Introduction
Parasites may increase their spread and transmission by using different egg excretion pattern strategies, e.g. a continuous or rhythmic release over time and space (Kennedy, Reference Kennedy and Kennedy1976; Platt et al., Reference Platt, Hussey and Zelmer2013). Excretion of nematode eggs from the gastrointestinal tract of their host into the external environment is, however, explicitly dependent on host animal feces excretion. A well-synchronized pattern of host-feces and parasite egg excretion has the potential to favour dispersal of eggs in the environment. Thus, one of the key factors in parasite spreading might be synchronization between the parasite egg excretion rate and host animal physiology (e.g. feces excretion) and behavioural activity. This synchronization is reasonable because any trait that might have favoured the spread and transmission success of parasites during the course of evolution would have been under positive selection pressure (Dolnik et al., Reference Dolnik, Metzger and Maarten2011); hence, this synchronization may have a favourable function in the parasite life history.
There is a large body of evidence indicating fluctuations, periodicity and diurnal rhythms in egg excretion by parasites. Several studies have indicated that parasite eggs are released at varying levels from day to day (Engels et al., Reference Engels, Sinzinkayo and Gryseels1996; Yu et al., Reference Yu, de Vlas, Yuan and Gryseels1998; Giver et al., Reference Giver, de Vlas, Johansen, Christensen and Nansen2000). Moreover, diurnal periodicity in parasite excretion of eggs/oocysts has also been shown for various species e.g. Aonchoteca caudinflata and Eimeria spp. (Villanоúa et al., Reference Villanоúa, Pérez-Rodriíguez, Gortázar, Höfle and Viñuela2006), Echinostoma caproni (Platt et al., Reference Platt, Hussey and Zelmer2013); Isospora spp. (Misof, Reference Misof2004; Morin-Adeline et al., Reference Morin-Adeline, Vogelnest, Dhand, Shiels, Angus and Šlapeta2011; Coelho et al., Reference Coelho, Berto, Neves, Oliveira, Flausino and Lopes2013) and Syngamus trachea (Oju and Mpoame, Reference Oju and Mpoame2006). We recently demonstrated that nematode egg excretion in chickens naturally infected with the most common nematodes, including Ascaridia galli, Heterakis gallinarum and Capillaria spp., collectively showed repeatable diurnal fluctuations (Wongrak et al., Reference Wongrak, Gauly and Das2015). Although periodicity was also confirmed for A. galli using experimentally mono-species-infected chickens, it has not yet been directly shown for H. gallinarum with mono-infections. Heterakis gallinarum resides in the caeca, which are two blind-ended sacs that project from the proximal colon at its junction with the small intestine (Clench and Mathias, Reference Clench and Mathias1995). The nematode itself is relatively less pathogenic to the chicken host (Daş et al., Reference Daş, Abel, Humburg, Schwarz, Rautenschlein, Breves and Gauly2011a), but it is the main vector for transmission of Histomonas meleagridis, a protozoon parasite that causes blackhead disease (McDougald, Reference McDougald2005). The caecal feces is the main transport-medium for eggs of H. gallinarum to the external environment, where they are embryonated and picked up by the definitive host. Uninfected healthy chickens, however, rarely excrete caecal feces during the dark period (Clarke, Reference Clarke1979), implying a potential periodicity in H. gallinarum egg excretion.
To investigate whether a pattern of periodicity in egg excretion by H. gallinarum exists, we quantified the effect of the sampling time on the concentration and a total number of H. gallinarum eggs excreted through host feces within 1 day. Furthermore, we tested whether a diurnal rhythm for per capita egg excretion (fecundity) existed for each worm infrapopulation. The aim of this study was to quantify patterns reflecting a periodicity or diurnal rhythm in both parasite egg excretion and host animal feces excretion.
Materials and methods
Heterakis gallinarum-infected chickens and feces collection
A total of 16 experimentally infected White Leghorn (Lohmann Selected Leghorn) chickens (Gallus gallus domesticus) were used. The birds were purchased as day-old-chicks and kept indoors under group conditions in an experimental stable that ensured helminth-free conditions during a pre-experimental period of 4 weeks.
Pooled faecal samples collected from the ground (n = 3) were negative for nematode eggs on the infection day. The birds were then infected at an age of 4 weeks with 1000 embryonated eggs of Heterakis gallinarum each. The infection procedures were similar to those used in previous reports for this nematode (Daş et al., Reference Daş, Abel, Humburg, Schwarz, Rautenschlein, Breves and Gauly2011a, Reference Daş, Savas, Kaufmann, Idris, Abel and Gauly2011b, Reference Daş, Abel, Rautenschlein, Humburg, Schwarz, Breves and Gauly2011c, Reference Daş, Abel, Savaş, Sohnrey and Gauly2014; Daş and Gauly, Reference Daş and Gauly2014) and excluded Histomonas meleagridis, a hyper-parasite vectored by H. gallinarum that can impair its growth and fecundity (Daş et al., Reference Daş, Abel, Humburg, Schwarz, Rautenschlein, Breves and Gauly2011a). The use of young birds and the experimental infection procedures were in line with the relevant guidelines of the WAAVP for inducing experimental infections in poultry (Yazwinski et al., Reference Yazwinski, Chapman, Davis, Letonja, Pote, Maes, Vercruysse and Jacobs2003).
At the start of the 5th week post-infection (wpi), the birds were placed individually into wire-bottomed cages for quantitative feces collection. The cages were in a half-open housing unit that provided protection from the sun and rain. The ambient temperature and humidity in the housing unit were similar to those of the outside climatic conditions. Following a 3-day period of adaptation to the cages, feces of individual chickens was collected quantitatively for 4 days (27–30.05.2013). The average day length was 16:12:29 h, with sunrise at approximately 05:12 (Anonymous, 2014). In addition to the natural light during the daytime, LED light bulbs were turned on between 18:00 and 22:00 h, which mainly fell within the daytime, so that the inside and outside conditions became more similar. During feces collection at night, the lights were turned on for a maximum of 30 min to remove feces from the cages. The barn temperature and relative humidity were measured (data available on request) every 15 min by a temperature/humidity data logger (Tinytag®). The birds were fed a commercial grower diet (18.4% crude protein; 11.4 MJ metabolizable energy) ad libitum and had free access to fresh water.
The sampling intervals, husbandry conditions, feces collection and sample processing procedure, as well as the egg counting method used in the present study, were the same as those previously reported in detail by Wongrak et al. (Reference Wongrak, Gauly and Das2015). Briefly, on each collection day, the total amount of feces excreted by each bird was collected every 4th h (i.e., at 06:00, 10:00, 14:00, 18:00, 22:00, 02:00 h) and mixed thoroughly and a sub-sample (4 g) was examined (sample size, N = 336) with the McMaster egg counting technique (sensitivity: 50 eggs per gram of feces) to determine the number of eggs per gram of feces (EPG). A saturated sodium chloride (NaCl) solution (density ⩾ 1.2 g mL−1) was used as the flotation fluid. Number of eggs excreted within 4 h (EP4h, eggs per 4 h) was calculated by multiplying EPG and the amount of feces excreted in the same period (4 h). A number of eggs per day (EPD) was calculated as the sum of six EP4h values within 24 h. In cases where the amount of feces collected within the 4-h period was above 4 g, another sub-sample (ca. 1 g) was oven-dried (24 h at 105 °C) to measure the dry matter content of the feces samples (N = 300).
At the end of the feces collection period, the birds were necropsied for a post-mortem parasitological examination. The average body weight of the birds at necropsy (5 wpi) was 732 g (s.d. = 36.3). Worms were collected from individual birds by wet-sieving. Briefly, the caeca were cut longitudinally and the contents washed out under low-pressure running tap water into a 100 µm pore sieve. The worms retained on the sieves were transferred to Petri-dishes, counted, sexed and measured for length using a dissecting microscope at 10 to 40 × magnification. The differentiation of larva from the mature worms was done based on general morphological traits (e.g. presence of spicules in males and presence of eggs in females) of the worms. The small intestines were also examined for the presence of A. galli and other helminths that might have accidentally infected the chickens.
Data management and statistical analyses
Considering the average fecundity of the nematode and feces excretion of the birds, data from two birds with only a few female worms (⩽6 females) were removed from the analyses to reduce the uncertainty of measurements arising from the relatively low sensitivity of the McMaster method (50 EPG). Per capita worm fecundity was defined as the number of eggs excreted per female worm within 4 h (EP4h per female) or within a day (EPD per female). To examine whether worm infrapopulations were sex-biased, a paired t-test was used to compare the number of female and male worms of the same hosts. Pearson correlation analysis was used to examine the relationship between the amount and dry matter content of feces using pooled data across four collection days (n = 300 samples). The paired t-test and correlation analysis were performed using the ‘ttest’ and ‘corr’ procedures of the SAS/STAT software, respectively [(SAS Institute Inc (2016)].
Two different statistical approaches were adopted to assess the existence, frequency and magnitude of periodicity in nematode egg excretion and host animal feces excretion parameters. The first statistical approach relied on classical analysis of variance fitted to the experimental design. For this analysis, egg excretion variables (EPG, Ep4h, EPD, fecundity estimates) were first transformed by using a natural logarithm (ln) function [Ln(y) = ln(y + 1)] to correct for heterogeneity of variance and to produce approximately normally distributed data.
The amount and dry matter content of feces, as well as the log-transformed nematode egg excretion data, were analysed with repeated measures ANOVA using the MIXED procedure in the SAS/STAT software of the SAS Institute Inc (2016). The statistical model included the fixed effects of the day (1–4), sampling time point (i.e., n = 6 from 02:00 to 22:00 at 4 h intervals) and interactions between these two factors. To account for the variability among measurements of the same experimental unit over time (i.e., a host or a worm infrapopulation residing in that host), a repeatedly sampled experimental unit (subject) was included in the model through the REPEATED statement of the MIXED procedure. For the log-transformed EPD and daily per capita worm fecundity data (EPD per female worm), the model excluded the fixed effects of the sampling time and interactions, factoring in day effects only. An adjusted Tukey test with a significance level of P < 0.05 was used for post-hoc comparisons where significant effects of days, sampling hours or interactions were encountered. Analysis of variance is capable of detecting time-dependent effects but does not strictly test for periodicity. Thus, we employed a second approach to rigorously assess the presence of diurnal rhythmicity and estimate the phases and amplitudes. For this approach, we focused on per capita egg excreted by the worms (log-fecundity, average number of eggs excreted per female worm within 4 h) and feces excretion (wet and dry) by the host within the given time (4 h intervals during 4 days). Analyses were performed separately for each host animal (i.e. worm infrapopulation). The amplitudes, phases, and P values were used to assess rhythmicity against a null hypothesis of non-rhythmic random variation over time using robust harmonic regression as described elsewhere (Thaben and Westermark, Reference Thaben and Westermark2016).
For quantification of how many of the 14 worm infrapopulations showed a diurnal rhythm, Benjamini-Hochberg-adjusted P values, interpretable as estimated false discovery rates (FDR), were calculated. The frequencies of infrapopulations and hosts showing a diurnal rhythm for fecundity and feces excretion were then calculated using two different thresholds of FDR <20% and FDR <5%, i.e. adjusted P values of <0.20 and <0.05, respectively. The relative amplitudes and phases of the diurnal rhythms were reported for infrapopulations or hosts falling below the thresholds.
Results
Characteristics of the worm infrapopulations
Mono-species infection with H. gallinarum was confirmed by the absence of helminth eggs other than those of ascarids, which are morphologically highly similar to those of H. gallinarum and A. galli (Kaufmann, Reference Kaufmann1996). The absence of A. galli was directly confirmed through a parasitological examination of the small intestines. Patency was confirmed in all worm infrapopulations at the beginning of the 5th wpi when the birds were placed in cages. The average number of H. gallinarum harvested from individual birds was 122 (s.d. = 90.6) (Table 1). As indicated by the low percentage of larvae (<2%), the worm infrapopulations mainly consisted of first-generation adult worms originating from the experimental infection. The few larvae were found only in two chickens and they were smaller than 2 mm in length. The average adult length was 10.8 and 8.5 mm in female and male worms, respectively. A paired t-test that compared female and male worm counts of the same hosts indicated a significant (df = 13, t = 2.53, P = 0.025) bias towards lower number of female worms in the same host, resulting in an average sex ratio of 46.1% (95% CI 43.0–49.3%).
Table 1. Descriptive statistics for average worm burdens, sex ratio and worm length in chickens (N = 14) experimentally infected with Heterakis gallinarum (N = 14)
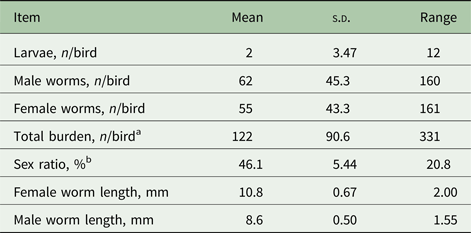
a Includes larvae, female and male worms as well worms that were cut or damaged during worm harvesting from the intestines, which could thus not be classified for sex.
b Defined as [(Female/Female + Male) × 100]. For this parameter, the lower and upper bounds of 95% confidence interval were 43.0 and 49.3%, respectively.
Nematode egg excretion
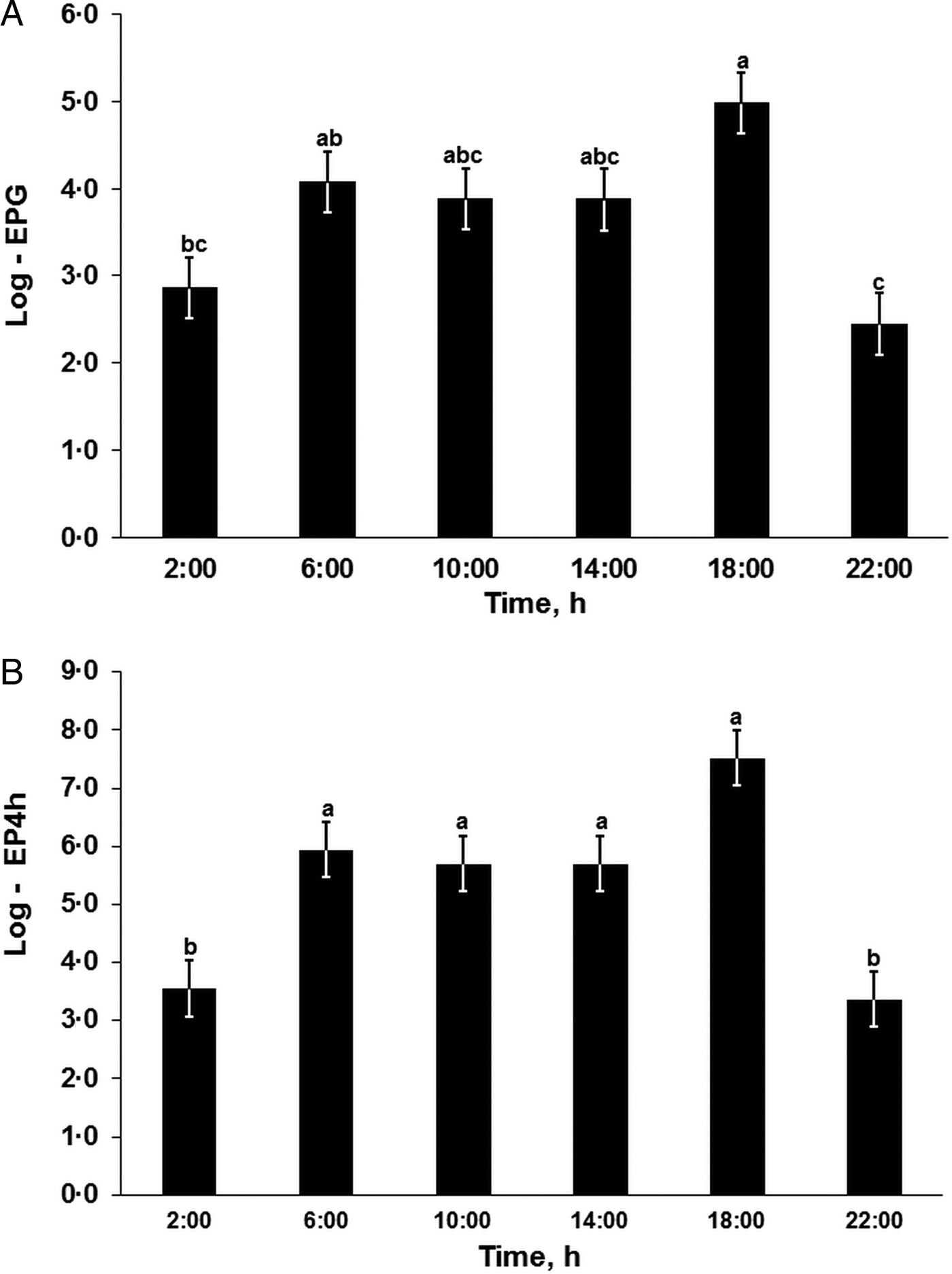
The nematode egg concentration in the feces (eggs per gram of feces, EPG) and cumulative egg excretion during the 4-h periods (EP4h) were affected significantly by the sampling time within 1 day (Repeated-Measures-ANOVA (RM-ANOVA), F (5, 65) = 6.7, P < 0.001; and F (5, 65) = 11.0, P < 0.001, respectively; Fig. 1) but not by the day or interaction effects (RM-ANOVA, P > 0.05; see Table 2 for DF and F-values), while the EPD did not differ significantly (RM-ANOVA, F (3, 39) = 0.9, P = 0.851) among the 4 days. The egg concentration in the feces (EPG, Fig. 1A) gradually increased from the night (02:00) to the late afternoon (18:00), followed by a sharp decrease in the evenings (22:00) with significant differences in the late afternoon (18:00) compared with the evening (22:00; Tukey, t (65) = 5.1, P < 0.001) and nights (02:00; Tukey, t (65) = 4.3, P < 0.001). The cumulative egg excretion during the 4-h periods (EP4h, Fig. 1B) was significantly higher during the daytime than during the evening or night, with a peak excretion quantified in the late afternoons (Fig. 1).
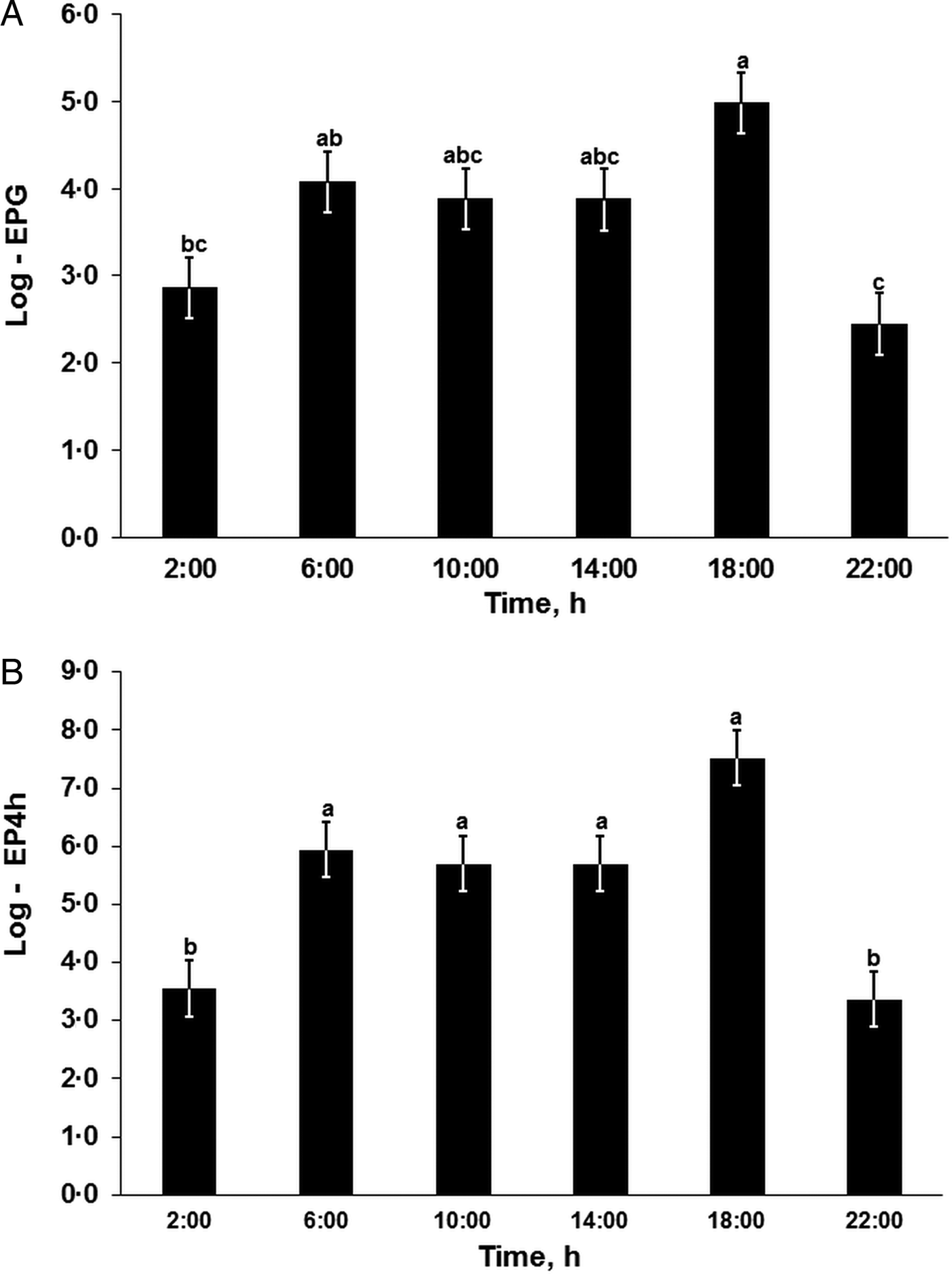
Fig. 1. Heterakis gallinarum egg excretion patterns within 1 day. EPG: Number of eggs per gram of feces; EP4h: total number of eggs excreted within 4 h. abc: Time points sharing no common letter differ significantly (Tukey, P < 0.05 following a significant effect of sampling time, Repeated-Measures-ANOVA, P < 0.001). For the corresponding degree of freedom (DF) and F-values see Table 2. Log-transformed data presented as LSMEANS with the s.e. in error bars.
Table 2. Time-related effects on nematode egg excretion through host feces in H. gallinarum-infected chickens
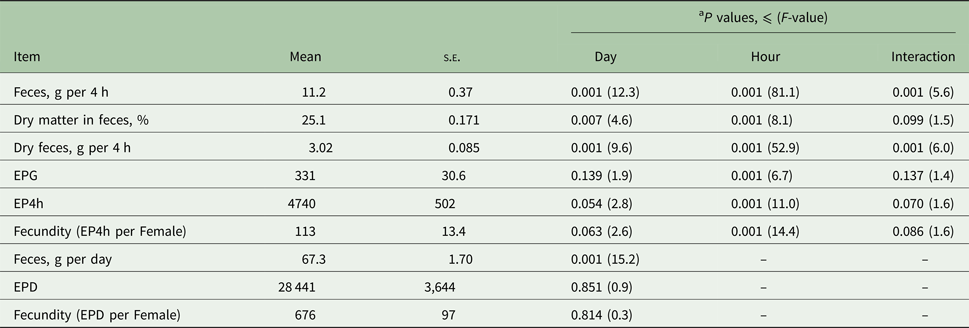
EPG, eggs per gram of feces; EP4h, number of eggs excreted within 4 h; EPD, number of eggs per day.
Sample size: n = 336 for parameters measured on a 4-h basis (except for dry matter and dry feces, n = 300); n = 56 estimates for parameters measured on a daily basis.
a Data were analysed with repeated measures ANOVA using a mixed model. P values for egg excretion and fecundity variables (EPG, Ep4H, EPD, fecundity) were derived from analysis of log-transformed data. Figures are presented as raw data (Mean ± s.e.). F-values derived from the repeated measures ANOVA are shown in brackets below the P values. Numerator degree of freedom (NDF) and denominator degree of freedom (DDF) are 3 and 39 for the days; 5 and 65 for the hours; 15 and 195 for the interactions, respectively. For variables with a smaller sample size (dry matter and dry feces, n = 300) DDF is 159 instead of 195.
Host feces excretion
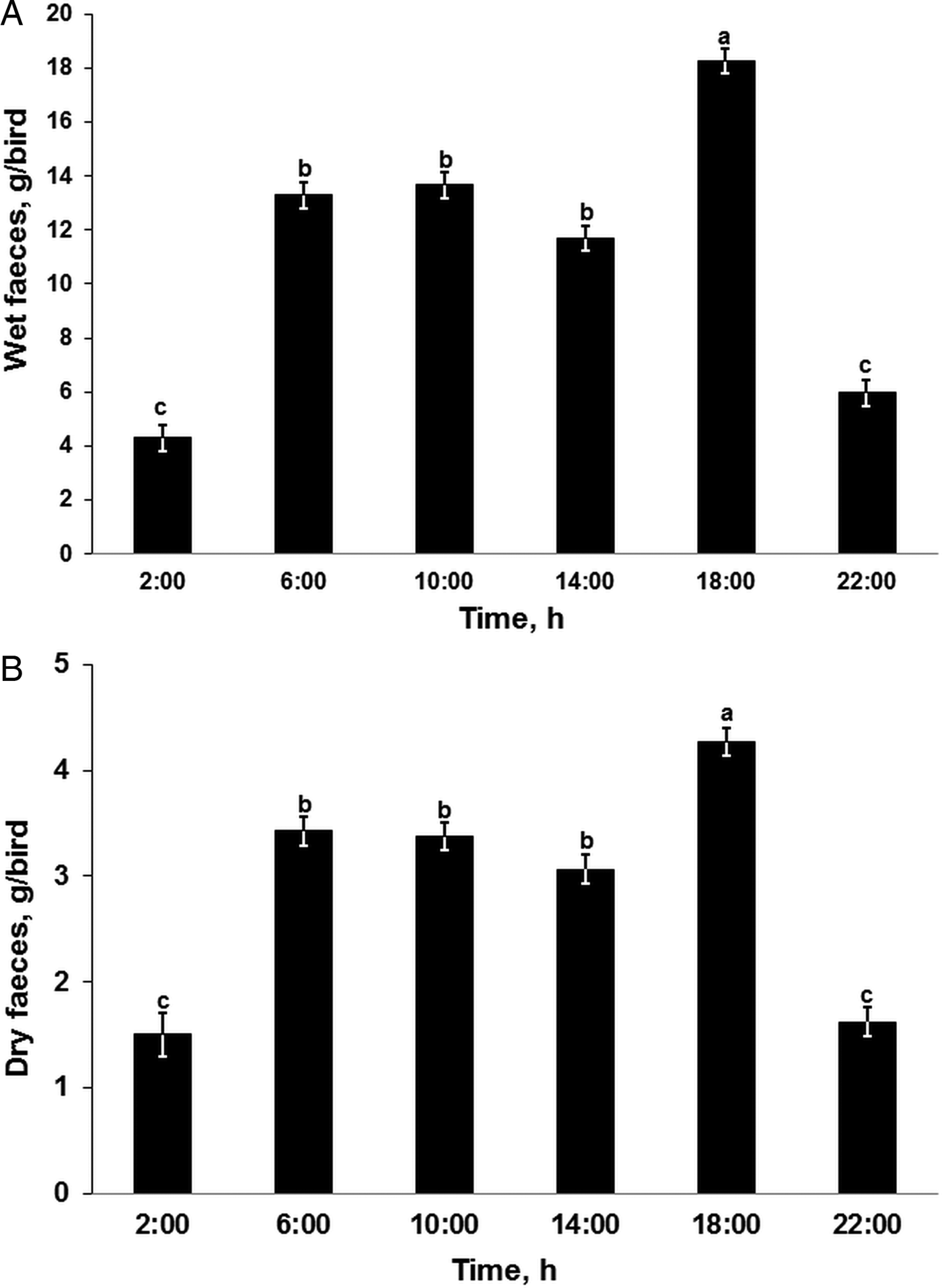
On average, 67.3 g (s.d. = 12.7) of feces, consisting of 25% (s.d. = 3.0) dry matter, was excreted by individual chickens within 24 h (Table 2). The amount of feces (as fresh substance) excreted during the 4-h periods increased conspicuously from the night to the early morning (Tukey, t (65) = 11.0, P < 0.001; Fig. 2A) and remained fairly constant until 14:00, followed by a sharp increase at 18:00 (Tukey, t (65) = 8.0, P < 0.001) before declining to initial (night) levels during the evening. A higher amount of wet feces was excreted during the daytime, particularly during the late afternoons, than during the evening or nights (Tukey, t (65) ⩾ 7.0, P < 0.001). The average dry matter contents of the feces samples varied within a narrow range (23.4 to 26.7%; Supplementary Fig. S1) each day, with the smallest and highest average values quantified in the late afternoons and evenings, respectively. Feces collected from 02:00 to 14:00 had similar (Tukey, t (65) ⩽ 0.9, P > 0.05) dry matter contents. Although there was a moderate negative correlation (Pearson r(300) = −0.37, P < 0.001) between the amount of feces and dry matter content, the impact of the dry matter content on the pattern of feces excretion during 1 day was relatively limited. As shown in Fig. 2B, the amount of water-free feces excreted within 1 day followed almost the same pattern as that of the fresh excreta. The amount of feces excreted within 4 h and the associated dry matter contents were different across the 4 days (RM-ANOVA, F (3, 39) = 12.3, P < 0.001; and F (3, 39) = 4.6, P = 0.007, respectively; Table 2).

Fig. 2. Amounts of wet (A) and water-free dry feces (B) excreted from Heterakis gallinarum-infected chickens at 4-h intervals. abc: Time points sharing no common letter differ significant (Tukey, P < 0.05 following a significant effect of sampling time, Repeated-Measures-ANOVA, P < 0.001). For the corresponding degree of freedom (DF) and F-values see Table 2. Data presented as LSMEANS with the s.e. in error bars.
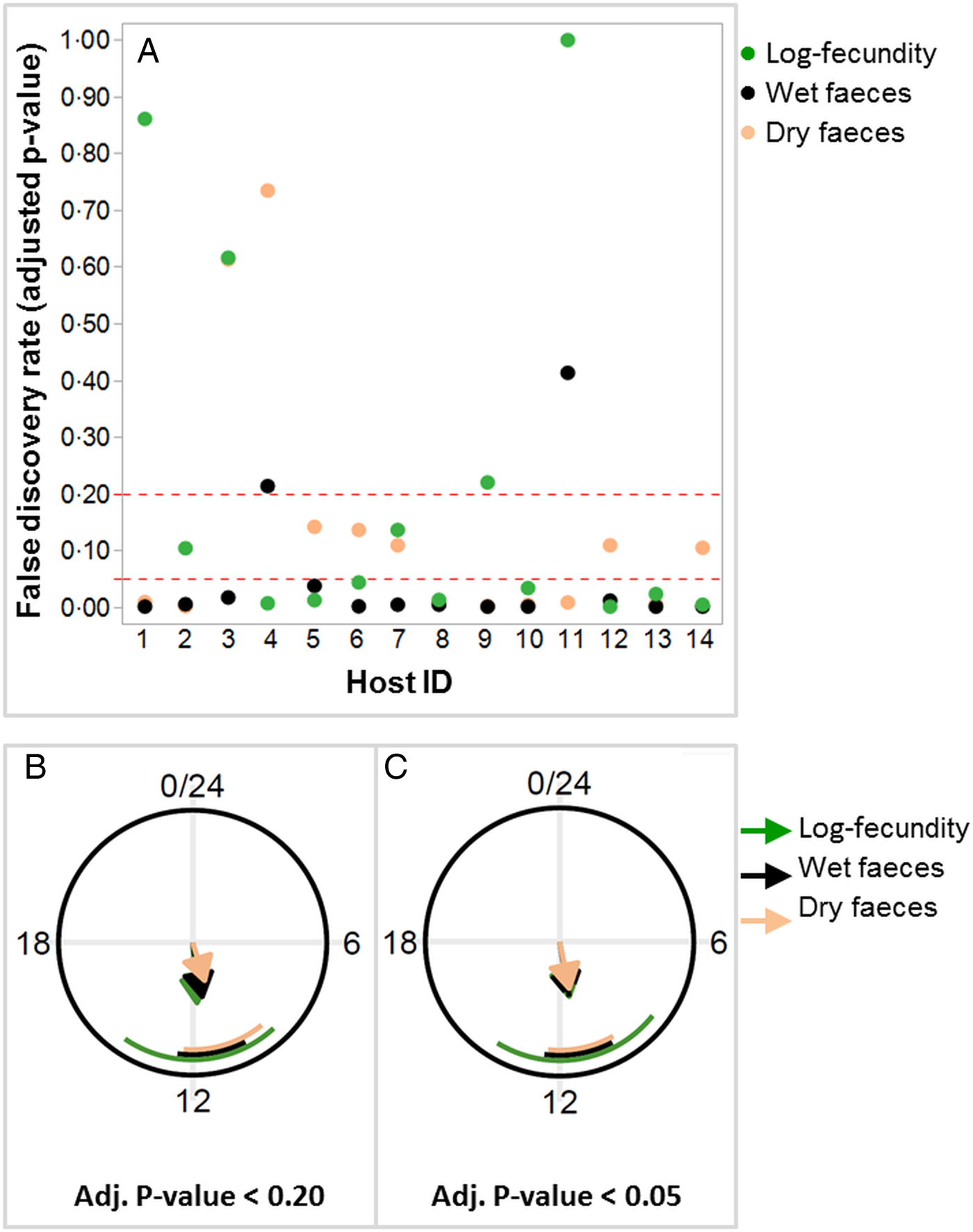
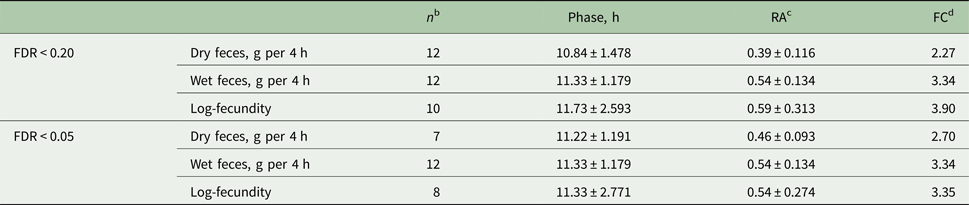
Out of 14 birds, 12 were shown to have a diurnal rhythm of dry or wet feces excretion, whereas log-fecundity in 10 worm infrapopulations exhibited a diurnal rhythm with a false discovery rate of FDR <0.20 (i.e. adjusted P value <0.20) (Fig. 3A; Table 3). Among four worm infrapopulations showing weaker or negligible evidence for diurnal rhythms (adjusted P value >0.20; Figure 3A), two were present in birds without a diurnal rhythm for dry or wet faeces excretion (Fig. 3A). Out of 12 birds with diurnal rhythm of dry or wet feces excretion, 9 showed also diurnal rhythm of parasite fecundity. Of the 12 birds, 11 were proven to have a diurnal rhythm for both wet and dry feces. A more conservative FDR as the cut-off (FDR <0.05) resulted in less numbers of hosts/infrapopulations with a diurnal rhythm of dry feces excretion (n = 7) or fecundity (n = 8), whereas number of birds for rhythmicity of wet feces excretion was not influenced (n = 12; Table 3).
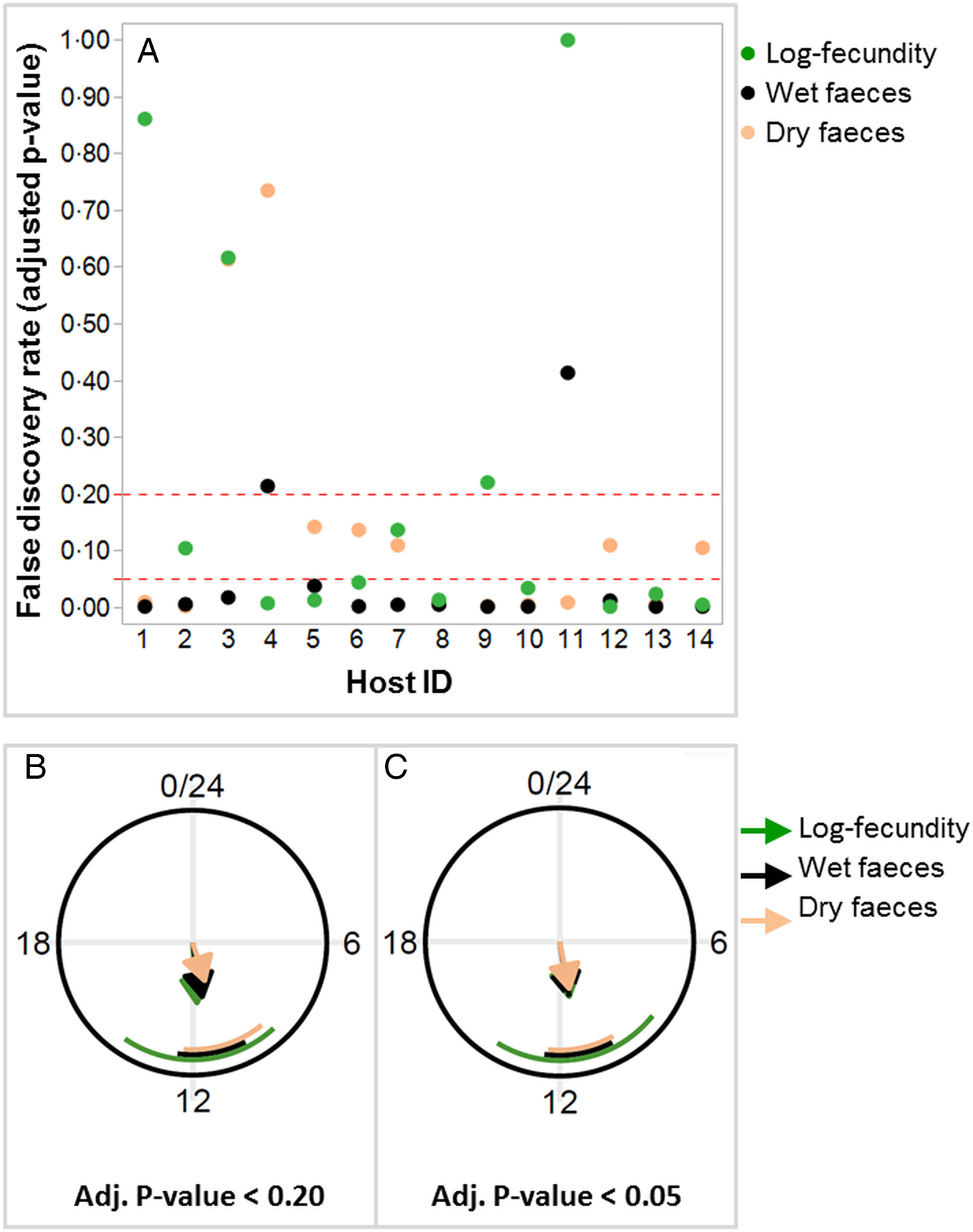
Fig. 3. False discovery rates (FDR) with two different thresholds separating hosts (or worm infrapopulations) with and without a diurnal rhythm for per capita worm fecundity (log-EP4h per female) and wet or dry feces excretion (A). Diurnal phase planes illustrating the phases and relative amplitudes of feces excretion (wet or dry) and worm fecundity (log-EP4h per female) in the host or worm infrapopulations with a proven diurnal rhythm using FDR < 0.20 (B) and FDR < 0.05 (C). Arrow lengths are proportional to the relative amplitudes of a given trait. Circular standard deviations of the phases are indicated by the circular segments.
Table 3. Average (mean ± s.d.)a phase and relative amplitude estimates for host feces excretion and worm fecundity in hosts and worm infrapopulations showing a persistent diurnal rhythm determined with two false discovery rates (FDR)
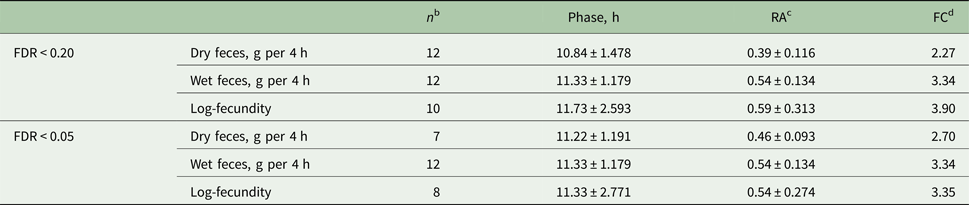
a The mean and s.d. values for phases are based on circular statistics and represent circular mean direction and dispersion, respectively.
b Out of 14 hosts and worm infrapopulations.
c RA, Relative amplitude.
d FC, Fold-change = [(1 + RA)/(1 − RA)].
For both feces (wet and dry) excretion variables and the fecundity, the phase (hits to maxima) estimates were approximately at around noon with both FDR cut-offs (Table 3; Fig. 3B and C). With FDR <0.20, the relative amplitude, a parameter assessing the magnitude of variation around the mean, ranged from 39 to 54% for host feces excretion, while it was 59% for nematode fecundity. FDR <0.05 resulted in more similar relative amplitude estimates, ranging from 46 to 59%, for all three variables.
Because the FDR cut-offs (<0.20 and <0.05) that were used to separate chickens with or without a diurnal rhythm of wet feces excretion did not change the number of animals below the thresholds (n = 12), the estimates for the phase and relative amplitude remained the same with both cut-offs (Table 3). Fold-changes were in general higher for fecundity (3.35 to 3.90) and wet feces excretion (3.34) than for dry feces excretion (2.27 to 2.70) independent of the cut-off used (Table 3).
Discussion
In this study, we investigated the periodicity patterns in both host feces and parasite egg excretion. The results showed that H. gallinarum excreted higher numbers of eggs during the daytime than during the dark period. We further demonstrated that nematode egg excretion had a synchronized association with host feces excretion and apparently was additionally regulated by the parasite itself through enhanced fecundity at the peak of host animal feces excretion. Because chickens are day-active hosts, our results support the hypothesis that the nematode makes efficient use of the host feces excretion pattern, which allows a maximal dispersal of the eggs in the environment, thereby facilitating greater spreading of the parasite.
Implications of periodicity in nematode egg excretion with practical relevance
As shown in the present study, the collection time of feces samples for estimation of the egg concentration in feces – and, thereby, the parasite infection intensity in the host animal – can cause a potential bias in estimates when samples from different animals are collected at different time points. These results are particularly important when assessing drug efficacy by faecal egg count reduction tests. Such a sampling bias has, however, already been reported in detail by others (Villanoúa et al., Reference Villanоúa, Pérez-Rodriíguez, Gortázar, Höfle and Viñuela2006; Wongrak et al., Reference Wongrak, Gauly and Das2015). For quantification of H. gallinarum eggs in the host feces, a 24-h long feces collection would be ideal to eliminate variations due to within-day fluctuations in egg excretion, although conducting sampling during the late afternoons may also be crucially important if the infection intensity is low and the sensitivity of the egg counting technique is not particularly high. We used a McMaster technique with a lower sensitivity (50 EPG) compared with that of new methods (e.g. Flotac, see Cringoli et al., Reference Cringoli, Rinaldi, Maurelli and Utzinger2010). Nevertheless, to reduce the uncertainty of measurements arising from the relatively lower sensitivity of the McMaster method, we excluded data from 2 birds with low worm burdens (⩽6 female worms), the worm presence of which would not clearly ensure a theoretically expected egg concentration in feces detected by the employed method (EPG ⩾50). For this exclusion, we relied on our previous estimates for daily per capita fecundity (416–436 eggs) for H. gallinarum shed through the daily amount of feces (Daş and Gauly, Reference Daş and Gauly2014; Daş et al., Reference Daş, Abel, Savaş, Sohnrey and Gauly2014). The overall average egg concentration (EPG = 331 ± 30.6) estimated in the present study was more than 6 times higher than the detection limit of the employed egg counting technique and suggests that the diurnal variation observed in egg excretion by H. gallinarum was attributable to factors other than methodological restrictions.
Time-related effects on host feces and nematode egg excretion
Nematode egg excretion, expressed as EPG or EP4h, was affected by the sampling time within 1 day but not by the day or interaction effects, whereas the EPD did not differ across the 4 sampling days. These findings clearly indicate the existence of a repeatable nematode egg excretion pattern within 1 day. The pattern was characterized by a gradual increase in EPG and EP4h from the night to the late afternoon, followed by a sharp decrease in the evenings, which in turn, resulted in a higher total egg excretion during the daytime than during the evening or night, with a peak excretion in the late afternoon. A very similar pattern was also quantified for the host feces excretion in both wet and dry forms. As already observed by Clarke (Reference Clarke1979), an important feature of caeca that has the potential to influence host–parasite relationships is the frequency and extent of the discharge of caecal feces. Although the amount of feces excreted within 4 h and the associated dry matter contents were different across the 4 days, neither EPG nor EP4h was influenced by the daily effects. Moreover, the EPD did not differ across the 4 days. These results may collectively indicate the persistence of the daily per capita fecundity of H. gallinarum over time, despite changes occurring in the quantity and water content of excreta. This persistence in egg excretion is also in agreement with our previous results showing high repeatability estimates (R = 0.67–0.84) for daily egg excretion by H. gallinarum after patency was fully achieved in the chicken host (Daş et al., Reference Daş, Abel, Rautenschlein, Humburg, Schwarz, Breves and Gauly2011c).
Regulation of egg excretion is beyond synchronization with host-feces excretion
As confirmed by the diurnal rhythm analysis, at least half the birds (n = 7–12) and their worms (n = 8–10 infrapopulations) showed diurnal rhythms for feces and per capita egg excretion, respectively, with either FDR cut-off. Feces excretion and worm fecundity showed overlapping diurnal rhythms, suggesting synchronization between host feces excretion and nematode egg excretion. The presence of synchronization was also supported by the phases (peaks) for feces and fecundity that overlapped at similar time points (Fig. 3B and C). Moreover, since the worm fecundity was standardized for feces excretion by considering the total eggs excreted within 4-h periods, the simultaneous changes (both increase and decreases) in both feces and per capita egg excretion not only indicated a well-balanced synchronization between parasite egg excretion and host feces excretion patterns but also provided evidence that the parasite may additionally regulate its fecundity so that the concentration of eggs in feces can be higher at the highest feces excretion rates. The relative amplitude of the diurnal rhythm was slightly higher (59%) for worm fecundity than for host feces excretion (39–54%), which approximately corresponded to 3.4–3.9-fold changes in fecundity, while smaller fold changes were calculated for feces excretion (i.e. 2.3–3.3). These fold differences indicate potential ranges in which feces excretion and fecundity can be regulated by the host and parasite, respectively. The fecundity and feces phases were estimated to occur around noon, which does not exactly coincide with the maxima. This difference is due to the use of harmonic regression to estimate the phase of an underlying first harmonic (i.e., sine curve). The first harmonic might better reflect the physiological processes that underlie the overt rhythmicity in feces and egg excretion, which show slightly more complicated waveforms with maxima between 14:00 and 18:00. Defining phases and amplitudes based on Fourier regression is much more robust than estimating them based only on maxima and minima. A fuller Fourier analysis could add more harmonics, to accommodate a wide spectrum of waveforms. However, it is not trivial to determine the number of harmonics to add, as well as how to define overall phase and amplitude in this case.
There is evidence that parasites can influence host feces excretion patterns, for instance, by altering caecal motility (Clench and Mathias, Reference Clench and Mathias1995) or inducing intestinal contractility (Oikawa and Kawaguchi, Reference Oikawa and Kawaguchi1974). In a 21 day-long study, Clarke (Reference Clarke1979) demonstrated that uninfected control birds never excreted caecal feces during the dark period of a day, whereas birds infected with Eimeria tenella, an apicomplexan protozoa parasitizing the caeca, excreted caecal feces during the dark period. Caecal feces excretion was still most frequent during the daytime, particularly between 16:00 and 19:00 (Clarke, Reference Clarke1979), which overlaps with the highest total number of eggs excreted by H. gallinarum in a comparable period of time (14:00–18:00) in our study. Thus, the increase in egg excretion by H. gallinarum during the late afternoon may partly be explained by the increase in excretion of caecal feces, which acts as the sole transport media releasing eggs into the external environment. Although we did not separate caecal feces from the non-caecal, regular feces, our data indicate that the increase in egg excretion in the late afternoon may not merely be due to an increase in host feces excretion but also due to an increased egg concentration in caecal feces, which might have been directly regulated by the parasite itself through a higher lay activity during the daytime. This assumption is based on two reasons. First, assuming a fairly constant worm fecundity within a day (i.e. no difference among sampling points), one would expect a lower faecal egg concentration in the late afternoons when the host total feces excretion was at the highest level. If the increase in egg excretion was solely due to an increased amount of caecal feces, which is excreted at lower rates during the dark period, the highest egg concentration in the feces samples would probably be quantified at the early mornings, because the eggs released by the nematode would accumulate during the night in the caecal lumen and be excreted with the first caecal droppings. Nevertheless, the hypothesis that fecundity is additionally regulated by the nematode itself needs to be tested in further studies that include (1) uninfected control chickens to quantify H. gallinarum-infection effects on host-feces excretion pattern, and (2) separation of caecal feces from the non-caecal feces. However, there are some methodological restrictions for separation of feces. As a precise and quantitative separation of caecal feces is not possible with time-interval sampling methods, particularly when the time-intervals are less frequent, other approaches may be employed. Although a more frequent time-interval sampling (e.g. every hour) or an event-sampling strategy during 24 h may sound reasonable, the more frequent the sampling occurs the more likely are the biological rhythms disturbed. Thus the use of an automatized feces collection system and/or the use of specific markers (e.g. caecal bacteria or fermentation products, ingestible and/or fermentable markers) may also be helpful.
Higher egg excretion by H. gallinarum during the daytime than during the dark period confirms previous results observed in A. galli (Wongrak, et al., Reference Wongrak, Gauly and Das2015), whose eggs are excreted through regular non-caecal feces. The only exception was that H. gallinarum additionally tends to excrete a higher number of eggs during the late afternoon period than during the rest of the daytime (e.g. from 06:00 to 14:00), whereas no difference was observed for egg excretion by A. galli in periods of daytime (Wongrak, et al., Reference Wongrak, Gauly and Das2015). The high similarities between the egg excretion patterns of A. galli and H. gallinarum might thus indicate a common strategy by the two genetically closely related species (Nadler et al., Reference Nadler, Carreno, Mejía-Madrid, Ullberg, Pagan, Houston and Hugot2007; Wang et al., Reference Wang, Gu, Yang, Wang, Lai, Zhong and Liu2016) in terms of a greater dispersal of the eggs in the environment that can facilitate parasite transmission (see below).
The function of periodicity in egg excretion by nematodes is likely to facilitate nematodes to benefit from host activity and increase their spatial distribution in the external environment. Because chickens are day-active and rest during the dark period (Ekesbo, Reference Ekesbo2011), it is of less advantageous for a parasite species to release its eggs during the night in the same place where the host animal is resting. By contrast, increased egg release during the host animal active period has the potential to extend the spatial distribution of the eggs, which in turn, can contribute to a greater parasite transmission via new or re-infections. Additionally, accumulation of large amounts of eggs in feces during the night may result in massive egg losses. As was shown for the eggs of Ascaris suum, the vast majority of nematode eggs (99.99%) do not develop into infective stages in the external environment when they remain in pen litter, which is a hostile environment to eggs due to the physico-chemical conditions caused by composting activity (Katakam et al., Reference Katakam, Thamsborg, Kyvsgaard, Dalsgaard and Mejer2014). The chickens given outdoor access during the daytime will leave their faecal droppings on the pasture area. Thus, another advantage of increased egg excretion during the daytime might be associated with finding suitable micro-environments in which embryonation of the excreted eggs may be favoured. Furthermore, because ascarids eggs (H. gallinarum + A. galli) can survive and remain infective up to 2 years when buried in soil under natural circumstances (Thapa et al., Reference Thapa, Thamsborg, Meyling, Dhakal and Mejer2017), an elevated egg excretion during the active-period of the diurnal host has the potential to favour environmental contamination and a long-term survival of the eggs. A completely oppositely synchronized relationship between parasite egg release with host feces excretion and activity has been shown for Echinostoma caproni residing in mice, a nocturnal host (Platt et al., Reference Platt, Hussey and Zelmer2013). In the current study, parasite egg excretion was lowest during the daytime and highest at night, corresponding to the highest and lowest host activities, respectively. Despite the opposite synchronization, this pattern also serves the same function, i.e., higher egg excretion when the host animal is most active, likely leading to greater parasite spread. Our data collectively support the hypothesis that the nematode is capable of benefiting from feces excretion and the activity patterns of the chicken host to extend its spread.
Concluding remarks
We conclude that egg excretion by H. gallinarum is synchronized with host feces excretion and is higher during the daytime than during the dark period. This overlaps with the maximum activity of the day-active host and allows a maximal dispersal of the eggs in the environment. Our data collectively support the hypothesis that the nematode is capable of benefiting from host feces excretion and its activity patterns to extend its spread.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018001075.
Acknowledgements
We thank Dr Kalyakorn Wongrak, Birgit Sohnrey and Erwin Tönges for their kind help with sample collection and processing. Comments of Dr Armin Tuchscherer on the statistics are particularly acknowledged.
Conflict of interest
The authors declare that no competing interests exist.
Financial support
Data presented in this study was obtained from a project that was funded by the German ‘Bundesministerium für Wirtschaft und Technologie’ through the funding programme ‘Zentrales Innovationsprogramm Mittelstand – ZIM‘(Project number: KF2484202).
Ethical standards
The ethical approval of the project was obtained from Lower Saxony State Office for Consumer Protection and Food Safety, Germany (permission no: 33.9-42502-04-12/0707).