitation have occurred (Krasnov, Reference Krasnov2008). Seasonal changes in environmental conditions (mainly temperature and precipitation) are strikingly distinct from the Pannonian Plain to the base of the Carpathians Mountains, and the relief of the lowlands, basins and mountains within this region varies substantially, with seasonal differences increasing at higher elevations (Slovak Hydrometeorological Institute, 2020). The fleas adapting to the changing conditions each season led us to divide them into several groups. These cover flea species whose adults are active and reproduce throughout the year, although some of the species reproduce just in warm months and others only during the hottest days of the year. This grouping also incorporates yet other species with active adults reproducing just during cold months (Vatschenok, Reference Vatschenok1988). In contrast to the majority of species which are all-seasonal, some flea species audits only thrive sporadically during a particular season. They are R. integella, R. mesoides, C. obtusus, P. kohauti, A. sibirica, H. talpae, H. orientalis (in winter) and M. rectangulatus (in summer). Species flourishing throughout the year, but with increased occurrence and abundance in autumn and winter are P. bidentata, R. pentacantha, R. isacantha, A. nuperus and N. fasciatus. In contrast, both M. turbidus and H. orientalis are more abundant in summer and autumn while M. walkeri and M. turbidus predominate in the warmer months of the year. All of the remaining species are all-seasonal, irrespective of the time of year. Although it is not entirely clear how these patterns of flea activity originated, they are thought to have adapted over time to the habitat's ecology, host behaviour, local environmental conditions and evolutionary pressure (Krasnov, Reference Krasnov2008). Depending on the type of parasitic relationship, the distribution of the different flea species accordingly changed within the observed elevational diversity gradient. Specific parasite types are strongly linked to their main hosts and any occurrence on another host species is considered to be a random occurrence (Krasnov, Reference Krasnov2008). For instance, P. similis, P. kohauti, C. bisoctodentatus, C. sciurorum, A. nuperus and R. mesoides are flea species that colonize a specific host. Host specialization is another type relationship that is affected by the elevation where the host ranges. Amalaraeus arvicolae is a flea species that specialized in common pine voles. Although the host's range covers lowlands and mountainous areas (Baláž et al., Reference Baláž, Ambros, Tulis, Veselovský, Klimant and Augustiničová2013), the fleas themselves prefer mountains and are absent from lower elevations along the gradient, something fully consistent with other studies (Rosicky, Reference Rosicky1957; Bartkowska, Reference Bartkowska1973; Dudich, Reference Dudich1995). A similar species, A. rossica, is specifically found on common voles whose range extends only to 1000 m elevation (Rosicky, Reference Rosicky1957; Skuratowicz, Reference Skuratowicz1967; Brinck-Lindroth and Smit, Reference Brinck-Lindroth and Smit2007; and our own study), although its host has spread to the higher elevations of the Eastern Carpathians because of past deforestation and grazing (Kratochvíl and Pelikán, Reference Kratochvíl and Pelikán1955; Ambros, Reference Ambros1998). Features in the outdoor environment may gradually be changing the fleas' host specialization, possibly different due to changing microclimatic conditions at various locations (Krasnov et al., Reference Krasnov, Stanko, Miklisova and Morand2006; Krasnov, Reference Krasnov2008).
Opportunistic flea species become parasites on a broader range of hosts, but to a different extent with each host (Krasnov, Reference Krasnov2008). Based on how the distribution of fleas has responded to changes in conditions along the elevational diversity gradient, the species have been divided into four groups. The first group comprises species that adapt to a wide range of environmental conditions and exhibit virtually no response to changing elevations. Opportunistic flea species like C. agyrtes and M. turbidus are generally considered to fall into this category (Rosicky, Reference Rosicky1957; Brinck-Lindroth and Smit, Reference Brinck-Lindroth and Smit2007). In contrast to them, species in second, third and fourth groups are all bound to specific parts of the elevational diversity gradient. Typical species restricted to lower elevations include C. solutus, N. fasciatus and C. assimilis, while those bound to higher elevations are A. penicilliger, A. sibirica and P. silvatica. On the other hand, H. talpae, a species that infests a wide range of small mammals, can be described as opportunistic in terms of elevation (Skuratowicz, Reference Skuratowicz1967; Dudich, Reference Dudich1989; Brinck-Lindroth and Smit, Reference Brinck-Lindroth and Smit2007), although such behaviour has not been documented at higher altitudes. Species occurring in moderate elevations like A. rossica, M. walkeri and L. segnis form a special section of fourth group. Leptopsylla segnis is a cosmopolitan species that thrives in different environments up to 900 m elevation and whose main hosts are rodents associating with humans. Amphipsylla rossica has penetrated into higher elevations up to 800 m along with common vole, but is nevertheless more abundant at lower altitudes. Megabothris walkeri's range covers Europe and Siberia and its main hosts, up to an elevation of 700 m, are root voles (Microtus oeconomus), bank voles and water voles (Arvicola amphibius). The distribution of fleas along the elevational diversity gradient among specific species therefore tends to be more influenced by the host, while environmental conditions play a greater role among opportunistic flea species.
Changes were observed by us in the flea community's response to changing elevations in four host species (common shrew, common pine vole, bank vole and yellow-necked mouse). These species have a wide range of habitats (Herczeg and Horváth, Reference Herczeg and Horváth2015; Mérő, et al., Reference Mérő, Lotnay and Lengyel2015), their occurrence is common in the conditions under which they have been studied and their populations tend to be numerous (Baláž et al., Reference Baláž, Ambros, Tulis, Veselovský, Klimant and Augustiničová2013), something also reflected in the highest level of parasitism among them and in the number of flea species collected. The results showed the community structure of flea hosts in the elevational gradient to be species-specific even in terms of diversity.
To conclude, this study focuses on diversity and distribution patterns at dozens of separate locations between the Pannonian Plain and the Carpathians Mountains, unlike most of the studies analysing elevational diversity gradients at specific localities or mountains. A significant advantage of this study is its consideration of seasonal effects, which recognizes the mid-peak pattern diversity of fleas, especially during the summer. A similar trend is also reflected in autumn and winter results. More information from higher elevations is needed to obtain complete confirmation of the impact during the remaining seasons. Analysis of individual host species showed interspecies differences, with some showing mid-peak pattern diversity and others flea diversity falling or even rising with increasing altitude. The distribution of fleas along the elevational diversity gradient led to the division of the species into four groups, influenced by either their specificity or opportunism to their hosts and because of environmental conditions. The study also pointed out the challenge of analysing the dual environment of parasites. It would be very interesting in future to monitor responses to diversity, distribution and seasonality of fleas, and parasites in general along the gradient, in the context of climate change, as well as in the outcome of changes in host distribution and the degree of vegetation.
Acknowledgement
Our sincere thanks to Michal Ambros, Alexander Dudich and Andrej Stollmann for providing a flea database. We thank anonymous reviewers for valuable comments and suggestions to an earlier version of the manuscript and we also thank American native speaker of English, Mr. Richard Budd, for English language correction.
Financial support
The study has been conducted with the support of grant projects VEGA 1/0277/19 and KEGA 026UKF-4/2020.
Conflict of interest
The authors each declare that they have no conflicts of interest.
Ethical standards
All applicable international, national and/or institutional guidelines for treatment of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Small mammals were caught according to the laws of the Slovak Republic. The research was conducted by virtue of Dr Michal Ambros's appointment (employee of the State Nature Protection of Slovak Republic) as mapping coordinator for important European small mammal species. This paper contains no studies by any of the authors involving human participants.