INTRODUCTION
Schistosoma mansoni, the trematode parasite that causes schistosomiasis in South American and sub-Saharan African countries alternates between humans and molluscs of the Biomphalaria genus in its complex life-cycle. Contaminated humans eliminate in their stools eggs that hatch in fresh water releasing the ciliated miracidium. This motile form actively swims towards the intermediate host, enters the snail where it begins an asexual proliferative stage that subsequently differentiates into primary sporocysts (Jourdane and Théron, 1987).
The fine structure of all parasite stages has been extensively studied for more than 50 years (see reviews in Rollinson and Simpson, 1987). S. mansoni eggs and hatching miracidia have been examined by optical microscopy (Kusel, 1970). Miracidium ultrastructure has been studied in great detail by transmission electron microscopy in the classic work by Pan (1980). In the 70's, the isolation of living sporocysts allowed the first ultrastructural observations of the miracidium to primary sporocyst transition (Basch and DiConza, 1974). With the advent of more advanced and powerful microscopical techniques such as confocal microscopy, S. mansoni developmental stages began to be re-examined. By labelling S. mansoni forms with fluorescently-labelled phalloidin, the detailed organization of the well-developed musculature of S. mansoni cercariae (Mair et al. 2003) and adult worms (Mair et al. 2000) has been described. In addition, in those studies it was possible to observe the special relationship between muscles and other components by using double-labelled samples (Mair et al. 2000). In this study we took advantage of the same approach and examined the distribution of actin filaments present in eggs, miracidia and sporocysts. By confocal microscopy, we confirmed the presence of well-organized longitudinal and circular muscle layers in these forms. Moreover, by careful observation of optical sections of parasites labelled with phalloidin-rhodamine and antibodies to myosin, it was possible to observe previously unidentified actin-rich tubular structures. By using specific antibodies we confirmed that these tubules are located inside flame cells and are probably contractile structures.
MATERIALS AND METHODS
Parasite forms
Eggs
S. mansoni eggs were obtained by homogenizing livers of S. mansoni-infected Swiss mice (LE strain, maintained at the Centro de Pesquisas René Rachou, FIOCRUZ), (Pellegrino and Katz, 1968) in phosphate-buffered saline (PBS). The homogenates were sequentially filtered through 190, 120 then 90 μm sieves, washed by centrifugation at 550 g for 1 min, then resuspended in PBS and fixed as described below.
Miracidia
These forms were obtained from eggs recovered from the liver of Swiss mice between 48 and 50 days after infection with approximately 100 S. mansoni cercariae. The miracidia were used to infect Biomphalaria glabrata snails originally collected in the metropolitan area of Belo Horizonte (Minas Gerais, Brazil) and currently maintained in the Laboratory of Malacology at the Centro de Pesquisas René Rachou (Pellegrino and Katz, 1968).
Primary sporocysts
In order to obtain primary sporocysts, miracidia were collected, washed twice with RPMI-1640 culture medium containing 100 μg/ml streptomycin and 5% fetal calf serum, and axenically transformed to primary sporocysts at 26 °C. Under these conditions, over 80% of the miracidia transformed into primary sporocysts within 24 h.
Immunofluorescence and detection of filamentous actin
In order to label the parasite forms, the following protocol was used: glass multi-well microscope slides were coated with 30 μl of poly-L-lysine (100 μg/ml in water) and allowed to air dry. Parasite suspensions were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 1 h in ice, washed in PBS and kept at 4 °C until use. Fifty μl drops of parasite suspensions were added to the pre-coated wells, and the parasites allowed to attach overnight in a humid chamber. Excess liquid was carefully drawn with a micropipette, and a blocking/permeabilizing solution (PBS, 0·2% gelatin, 0·1% saponin, 0·1% NaN3, solution henceforth referred to as PGN) was added to each well and incubated for 1 h. Excess solution was again carefully removed and the parasites incubated for 1 h with one of the following monoclonal antibodies directed against myosin: MF20, raised against chicken light meromyosin; CMII 23 specific for non-muscle myosin IIB heavy chain (chicken), and A4.1025, directed to human myosin II, all used as undiluted culture supernatants obtained from the Developmental Studies Hybridoma Bank (DSHB, under the auspices of NICHD, at the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA). Specificity controls were carried out by using monoclonal antibodies to α-tubulin (Clone DM1A, Sigma Chemical Co. St Louis, MO, USA) or to vimentin (AMF-17b, also from DSHB). Anti-tubulin gave intense labelling of epidermal plate cilia (see below) whereas anti-vimentin, used as a negative control, did not react with any of the S. mansoni forms (not shown). Flame cells were labelled with a rabbit antiserum raised against a protein expressed by a clone that was immunoselected from a S. mansoni gene library using a monoclonal antibody (51-4H8-A) to flame cells (Bogers et al. 1994). This clone represents part of the gene that codes for a giant protein with several repeats and the repeat-rich region was expressed and used to obtain the antiserum (unpublished observations). The antibody reacts by immunoblotting with a high-molecular weight component in schistosome extracts that co-migrates with the band recognized by Mab 51-4H8-A and both reagents yield identical staining patterns by immunofluorescence (not shown). The rabbit antibody was diluted 1[ratio ]100 in PGN, and after careful washings in PGN, samples were incubated for 1 h with a mixture of the appropriate anti-Ig conjugated to FITC (Sigma, diluted 1[ratio ]50), 2 μg/ml phalloidin-rhodamine (Molecular Probes, Eugene, OR,USA) and 20 μM DAPI (4′, 6-diamidino-2-phenylindole dihydrochloride, Molecular Probes) diluted in PGN, to label filamentous actin and nuclei, respectively. Control samples were incubated with normal rabbit serum diluted 1[ratio ]100. The wells were then carefully washed with PBS, and the slides mounted in glycerol buffered with 0·15 M Tris, pH 8·6 containing 0·1% p-phenylenediamine. Slides were examined on a Bio-Rad 1024UV confocal system, attached to a Zeiss Axiovert 100 microscope using either a 40×1·2 NA water immersion or a 100×1·4 NA DIC objective (DeMarco et al. 2003; Procópio et al. 1999). Images were processed using NIH-Image J (http://rsb.info.nih.gov/ij/) or Adobe Photoshop 7.0 softwares.
RESULTS
Actin distribution in S. mansoni eggs
S. mansoni eggs containing miracidia were labelled with phalloidin-rhodamine as described and imaged under confocal microscopy. Serial optical sections were taken through the entire egg and although somewhat distorted and not very well defined as in the other forms (see below), the longitudinal and circular muscle layers can be visualized (Fig. 1). In a projection of all images obtained using Image J software, the overall distribution of actin in the longitudinal and circular muscle fibres of the shelled miracidium can be visualized (arrowheads and arrows, respectively, in Fig. 1B). Also, 4 tubular structures (asterisks, in Fig. 1B) can be seen among the muscle fibres. Nuclei of S. mansoni eggs labelled with DAPI and subjected to the same optical sectioning procedure enable the visualization of a region where circular-shaped nuclei (arrowheads in Fig. 1C) are visible.
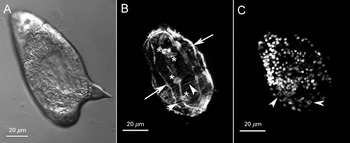
Fig. 1. Filamentous actin labelling in Schistosoma mansoni eggs. (A) Differential interference contrast (DIC) and (B) Z-axis projection (maximum pixel brightness mode) of 42 sequential optical sections taken 0·72 μm apart. Arrows indicate the longitudinal muscle layers and arrowheads the circular muscle layer. Asterisks indicate the actin tubular structures. (C) Nuclei labelled with DAPI. Arrowheads indicate circular shaped-nuclei.
Actin distribution in S. mansoni miracidia
When S. mansoni miracidia were labelled with phalloidin-rhodamine, the prominent and well-organized circular and longitudinal muscle layers were clearly observed, since they are the most abundant actin-rich structures in these forms (Pan, 1980) (Fig. 2). Muscles from the circular layers appeared as 1 μm thick labelled bands at approximately 1·4 μm intervals (Fig. 2B, arrows). Longitudinal muscles were grouped as six 2·4–4·3 μm wide ribbon-like structures (Fig. 2B, arrowheads) that converge towards the miracidium's posterior end (Fig. 2B, double asterisks). The longitudinal muscles were regularly spaced at 2–5 μm and at the anterior and posterior regions of the miracidium, they appeared as heavily labelled patches (Fig. 2B, asterisks), probably corresponding to the ‘tight’ junctions described by Pan (1980). Nuclei of cells located at the posterior end of the parasite appeared as circular-shaped structures (Pan, 1980) (arrowheads in Fig. 2C). This typical circular arrangement of the neural mass (Pan, 1980) was also apparent in DAPI-labelled miracidium (arrow in Fig. 2C). By step-wise visualization of optical sections through this phalloidin-labelled miracidium, it was possible to detect approximately 4 μm thick longitudinal muscle fibres (Fig. 3A). In the following 7–8 sections (0·51 μm thick) the first pair of tubules was visible (Fig. 3, arrowheads in B-D). From this focal plane (approximately 10·7 μm from the outer muscular layer) there were about 8·2 μm until the next pair of tubules begins to appear (Fig. 3, arrowheads in E-H). These values varied from specimen to specimen, but the overall disposition and occurrence of the actin-rich tubules was consistent in all specimens imaged so far (n=12). The tubules were up to 10 μm long and approximately 2·5 μm wide. In some parasites, the first pair of tubules appeared in adjacent optical planes at the front edge close to the terebratorium, whereas the second pair was visualized near the posterior end, at another focal position (Fig. 4).

Fig. 2. Distribution of F-actin in Schistosoma mansoni miracidium. (A) DIC image, and (B) Z-axis projection (maximum pixel brightness mode) of 51 sequential optical sections taken 0·51 μm apart. Circular (arrows) and longitudinal muscle layers (arrowheads) are indicated. (*) Patched accumulation of actin filaments in longitudinal layers; (**) point of convergence of actin ribbons. (C) Projection of DAPI-stained nuclei. Arrow indicates neural mass, and arrowheads indicate nuclei from the cyton ridge.

Fig. 3. Presence of actin-rich tubular structures in Schistosoma mansoni miracidium. Sequential optical sectioning through S. mansoni miracidium enabled the visualization of the actin tubules. Of the 51 images collected through the entire miracidium that were used to generate Fig. 3B, 8 planes were arbitrarily selected to delineate the actin tubular structures (arrowheads).

Fig. 4. Visualization of renderized actin-rich tubules in Schistosoma mansoni miracidium. (A) Phalloidin-labelled miracidium was subjected to optical sectioning and the entire stack (71 sections) projected for maximum pixel intensity. For the boxed areas corresponding to the anterior (B) and posterior (C) ends, sections were cropped from the original image, selected for the optical planes in which the tubules were visible and then subjected to Volume J (Image J plug-in) rendering in 360 degrees. Then, from this renderized sequence, images in which tubules were clearly visible (arrowheads) were selected.
The actin-rich tubules are components of flame cells
Based on their overall distribution it became apparent that the 4 actin-rich tubules could be related to flame cells (Pan, 1980). In order to examine this possibility, we performed double-labelling experiments using an anti-flame cell antibody. The reactivity of this antibody on S. mansoni miracidia confirmed the presence of 4 regions of reactivity that corresponded to the 2 pairs of flame cells (Fig. 5). Co-localization analysis obtained after optical sectioning and 3D rendering, confirmed that the antibody reactivity embraces the actin-rich tubule (Fig. 6). As previously described, flame cells contain a bundle of densely packed microtubular axonemes (1980). We performed double labelling experiments with an anti-α-tubulin monoclonal antibody and detected, besides intense labelling of epidermal plate cilia (not shown), the presence of tubulin inside the actin-rich tubules. This result confirmed that the actin-rich tubules are indeed part of the flame cells (Fig. 7). In most of the images obtained in this study, the tubules appeared hollow but the image in Fig. 7 suggests that they may also appear closed at the narrower end.

Fig. 5. Actin-rich tubules co-localize with flame cells. Schistosoma mansoni miracidia (DIC image in A) were double labelled with an antibody to flame cells (B) and phalloidin-rhodamine (C). Serial optical sections covering the region occupied by the tubules were obtained and the generated z-projections (maximum pixel brightness mode) are shown. Arrowheads indicate flame cells (B) and the actin-rich tubules (C).

Fig. 6. Flame cell marker in Schistosoma mansoni miracidia surrounds actin-rich tubules. Serial optical sections through miracidia labelled for flame cells (green), actin (red) and DNA (with DAPI, in blue) were generated, the labelled sections were selected and the z-projections renderized using Image J.

Fig. 7. Actin-rich tubules are filled with tubulin. Serial optical sections through miracidia double labelled for actin and tubulin were generated, the labelled sections were selected and the z-projections renderized using Image J. (A) Actin; (B) α-tubulin; (C) merged image of α-tubulin (green) and actin (red).
Myosin is a component of the actin-rich tubules
In order to investigate for the possible presence of myosin associated with the actin-rich tubules, we performed double-labelling experiments with 3 distinct anti-myosin monoclonal antibodies in S. mansoni miracidia. MF 20 anti-myosin monoclonal antibody provided a diffuse labelling in the parasite and also recognized the actin-rich tubules (Fig. 8). Similar diffuse myosin labelling was observed with the other 2 anti-myosin monoclonal antibodies tested (not shown).
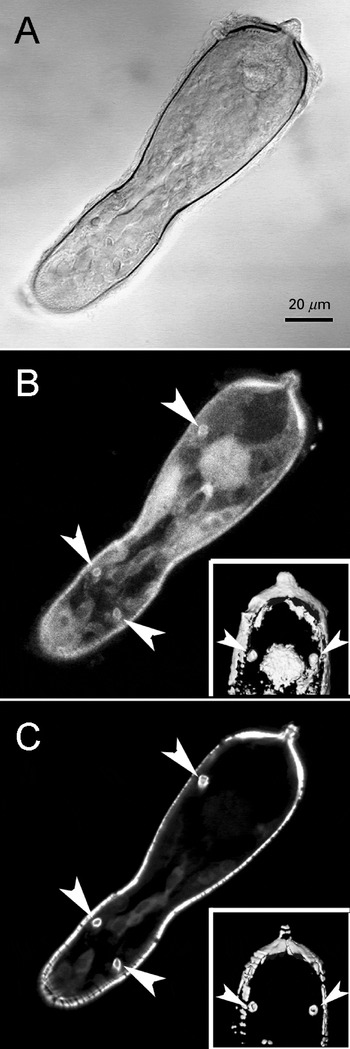
Fig. 8. Myosin is present in Schistosoma mansoni actin-rich tubules. Serial optical sections through miracidia labelled for myosin and actin were generated, the labelled sections were selected and the z-projections obtained with Image J (maximum pixel brightness mode) are shown. (A) DIC image. (B) Myosin labelling (MF 20 monoclonal antibody). (C) Actin labelling. Inserts in B and C, are renderized images of a pair of tubules close to the terebratorium. Arrows indicate positive labelling for myosin and actin on the tubules.
Actin distribution in S. mansoni primary sporocysts
When miracidia differentiate into primary sporocysts, the epidermal plate that comprises the outer ciliated layer gives rise to a simpler tegument (Basch and DiConza, 1974). Along with this modification, phalloidin labelling of primary sporocysts revealed that major rearrangements in actin-rich structures also occurred in this transition (Fig. 9). The longitudinal muscular ribbons became less well developed than the circular layers (Fig. 9B and 10, arrows). The circular muscle layers appear as approximately 0·7–1 μm thick doublets (Fig. 10, arrows). The actin-rich tubules in primary sporocysts are visible both at the posterior (Fig. 9B and 10A, small arrowheads) and anterior (Fig. 10B, small arrowheads) ends. Internal thin tubules approximately 2·5 μm thick and up to 9·8 μm long, that were absent in the miracidium became apparent (Fig. 9B and 10, asterisks). At both extremities of the primary sporocysts, actin filaments coalesced to form heavily labelled protrusions of the tegument, particularly at the former terebratorium region (Fig. 9B and 10, large arrowheads). The circular-shaped nuclei previously observed at the posterior end of the miracidia appeared as well-defined flower-like arrangements (Fig. 9C, arrowheads), located behind the neural mass (Fig. 9C, arrow).

Fig. 9. Overall distribution of F-actin in Schistosoma mansoni sporocyst. (A) DIC image, and (B) Z-axis projection (maximum pixel brightness mode) of 38 sequential optical sections taken 0·72 μm apart. Circular muscle layers (arrows) and actin tubules (small arrowheads) are indicated. (*) Indicates long actin tubules. Actin accumulation at the anterior region (former terebratorium) is indicated by large arrowhead. (C) Projection of DAPI-stained nuclei. Arrow indicates neural mass, and arrowheads indicate nuclei from the cyton ridge. Insert: renderized image of DAPI-labelled nuclei from the region indicated by the arrowhead.
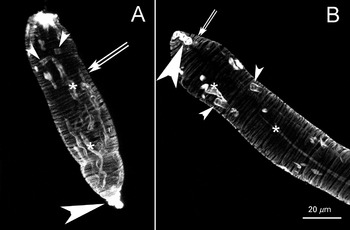
Fig. 10. Circular muscle layers predominate in Schistosoma mansoni primary sporocyst (A). (B) Z-axis projections (maximum pixel brightness mode) of 38 and 51 sequential optical sections taken 0·72 μm apart. Circular muscle layers (arrows) and actin tubules (small arrowheads) are indicated. (*) Indicates long actin tubules. Actin accumulation at the anterior region (former terebratorium) is indicated by large arrowhead.
DISCUSSION
Throughout its life-cycle, S. mansoni has to adapt to extreme environmental conditions ranging from blood vessels in the vertebrate host to the intermediate mollusk host. In all stages, movement is accomplished through well-developed muscular arrangements that have been very well documented both in adult worms (Mair et al. 2000) as well as in the infective form for humans, the cercaria (Mair et al. 2003). In spite of detailed ultrastructural studies on miracidia and their transformation into primary sporocysts (Basch and DiConza, 1974; Pan, 1980) little information on the overall distribution of muscular components of these forms was available. This is probably due to the fact that because of the abundant cilia at the miracidium epidermal plate, that cover most of the parasite tegument and whose movement drives the larva towards the mollusk in fresh water (Rollinson and Simpson, 1987), the muscular components of this developmental stage have not been properly studied so far. By using an approach that has been previously applied to S. mansoni adult worms and cercariae, we examined the distribution of filamentous actin in eggs, miracidia and primary sporocysts. Use of optical sectioning under confocal microscopy enabled visualization of the overall distribution of longitudinal as well as the circular muscles in miracidia inside the eggshell. Already in that form, by careful examination of sequential optical sections, it was possible to observe previously undescribed actin-rich tubules. These structures appeared as 2 pairs located towards the anterior and posterior edges. Surprisingly, the miracidium muscle layers were well developed, and may display a complexity comparable to that observed in cercariae (Mair et al. 2003). Longitudinal muscle fibres cross circular layers at densely packed patches, similar to what has been described for the intersection of diagonal with longitudinal and circular fibres in cercariae (Mair et al. 2003). In the course of transformation to primary sporocysts, the well-developed longitudinal muscle layers were lost and the circular layers became the predominant organization. A previously undetected long and thin tubular arrangement of actin-labelled material also appeared in sporocysts but the nature of these structures remains to be determined.
Flame cells are thought to be protonephridial elements involved in the excretion process by S. mansoni forms and can also be found in other Trematoda (Pan, 1980; Pinheiro et al. 2005; Rohde and Watson, 1992; Rollinson et al. 1987). A number of components have been localized to S. mansoni flame cells such as neurosubstances (Solis-Soto and De Jong, 1994), proteases (Skelly and Shoemaker, 2001), glutathione-S-transferase (Trottein et al. 1990) and calcineurin, a parasite phosphatase (Mecozzi et al. 2000). Flame cells have been extensively characterized by unique ultrastructural features that include a structure referred to as a ‘hollow barrel’ that surrounds numerous axonemes that comprise the so called ‘flame’ (Pan, 1980). By double immunolabelling we confirmed that the actin-rich tubules were encircled by the flame cell label, and also contained tubulin in their interior, thus confirming that the tubules corresponded to the previously described ‘hollow barrel’ (Pan, 1980). The cylinder or ‘hollow barrel’ that holds the axonemes is derived from the interdigitation of fingers from the cap cell and the first tubule cell (Wilson and Webster, 1974) and Pan (1980) had noticed that they contain fibrils in their interior. According to our results, the fibrils are probably acto-myosin filaments. It has been hypothesized that motion of microtubular bundles creates the negative pressure that promotes fluid filtration through the regions of adhesion between the interdigitations that comprises the ‘hollow barrel’ (Wilson and Webster, 1974). Since we have demonstrated that the ‘hollow barrel’ contains not only actin but also myosin, it is highly probable that this previously undescribed feature provides the ‘hollow barrel’ with a form of motility that might also play a role in flame cell function.
We thank the Brazilian agencies CNPq, FAPESP, FAPEMIG and CAPES for financial support. We are also indebted to Dr Marcos André Vannier-Santos for his insightful idea that the tubules were related to flame cells, and to Dr John Kusel for his suggestions and encouragement in the initial stages of this work. We are also grateful to Dr Alan Wilson for his insightful comments to the manuscript.












