INTRODUCTION
All species of parasites are restricted to particular host species and specific habitats on or in their hosts. The phylogenetic constraints and selective pressures leading to apparent habitat restrictions within the host depend on the scale at which the habitat-selection behaviour of the parasite is observed (Lymbery et al. 1989; Rohde, 2002). It is often assumed that patterns of intrahost habitat selection result from parasite decisions with a functional meaning, e.g. efficient host exploitation associated with trophic specialization, enhancement of mating opportunities or avoidance of interspecific competition (Holmes, 1990; Rohde, 2002 and references therein). However, in this study we document the case of a parasite that infects several host species but exhibits contrasting patterns of habitat restriction within each of them and, interestingly enough, parasite decisions seem to be almost inconsequential in generating these patterns.
Pholeter gastrophilus is a gastric digenean that has been reported in at least 17 cetacean species world-wide (Aznar et al. 1992; Raga, 1994). The parasite infects most commonly coastal species (e.g. Dollfus, 1974; Van Waerebeek et al. 1993; Aznar et al. 1994; Gibson et al. 1998; Berón-Vera et al. 2001), but has also been reported in oceanic species (Aznar et al. 1992; Raga and Balbuena, 1993; Fernández et al. 2003), and even in freshwater species (Zam et al. 1970). It is not known how cetaceans become infected with the parasite, but Gibson et al. (1998) suggested that molluscs and fish would act as first and second intermediate hosts, respectively. In cetaceans, P. gastrophilus burrows into the stomach wall and lives within submucosal fibrotic nodules formed by the host (Woodard et al. 1969; Migaki et al. 1971; Howard et al. 1983). Nodules can contain from one to several hundred worms and have a narrow duct that opens into the stomach lumen to void the eggs (Gibson et al. 1998). Within each nodule, worms are typically isolated in pairs (Raga et al. 1985; F. J. Aznar, personal observations), suggesting that this aggregative behaviour is related to enhancing mating (Lymbery et al. 1989).
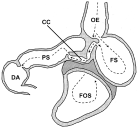
In contrast, little is known about the habitat selection of P. gastrophilus in the whole stomach. The stomach of odontocetes is a complex multichambered organ (Fig. 1) that seems to be adapted to opportunistic feeding on prey discontinuously available in space and time (Gaskin, 1978). It typically consists of (1) a non-glandular forestomach where whole prey are stored and partially digested by enzymes coming from the next chamber; (2) a fundic stomach, which receives the semi-digested food and carries out the major chemical breakdown; (3) a narrow connecting channel, which appears to act as a valve to regulate the flow of chyme into the next chamber, and (4) a pyloric stomach, which produces mucus that regulates the pH of the chyme before it is passed to the first region of the duodenum, the duodenal ampulla (Harrison et al. 1970; Smith, 1972; Gaskin, 1978; Desportes, 1985; Mead, 2002).

Fig. 1. Schematic drawing of the stomach of a typical odontocete, the spinner dolphin, Stenella longirostris (redrawn after Harrison et al. 1970). Broken arrows indicate the path of food flow (see text for details). OE, oesophagus; FOS, forestomach; FS, fundic stomach; CC, connecting channel; PS, pyloric stomach; DA, duodenal ampulla.
Pholeter gastrophilus has been reported in all stomach chambers, and the duodenum, of several odontocete species (Zam et al. 1971; Dollfus, 1974; Aznar et al. 1992; Van Waerebeek et al. 1993; Raga and Balbuena, 1993). However, 3 major questions about the habitat selection at this scale remain unanswered. What factors drive the distribution of P. gastrophilus among stomach chambers? Does P. gastrophilus favour particular chambers? Does habitat selection differ among host species? In this paper, we address these questions by comparing the distribution of P. gastrophilus in the stomach and duodenal ampulla of 4 odontocete species, namely, the harbour porpoise, Phocoena phocoena, the striped dolphin, Stenella coeruleoalba, the bottlenose dolphin, Tursiops truncatus, and the long-finned pilot whale, Globicephala melas. The results suggest that peculiarities in the diet and digestive physiology of each host species might play a fundamental role in the distribution of P. gastrophilus among stomach chambers.
MATERIALS AND METHODS
Samples
Sampling details from the 4 cetacean species are provided in Table 1. Pilot whales were killed in the aboriginal fishery of the Faroe Islands and were obtained fresh (Raga and Balbuena, 1993). In the other species, the condition of carcasses ranged from very fresh to moderately decomposed (Codes 1-3 sensuGeraci and Lounsbury, 1993), but post-mortem movements of worms were unlikely since they occurred within nodules.
Table 1. Sampling details of the four odontocete species analysed for Pholeter gastrophilus in this study (N, number of hosts examined; n>1 year, number of hosts older than 1 year.)

Depending on the cetacean species, animals were necropsied in situ or transported to the laboratory, where they were either immediately necropsied or frozen. The stomach of each animal was generally kept frozen before parasitological analysis. After thawing, each stomach chamber and the duodenal ampulla were examined separately for P. gastrophilus. Nodules were detected through a careful visual and tactile screening of the stomach wall. Most nodules were easily identifiable because they protruded prominently; small nodules were detected based on their harder texture with respect to the surrounding tissue. An incision was made to every nodule to confirm the presence of P. gastrophilus. Some nodules contained only calcified material and/or debris, but exhibited the same structure of the typical nodules of P. gastrophilus and were assumed to be positive for the parasite. Most nodules were found separated from each other; when in contact, nodules were considered as independent if they were surrounded by soft, non-fibrotic tissue on at least 90% of their contour. In harbour porpoises and pilot whales, we recorded only the number of nodules found in each stomach chamber. In striped and bottlenose dolphins, each individual nodule was removed and all worms collected and counted. The number of worms in calcified nodules was estimated with the following regression: log (no. worms)=0·43+0·80 log (nodule weight) (r2=0·55, P<0·001). A mixed nested-ANOVA model indicated that this single equation was suitable, regardless of host individual, host species and stomach chamber. Also, the number of nodules appeared to be an acceptable proxy for parasite intensity; in striped and bottlenose dolphins, an ANCOVA indicated that the total number of nodules per host was a highly significant predictor of the total number of worms (log-transformed variables, F(1,43)=12·71, P=0·001; r2=0·23) and the regression did not differ significantly between both species.
Comparison of infection levels
We used Sterne's exact method to set 95% confidence limits (CI) for the prevalence of P. gastrophilus (Reiczigel, 2003), and 5000 bootstrap replications to set 95% CIs for the mean and median number of nodules, and the number of worms in striped and bottlenose dolphins (Rózsa et al. 2000). Hosts <1 year old (Table 1) were excluded from these calculations because they feed mainly on milk. Prevalence was compared among host species with a Fisher's exact test, and the number of nodules and worms with pairwise Brunner-Munzel tests (Neuhäuser and Poulin, 2004). These analyses were carried out with Quantitative Parasitology 3.0 (Reiczigel and Rózsa, 2001).
Stomach distribution patterns
For each cetacean species, we first examined whether the distribution of P. gastrophilus among stomach chambers was predictable at the infrapopulation level; this was taken as a rough indication of chamber preference. Predictability was assessed with Kendall's concordance tests (Conover, 1999) that examined whether the number of nodules or worms per chamber tended to be similarly ordered among chambers from host to host. To assess the potential impact of infrapopulation size on predictability, we repeated the analyses using only a subsample of lightly infected hosts, i.e., hosts having values of no. of nodules or no. of worms in the lower half of the entire host sample distribution.
These analyses suggested that P. gastrophilus might favour the fundic stomach in the bottlenose dolphin (see Results section). Therefore, we used 2 procedures to test whether the occupancy of posterior chambers was driven by density-dependence. First, we used a logistic regression to examine whether the likelihood of colonization of the connecting channel and/or the pyloric stomach was dependent on the density of worms in the fundic stomach. To estimate density, we measured the area from digital photographs of opened stomachs of all individuals with the fundic stomach infected (n=17), using Image Tool 3.0 (University of Texas Health Science Center, http://ddsdx.uthscsa.edu/dig/itdesc.html). Second, we used a least squares linear regression to test whether total intensity predicted worm density in the fundic stomach through a curvilinear relationship.
In each host species, the prevalence of P. gastrophilus among chambers was compared with Cochran' tests and post hoc comparisons were carried out with MacNemar tests (Conover, 1999). The number of nodules or worms per chamber was compared excluding non-infected chambers to obtain independent evidence about distribution patterns (see Rózsa et al. 2000). A test for related, unbalanced observations was required because the number of infected chambers varied among individual hosts. Such a (nonparametric) test is described in eq. 2.13 of Akritas et al. (2002) but can only be used for paired data. Thus, pair-wise comparisons between chambers were made for each host species.
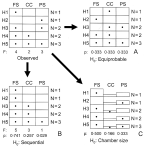
The above comparisons assume that the colonization of stomach chambers is equiprobable (Fig. 2A). However, even if the parasite shows no chamber preference, this null hypothesis might be unrealistic because chambers differ in size and follow a sequential arrangement. We therefore devised 2 more restrictive null hypotheses (see e.g. Péres-Neto et al. 2001; Gotelli and Rohde, 2002). (1) The parasite tends to colonize chambers sequentially, from the fundic stomach to the pyloric stomach (the ‘sequential’ hypothesis, Fig. 2B). (Note that the fundic stomach, the connecting chamber and the pyloric stomach were the only chambers regularly colonized; see the Results section.) (2) The likelihood of colonization of a chamber depends on its size. Accordingly, the largest chamber should be colonized first and so on (‘chamber-size’ hypothesis, Fig. 2C).

Fig. 2. Null hypotheses accounting for the colonization of Pholeter gastrophilus in the fundic stomach (FS), connecting channel (CC) and pyloric stomach (PS) of the 4 odontocete species examined in this study. A hypothetical example of the observed distribution in 5 host individuals is shown in the upper left matrix; Hi represents the ith host in the sample; N is the number of infected chambers; F is the total incidence per chamber; p is the probability of colonization. (A). Equiprobable hypothesis: the probability of chamber colonization is equal for the 3 chambers; (B). Sequential hypothesis: the parasite colonizes chambers sequentially and the probability of colonization is derived from total incidence values (see text for details); (C). Chamber-size hypothesis: the probability of colonization is proportional to the relative size of each chamber. The number of infected chambers per host is preserved in the simulations of the 3 hypotheses.
Colonization probabilities under each null hypothesis were generated as follows. Rows and columns in the data matrix were defined by individual hosts and stomach chambers, respectively (Fig. 2). For the ‘sequential’ hypothesis, we first re-arranged the observed matrices as shown in Fig. 2B and then calculated incidence values per chamber in the re-arranged matrix. Colonization probabilities per chamber were obtained with the procedure described by Wright et al. (1998): the incidence value in each chamber was squared, and divided by the sum of the squared incidence values in all chambers (see Wright et al. 1998 for details). For the ‘chamber-size’ hypothesis, we measured, as described above, the area of each stomach chamber in 5 individual hosts of each cetacean species. Chamber areas were then averaged for the 5 individuals and transformed into probability values as pi=Ai/AT, where Ai is the area of chamber i and AT is the summed area of all chambers (Fig. 2C).
We generated 10000 random matrices under each null hypothesis with EcoSim 7 (Gotelli and Entsminger, 2001). The observed row incidence totals were fixed to preserve the influence of parasite population size on colonization patterns (Fig. 2); columns (chambers) were filled randomly according to the probabilities calculated above (Fig. 2, Table 2). We then tested whether there were statistically significant departures of the empirical colonization patterns with respect to those obtained under each null hypothesis.
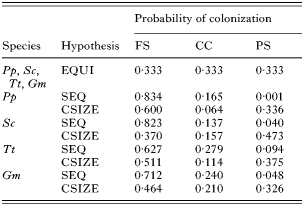
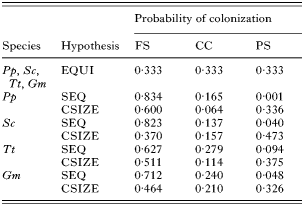
Table 2. Probability values that Pholeter gastrophilus colonizes the fundic stomach (FS), the connecting channel (CC) and the pyloric stomach (PS) of four odontocete species: harbour porpoise Phocoena phocoena (Pp), striped dolphin Stenella coeruleoalba (Sc), bottlenose dolphin Tursiops truncatus (Tt) and long-finned pilot whale Globicephala melas (Gm), according to three null hypotheses: equiprobable (EQUI), sequential (SEQ) and chamber size (CSIZE) (See text for details.)
The above procedure allowed us to investigate a common cause for the stomach distribution of P. gastrophilus through tests applied to each host species. However, we used an interspecific comparison to assess an additional hypothesis, i.e., that the distribution of P. gastrophilus was dependent on the passage time of food through the stomach (or, conversely, the retention time of food in the stomach). As far as we are aware, information from this variable is not available from any cetacean species, yet its potential influence can be evaluated indirectly. In other vertebrates, the passage time of food through the stomach correlates negatively with stomach size (e.g. Hilton et al. 2000a), and with the energy content of food, because this variable delays gastric evacuation rate (e.g. Jobling, 1986; Olson and Boggs, 1986; Maerz et al. 1994; Andersen, 1999; Peracchi et al. 2000; Olson and Galván-Magaña, 2002). If passage time drives the distribution of P. gastrophilus, we would expect a lower colonization of posterior chambers in the cetacean species with smaller stomachs and/or that consume prey with higher energy contents. To test these hypotheses, we firstly obtained a single distribution value of P. gastrophilus per individual host. Following the protocol of Moore and Simberloff (1990), each nodule was scored as to the stomach chamber where it was found (from 1, fundic stomach, to 4, duodenal ampulla); then, scores were averaged (for brevity, we will hereafter refer to average scores as AS). Apparently, the use of nodules did not bias the true distribution of worms: in the striped dolphins and bottlenose dolphins, a comparison of the AS values calculated from nodule distribution with those calculated from worm distribution evidenced no significant differences (Wilcoxon tests, both P>0·5).
The average area of the glandular region of the stomach (fundic stomach+connecting channel+pyloric stomach) from 5 individual hosts per species (see above) was used as a proxy for stomach size. Values of stomach area were corrected for host body size by using residuals obtained from a least squares log-log regression between average stomach area and average body length per species. Dietary information came from the same samples used in this study, except for one species, while values of energy for each prey item were obtained from bibliographic sources (Table 3). In the latter, we attempted to use energy values from entire prey, not just from the edible part for humans (Table 3). To get an overall value of energy content per cetacean species we weighted energy values by the relative biomass of each prey item (Table 3). The AS data did not satisfy the requirements of normality and constant variance even after transformations. Therefore, we ordered host species according to their values of prey energy content or residual stomach size, and used one-tailed Jonckheere-Tespstra non-parametric tests for trends with the AS data (Conover, 1999).

Confounding effects
The interpretation of results derived from the above hypotheses might potentially be confounded by intra- or interspecific effects between parasites. However, this did not appear to be likely. With regard to intraspecific effects in the simulations, we explicitly considered intraspecific density-dependent effects by fixing row totals (see above). Also, although the number of nodules differed significantly between host species (see the Results section), an ANCOVA using ‘log (AS)’ as the dependent variable, ‘host species’ as a factor, and ‘log (total no. of nodules)’ as a covariable, did not reveal any significant main effect of the number of nodules upon AS, or any significant interaction with species (both P=0·77). With regard to interspecific effects, the nematode Anisakis simplex was the only species that regularly co-occurred in the stomach, in harbour porpoises (in all but 2 hosts) and pilot whales (in all but 1 host). We generated a null niche overlap distribution between both species using 1000 simulations in which the (nodule) infrapopulations of P. gastrophilus were randomly associated with the infrapopulations of A. simplex (Moore and Simberloff, 1990). The observed overlap was not significantly different from the overlap expected by chance (P>0·3 in both host species). Moreover, the mean nodule location or locational variance of P. gastrophilus did not change with the intensity of A. simplex and/or the combined intensity of A. simplex and P. gastrophilus (all Spearman correlation tests <0·16, P>0·1) (see Moore and Simberloff, 1990).
Statistical criteria and terminology
In multiple and pair-wise comparisons, we corrected critical probability values by the sequential Bonferroni procedure (Rice, 1989). Ecological terminology follows that of Bush et al. (1997). However, we used the term incidence as the number of hosts with the ith chamber infected (similar to island biogeography studies) and applied the terms ‘prevalence’ and ‘intensity’ also to samples of P. gastrophilus in specific chambers.
RESULTS
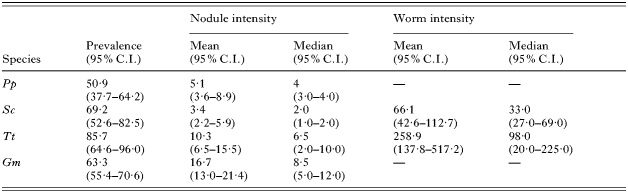
Values of infection parameters of P. gastrophilus in the 4 host species are shown in Table 4. Prevalences were >50% in all cases, and only marginally significant differences were observed among species (Fisher's exact test, P=0·03), because of the high prevalence in bottlenose dolphins (Table 4). Nodule intensity varied clearly among species (Table 4): it was significantly higher in pilot whales than in the other species (Brunner-Munzel tests, all P<0·001), and significantly higher in bottlenose dolphins and harbour porpoises compared with striped dolphins (P<0·001 and <0·04, respectively). Worm intensity also differed significantly between bottlenose and striped dolphins (P<0·001) (Table 4).
Table 4. Infection parameters of Pholeter gastrophilus in four odontocete species (Abbreviated as in Table 2. C.I.: Confidence interval.)

In all host species, P. gastrophilus was largely restricted to the fundic stomach, the connecting channel and the pyloric stomach. In striped dolphins, nodules seldom occurred in the limit between the forestomach and the fundic stomach, but it was evident that worms had entered through the fundic stomach. Nodules were also found in the duodenal ampulla of 8 pilot whales (8% of infected hosts; range: 1–14; mean intensity: 5·0; 95% C.I.: 2·6–8·8) and 1 harbour porpoise. In the former, the number of nodules in the duodenal ampulla correlated weakly, but significantly, with that of the stomach (Spearman correlation test, rs=0·23, n=100, P=0·019). We excluded data from infections of the duodenal ampulla in subsequent analyses.
Patterns of distribution among stomach chambers at increasing intensities are shown in Fig. 3. The number of infected chambers increased significantly with nodule intensity in all host species (Spearman correlation test, harbour porpoise: rs=0·70; striped dolphin, rs=0·79; bottlenose dolphin, rs=0·85; pilot whale, rs=0·77; all P<0·001). However, predictability of chamber occupation was low except in bottlenose dolphins, where P. gastrophilus clearly favoured the fundic stomach (Fig. 3). This was confirmed with Kendall concordance coefficients which, although generally significant, were from low to moderate (number of nodules: harbour porpoise: W=0·35, P<0·001; striped dolphin, W=0·03, P<0·48; bottlenose dolphin, W=0·60, P<0·001; pilot whale, W=0·09; P<0·001; number of worms: striped dolphin, W=0·06, P<0·19; bottlenose dolphin, W=0·65, P<0·001). The same result was obtained at low intensities (number of nodules: harbour porpoise, W=0·15, P=0·126; striped dolphin, W=0·06, P=0·395; bottlenose dolphin, W=0·65, P=0·003; pilot whale, W=0·115, P=0·003; number of worms: striped dolphin, W=0·089, P=0·29; bottlenose dolphin, W=0·581, P=0·005). In the bottlenose dolphin, the likelihood that P. gastrophilus colonized the connecting channel and/or the pyloric stomach was not related to the density in the fundic stomach (Wald statistic: 0·424, n=17, 1 D.F., P=0·515), and the density in the fundic stomach increased linearly with total intensity (slope of the log-log regression: 0·911, 95% C.I. 0·755–1·068; not significantly different from 1).

Fig. 3. Observed patterns of colonization of Pholeter gastrophilus in the fundic stomach, connecting channel and pyloric stomach of 4 odontocete species at increasing intensities. Abbreviations as in Table 2.
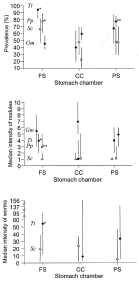
The prevalence of P. gastrophilus per stomach chamber differed among cetacean species (Fig. 4A). In harbour porpoises and bottlenose dolphins, the fundic stomach was the chamber most frequently infected, followed by the pyloric stomach and the connecting channel; the difference between the fundic stomach and the connecting channel was significant (Fig. 4A). In striped dolphins, the highest prevalence also occurred in the fundic stomach, but was not significantly different from those of the connecting channel and the pyloric stomach; the latter two had the same values (Fig. 4A). In pilot whales, the highest prevalence occurred in the pyloric stomach, and was significantly different from those of the connecting channel and the fundic stomach, which did not differ from each other (Fig. 4A). Differences in the intensity of nodules and the intensity of worms per chamber agreed with those of prevalence (Fig. 4B,C), with the exception of the number of nodules per chamber in pilot whales, which did not differ among chambers (Fig. 4B).
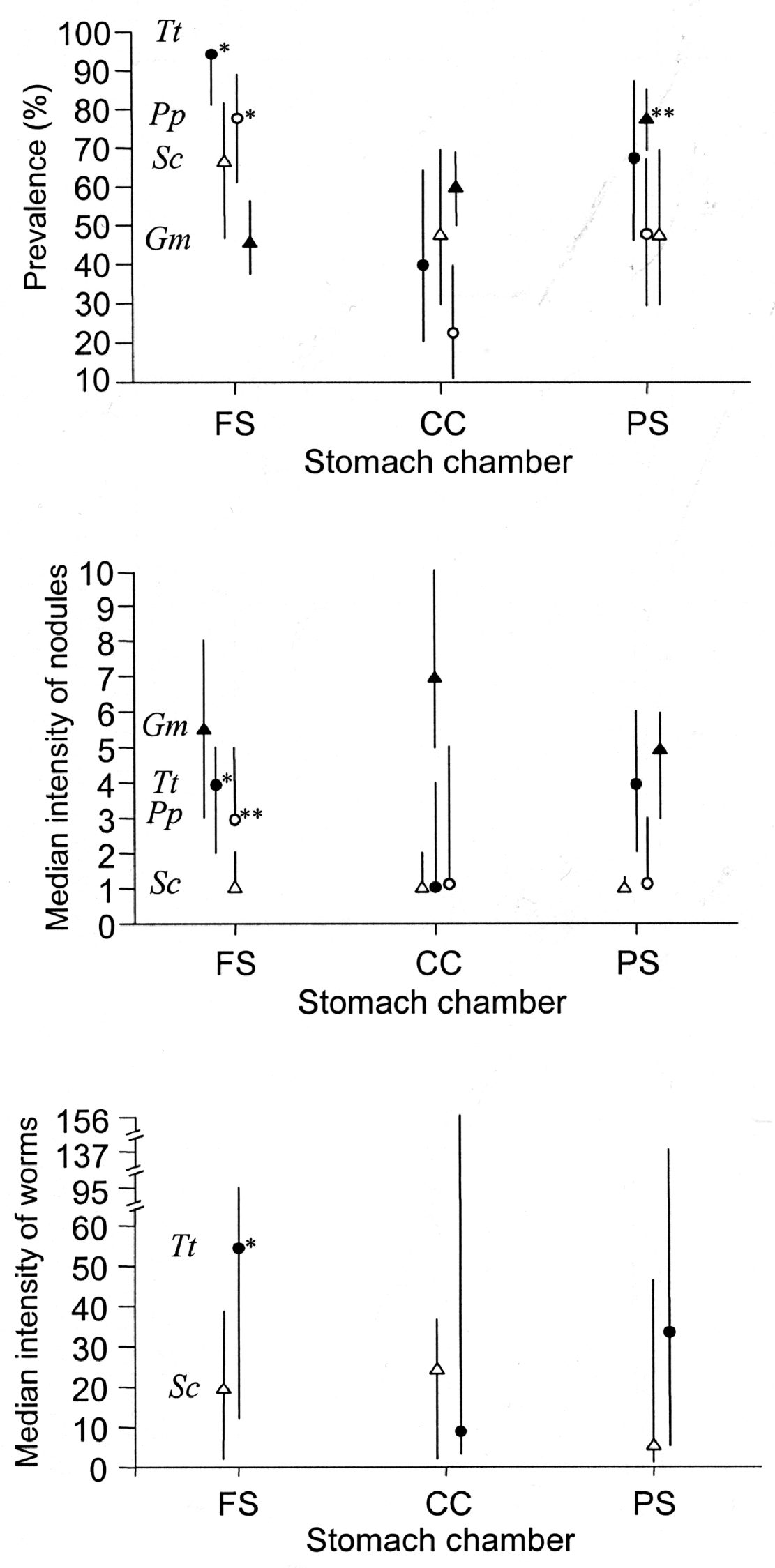
Fig. 4. Prevalence, median intensity of nodules and median intensity of worms in the fundic stomach, connecting channel and pyloric stomach (PS) of four odontocete species (abbreviations as in Table 2). For each host species, significant differences between chambers are indicated with asterisks: (*) indicates a significant difference with the chamber with lowest value; (**) indicates a significant difference with the other two chambers. Bars represent 95% C.I.
Results of simulations for each null hypothesis of chamber colonization are shown in Table 5. In harbour porpoises and bottlenose dolphins, the empirical incidence values of P. gastrophilus were compatible with those obtained under the ‘chamber’ size hypothesis (Table 5). However, in striped dolphins, incidence values were most compatible with the ‘equiprobable’ hypothesis and, in pilot whales none of the null hypotheses agreed with real data (Table 5).
Table 5. Observed and predicted incidence values of colonization of Pholeter gastrophilus in the fundic stomach, connecting channel and pyloric stomach of four odontocete species (Abbreviations as in Table 2. Predicted values were obtained based on 10000 Monte Carlo simulations using probability colonization values for each null hypothesis (abbreviated as in Table 2); n is the number of individual hosts of each species; C.I. is confidence interval of simulation; (*) indicates a suitable fit with the empirical data.)

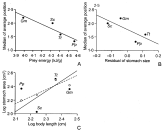
We found a significant negative trend between the AS values of each host species and their residual stomach area (J=−1·83, k=4, one-tailed P=0·033) (Fig. 5A), and, specially, the energy content of prey (J=−4·98, one-tailed P<0·0001) (Fig. 5B). The fundic stomach was the chamber with largest residuals in the regressions between body length and the area of stomach chambers (Fig. 5C). The two mostly piscivorous species, the harbour porpoise and the bottlenose dolphin, had the largest absolute area of this chamber regardless of body size.

Fig. 5. Relationships between relevant variables that account for the distribution of Pholeter gastrophilus in four odontocete species (abbreviations as in Table 2). (A) Energy content of average prey against median of average chamber position of the parasite; (B) body length-corrected residuals of stomach area against median of the average chamber position of the parasite; (C) log-body length against log-main stomach area (solid points, solid line) and log-connecting channel plus pyloric stomach area (open points, broken line). In (A) and (B), lines represent least squares regressions; in (C), geometric mean regressions.
DISCUSSION
Our results indicate that P. gastrophilus consistently infects the glandular part of the stomach in all host species examined, which conforms to the available evidence from other cetacean species (references in Raga, 1994). Records in the forestomach are exceptional (Raga and Balbuena, 1993) and could likely be accounted for by worm penetration through the region of the fundic stomach immediately adjacent to the forestomach, as indicated by the present study. The parasite was also found occasionally in the anterior duodenum, as was shown in other studies (e.g. Dollfus, 1974; Beverley-Burton, 1978; Conti and Frohlich, 1984). However, our data suggest that the likelihood of infecting the duodenum depends on both the pattern of stomach distribution (most posteriad in pilot whales) and parasite intensity. The conditions in the anterior duodenum are comparable to those of the pyloric stomach (Mead, 2002), and might offer appropriate cues eliciting the settlement behaviour of the parasite.
At the scale of stomach chambers, the distribution pattern of P. gastrophilus differed clearly among host species, particularly between the pilot whale and the other species. Given the cosmopolitan distribution and the extensive exploitation of host species by P. gastrophilus, one may wonder whether it may actually represent a complex of sibling species (see, for example, Knowlton, 1993), each one adapted differently to its own host species (see Mattiucci et al. 1997; 2001, for examples in other parasites of cetaceans). However, our analyses provide evidence that the observed variability in the distribution of P. gastrophilus is best interpreted as the result of a process common to all host species. Firstly, P. gastrophilus tends to behave as a generalistic parasite regarding chamber selection. At the infrapopulation level the parasite did not exhibit a strong tendency to infect a specific chamber in any species except in the bottlenose dolphin. However, even in this case, we found no signs of density-dependence that could support the idea of an active chamber choice by the parasite.
Secondly, two patterns suggested that the host's diet and digestive physiology were involved in driving the stomach distribution of P. gastrophilus. We found that the distribution of the parasite was more anteriad when the average caloric content of prey increased, and there is ample consensus that this factor delays gastric evacuation (Jobling, 1986; Olson and Boggs, 1986; Maerz et al. 1994; Andersen, 1999; Peracchi et al. 2000; Olson and Galván-Magaña, 2002). The reasons for this phenomenon are not fully understood, but the stomach seems to regulate the volume of digesta through a feed-back mechanism that ensures that the intestine receives a constant amount of energy per unit of time (Jobling, 1986; Maerz et al. 1994). One could object that using a single overall value of energy content per cetacean species may be inadequate: if P. gastrophilus is recruited with a specific prey item (e.g. a fish species, see Gibson et al. 1998), it would then be the energy content of this prey which should be relevant for the hypothesis. This objection seems weak for two reasons. First, P. gastrophilus is presumably recruited with many species of fish and cephalopods; otherwise, it would be difficult to understand why comparable infection levels were found in host species with such diverse feeding habits. Second, cetaceans are opportunistic predators that usually concentrate feeding in specific daily periods (e.g. Desportes and Mouritsen, 1993; Blanco et al. 1995). Therefore, the stomach contents at any time are usually made up of many different prey types that contribute together to determine an overall rate of digestion and gastric emptying.
The distribution of P. gastrophilus was also negatively related to the area of the glandular region of the stomach, particularly that of the main digestive chamber, the fundic stomach. In other vertebrates, the possession of a large stomach has been related to an increased capacity for storage and/or physical rupture of resistant prey (Piersma et al. 1993; Hilton et al. 2000b). Since in most cetaceans both tasks are carried out in the forestomach (see below), the larger glandular stomachs of harbour porpoises and bottlenose dolphins could reflect a slower and more difficult chemical digestion of their fish prey (see Hilton et al. 2000a) (Table 3). Many authors consider that fish are generally less digestible than squid because of their higher lipid content and their often greater tissue resistance to enzymatic action (Santos et al. 2001; Chase, 2002; Olson and Galván-Magaña, 2002; but see Jackson and Ryan, 1986; Forero et al. 2002). Accordingly, a longer digestion time in the fundic stomach would result in more anteriad distributions of P. gastrophilus in the two piscivorous species, such as observed.
The two above hypotheses rely on two key assumptions. The most critical one is that the period of excystment and settlement of metacercariae of P. gastrophilus in the stomach must be long enough for small changes in the time of passage of food affect the linear distribution of the parasite. This assumption seems plausible. We can assume that metacercariae of P. gastrophilus excyst mostly in the fundic stomach; this is the chamber where the major chemical digestion with hydrochloric acid and pepsine is carried out (Harrison et al. 1970; Smith, 1972; Gaskin, 1978), and acidified pepsin and low pH are known to be major excystment cues for many digeneans (Fried, 1994). However, digestions are extremely fast in cetaceans because of their high metabolic requirements (Williams et al. 2001). For instance, the passage time of food through the entire gut in 8 odontocete species ranged from 2·5 to 4 h (Kastelein et al. 1997). Thus, even considering a minimum excystment time of 0·5 h (see Fried, 1994) for all metacercariae of P. gastrophilus included in a meal, small variations in the transit time of food might affect their final settlement. In this context, a single figure is illustrative. In a study in humans, when the energy content of a meal was doubled, the total evacuation of food from the stomach to the duodenum delayed from 120 to 195 min, or ca. 1·5 times (Peracchi et al. 2000). The differences of dietary energy content among cetacean species were more modest (a maximum of ca. 1·17 times between harbour porpoises compared to pilot whales), yet their digestions are exceedingly more rapid.
A second assumption is that the potential allometric effects upon digestion and evacuation rates did not bias the results. Larger cetacean species are expected to have comparatively longer digestion periods and lower rates of gastric emptying (e.g. Kastelein et al. 1997). However, the distribution of P. gastrophilus was most proximal in the smallest host species, and most distal in the largest species. Thus, apparently, the allometric effect did not overcome the stronger effect of the other variables.
This hypothesis of digestive differences between host species might also explain the apparently conflicting results obtained from the simulations of chamber colonization. The actual incidence data fitted the ‘chamber size’ hypothesis in case of the harbour porpoise and the bottlenose dolphin, the ‘equiprobable’ hypothesis in the striped dolphin, and none in the pilot whale. Interestingly, in the tree-former species there was a coarse agreement between the expected distribution of P. gastrophilus according to the ‘digestive physiology’ hypothesis (see above) and the relative size of the fundic stomach with respect to other chambers. In contrast, the pilot whale tended to accumulate worms in the pyloric stomach (as might be expected due to its relatively faster digestion rate), but this was not accompanied by a larger size of pyloric stomach relative to other chambers.
In conclusion, this study documents an interesting example of a gastrointestinal parasite, the distribution of which, at a certain scale, seems to be driven passively by host anatomical and physiological features. We are aware that our analysis conveys a rather static picture of the diet and digestion process in each cetacean species (see, for example, Pennisi, 2005, and references therein). However, with more detailed data about the diet, stomach size, and distribution of P. gastrophilus in these and other odontocetes, more refined hypotheses can be tested in the future. In addition, this study provides also a more general framework for studies on microhabitat choice by parasites. We propose that the composition of prey and digestion style of hosts should be routinely considered when assessing evidence about distribution patterns of gastrointestinal parasites. For instance, Aznar et al. (2003) wondered why the same species of the Anisakis simplex complex occurred in different stomach chambers in dolphins and minke whales, without considering that the answer might lie just in hosts' dietary differences.
We thank our colleagues from the Marine Zoology Unit, I.C.B.I.B.E., University of Valencia, especially M. Fernández and M. V. Herreras, for their assistance in the processing of samples, and C. Blanco for allowing us to use unpublished diet data. Charo Luque helped us to find some references. The comments of three anonymous referees significantly improved the paper. Cetaceans were collected thanks to an agreement between the Conselleria de Medio Ambiente (Generalitat Valenciana) and the University of Valencia. This work has been supported by projects REN2003-01758 from the Spanish Government and GV4B-304 from the Valencian Government. The first author benefits from a ‘Ramón y Cajal’ contract from the M.E.C. of Spain.