INTRODUCTION
Leishmaniasis is a group of diseases with a large spectrum of clinical manifestations, from self-healing lesions to severe visceral injury, which can lead to death. There are approximately 350 million people living in endemic areas around the world. Leishmania braziliensis is one of the aetiological agents of leishmaniasis in the New World (WHO, 2000) that is transmitted by the bite of sand flies. The leishmania life cycle comprises the multiplicative promastigotes, a flagellated motile form that multiplies in the gut of the sand fly, the metacyclic promastigotes, virulent form that resides in the mouthparts of the insect vector, and amastigotes, non-motile form that lives and replicates in the phagolysosomal compartment of mammalian macrophages (Parsons and Ruben, Reference Parsons and Ruben2000).
Peptidases participate in several phases of Leishmania development and virulence, including proliferation, nutrition, differentiation, interaction with both invertebrate and vertebrate hosts, and evasion of host immune response. For these reasons, this class of hydrolytic enzymes has attracted the attention of the scientific community to exploitation as targets for rational chemotherapy against leishmaniasis. Moreover, leishmania peptidases are highly immunogenic and have been exploited for serodiagnosis and as vaccine targets (McKerrow et al. Reference McKerrow, Sun, Rosenthal and Bouvier1993; Mottram et al. Reference Mottram, Brooks and Coombs1998; Yao et al. Reference Yao, Donelson and Wilson2003; Vermelho et al. Reference Vermelho, Giovanni-de-Simone, d'Avila-Levy, Santos, Nogueira de Melo, Silva-Junior, Bom and Branquinha2007).
In this sense, the best characterized Leishmania metallopeptidase is a glycoprotein of 60–66 kDa, named leishmanolysin or gp63, abundantly expressed on the promastigote surface that is involved in several steps of parasite–host interaction, including a protective role against the parasite degradation within macrophage fagolysosomes. Furthermore, the disruption of all seven gp63 genes of L. major generated a strain mutant able to development inside the invertebrate vector; however, this parasite was more susceptible to complement-mediated lysis as well as incapable in inducing vigorous lesions in BALB/c mice. Collectively, these results confirm that the gp63 molecules participate actively in the pathogenesis and virulence in Leishmania (Joshi et al. Reference Joshi, Kelly, Kamhawi, Sacks and McMaster2002). Moreover, gp63 is capable of hydrolysing several protein substrates including serum proteins such as albumin, casein, complement components, fibrinogen, haemoglobin and immunoglobulins (Bouvier et al. Reference Bouvier, Schneider, Etges and Bordier1990), as well as extracellular matrix components including type IV collagen and fibronectin, which could facilitate the migration through the subendothelial basement membrane, facilitating the dissemination of the parasite in the tissues (McGwire et al. Reference McGwire, Chang and Engman2003). In addition, gp63 has been reported to be a major acceptor for C3b and C3bi, assisting in the parasite's resistance to complement and indirectly interfering with the transduction of signals from the host cell, since the entry into macrophages by CR3 (receptor of C3bi) inhibits the expression of IL-12 gene, an interleukin capable in activating the macrophage cells (reviewed by Sacks and Sher, Reference Sacks and Sher2002). Gp63 also degrades surface CD4, diminishing the T cell responses (Hey et al. Reference Hey, Theander, Hviid, Hazrati, Kemp and Kharazmi1994), as well as cleaving intracellular peptides presented by MHC class I molecules (Garcia et al. Reference Garcia, Graham, Harris, Beverlwy and Kayne1997).
Cysteine peptidases are also produced by all Leishmania species. Several reports have shown that the expression of cysteine peptidases in leishmania is stage-specific, being predominantly expressed in the amastigote stage and to a lesser extent in promastigote forms (Souza et al. Reference Souza, Waugh, Coombs and Mottram1992; Mottram et al. Reference Mottram, Frame, Brooks, Tetley, Hutchison, Souza and Coombs1997). Interestingly, cysteine peptidases are preferentially expressed in virulent as opposed to avirulent L. amazonensis promastigotes (Soares et al. Reference Soares, Santos, Bonaldo, Andrade, Alviano, Angluster and Goldenberg2003). Information about the roles and importance of these enzymes in host–parasite interactions was also obtained by the generation of a cysteine peptidase B (cpb)-deficient (Δcpb) mutant. It was shown that Δcpb promastigotes are less infective to macrophages than wild-type parasites in vitro and that they are able to form only small, slow-growing lesions in BALB/c mice (Mottram et al. Reference Mottram, Souza, Hutchison, Carter, Frame and Coombs1996; Frame et al. Reference Frame, Mottram and Coombs2000), indicating that cpb could be considered a virulence factor (Denise et al. Reference Denise, McNeil, Brooks, Alexander, Coombs and Mottram2003). Subsequent studies indicated that the absence of the cpb genes resulted in a protective Th1 immune response, contrary to the Th2 response normally observed when the cpb isoenzymes are present (Alexander et al. Reference Alexander, Coombs and Mottram1998). Furthermore, inhibitors of cysteine peptidases affect these parasites dramatically, impairing the in vitro transformation of promastigotes into amastigotes, and diminishing macrophage infection (Selzer et al. Reference Selzer, Pingel, Hsieh, Ugele, Chan, Engel, Bogyo, Russel, Sakanari and McKerrow1999).
The present study reports the production of metallo- and cysteine peptidases in virulent and avirulent promastigote forms of L. braziliensis. In addition, the distinct susceptibility of these two strains against proteolytic inhibitors, the dissimilar recognition by antibodies against the major metallo- (gp63) and cysteine (cpb) leishmania peptidases and the interaction capability with murine macrophages were reported.
MATERIALS AND METHODS
Parasites and growth conditions
The avirulent strain of Leishmania braziliensis (CT–IOC 238/L566) was acquired at the Coleção de Tripanossomatídeos, Instituto Oswaldo Cruz (IOC), Fundação Oswaldo Cruz (FIOCRUZ), Rio de Janeiro, Brazil. The virulent strain of L. braziliensis (MHOM/BR/2002/EMM IOC-L2538), originally isolated from a human case of cutaneous leishmaniasis, was supplied by Dr Léa Cysne (Laboratório de Imunoparasitologia, IOC, FIOCRUZ, Rio de Janeiro, Brazil). The promastigote forms of the avirulent strain have been maintained for over 3 years only in axenic cultures and have lost their virulent capability as judged by their inability to induce lesions in the susceptible host (hamster) during a 6-month-period of observation after a subcutaneous injection of 1×107 cells. On the other hand, virulent promastigote forms were obtained by in vitro culture from linfonodes of 8 to 10-week infected hamsters, and maintained in culture for no more than 3 passages. For the comparative experiments, both avirulent and virulent promastigotes were grown in Schneider's medium supplemented with 10% fetal calf serum (FCS; Gibco) at 26°C up to 120 h. Parasite growth was estimated by determining the cell concentration in a Neubauer chamber. Cellular viability was assessed by motility and exclusion of trypan blue dye from parasite cells. Alternatively, after isolation of L. braziliensis from infected hamsters, the parasites were maintained by various passages in Schneider's medium containing 10% FCS at 26°C, in order to verify the possible modulation of peptidase production during in vitro cultivation.
Parasite extracts
Parasites were collected by centrifugation (500 g/5 min/4°C) and washed 3 times with cold phosphate-buffered saline (PBS; 150 mM NaCl, 20 mM phosphate buffer, pH 7·2). Promastigotes (1×108 cells) were resuspended in 200 μl of PBS and lysed by the addition of 1% sodium dodecyl sulfate (SDS). The cells were broken in a vortex mixer by alternating 1 min shaking and 2 min cooling intervals (3 cycles), followed by centrifugation (5000 g/15 min/4°C), leaving the parasite cellular extracts in the supernatants (Santos et al. Reference Santos, Alviano and Soares2005). Alternatively, culture supernatant fluids were filtered in a 0·22-μm membrane (Millipore) and then concentrated 10-fold by ultrafiltration in a 10 000 molecular mass cut-off Centricon microconcentrator (AMICON, Beverly, MA, USA) at 4°C (Santos et al. Reference Santos, Alviano and Soares2005). The same volume of Schneider's medium containing 10% FCS was also concentrated and used as a control to check for possible proteolytic activity, presenting negative results. Protein concentration was determined by the method described by Lowry et al. (Reference Lowry, Rosebrough, Farr and Randall1951), using bovine serum albumin (Sigma) as standard. The parasite extracts were aliquoted and the fractions stored at −80°C for further analyses.
Peptidase activity assay
The proteolytic activities were assayed and characterized by 10% SDS-PAGE with 0·1% gelatin incorporated into the gel as substrate (Heussen and Dowdle, Reference Heussen and Dowdle1980). The gels were loaded with 25 μg protein from both parasite and supernatant extracts that were added to SDS–polyacrylamide gel electrophoresis (PAGE) sample buffer (125 mM Tris, pH 6·8, 4% SDS, 20% glycerol and 0·002% bromophenol blue). Electrophoresis was performed at a constant current of 60 mA at 4°C for 2 h. After electrophoresis, SDS was removed by incubation of the gels with 2·5% Triton X-100, for 1 h, at room temperature under constant agitation. Then, the gels were incubated for 40 h at 37°C in 50 mM sodium phosphate buffer supplemented with 2 mM dithiothreitol (DTT), pH 5·5, in the absence or presence of 1 μMtrans-epoxysuccinyl l-leucylamido-(4-guanidino) butane [E-64] (Sigma) or in 10 mM glycine-NaOH buffer, pH 9·0, added or not with 1 mM ethylene glycol-bis (β-aminoethyl ether) [EGTA] or 1 mM 1,10-phenanthroline (Sigma). The gels were stained for 2 h with 0·2% Coomassie Brilliant Blue R-250 in methanol-acetic acid-water (50:10:40) and destained overnight in a solution containing methanol-acetic acid-water (5:10:85), to intensify the proteolytic halos. Prior to electrophoresis, molecular mass standards (GIBCO) were boiled in SDS–PAGE sample buffer and applied in the same gel. The molecular mass of each parasite peptidase was calculated by comparison with the mobility of GIBCO BRL SDS-PAGE standards. The gels were photographed and digitally processed (Santos et al. Reference Santos, d'Avila-Levy, Dias, Ribeiro, Pereira, Elias, Souto-Padrón, Lopes, Alviano, Branquinha and Soares2006).
Immunoblotting
Samples containing both cellular and supernatant extracts, equivalent to 50 μg of protein, were added to SDS–PAGE sample buffer and mixed with 10% β-mercaptoethanol, followed by heating at 100°C for 5 min. Thereafter, protein extracts were separated in 12% SDS–PAGE and the polypeptides electrophoretically transferred at 4°C at 100 V/300 mA for 2 h to a nitrocellulose membrane. The membrane was blocked in 5% low-fat dried milk in PBS containing 0·5% Tween 20 (PBS/Tween) for 1 h at room temperature. Then, membranes were washed 3 times (10 min each) with the blocking solution and incubated for 1 h with the following polyclonal antibodies at 1:500 dilution:anti-cpb raised against Leishmania mexicana cysteine peptidase b (kindly provided by Dr Mary Wilson, Department of Internal Medicine, Biochemistry, Microbiology and Epidemiology, Program in Molecular Biology, University of Iowa, USA), anti-gp63 raised against L. mexicana (kindly provided by Dr Peter Overath – Interfakultäres Institut für Zellbiologie, Abteilung Immunologie, Universität Tübingen, Tübingen, Germany) and anti-α-tubulin monoclonal antibody (Sigma). The secondary antibody used was peroxidase-conjugated goat anti-rabbit IgG at 1:25000 followed by chemiluminescence immunodetection after reaction with ECL reagents (Santos et al. Reference Santos, d'Avila-Levy, Dias, Ribeiro, Pereira, Elias, Souto-Padrón, Lopes, Alviano, Branquinha and Soares2006). The relative molecular masses of the reactive polypeptides were calculated by comparison with the mobility of boiling-heated molecular mass standards, which were applied in the same gel.
Densitometric analyses
The densitometric scanning analysis was performed with the use of the Kodak Digital Science EDAS 120 software. In these analyses, bands in each gel were manually selected using the free selection tool provided by the software. Band areas were then determined by repeating this process 3 times, to diminish the probability of errors in these estimations. Values of band area were further integrated with means of grey level in selected bands, generating densitometric values that were used in the comparison between corresponding bands from the different gels. For proteolytic bands analyses, first the images were inverted using the tool provided by the software and then the measurements were performed (Elias et al. Reference Elias, Pereira, Silva, Alviano, Soares and Santos2006; Pereira et al. Reference Pereira, Elias, d'Avila-Levy, Branquinha and Santos2009).
Fluorescence microscopy and flow cytometry analyses
Parasites (1×107 cells) were collected by centrifugation (500 g/5 min/4°C), washed 3 times with cold PBS and fixed at 4°C in 0·4% paraformaldehyde in PBS for 20 min, followed by extensive washing in the same buffer. The fixed cells maintained their morphological integrity, as verified by optical microscopic observation. Subsequently, the cells were incubated for 1 h with a 1:500 dilution of anti-cpb and anti-gp63 antibodies and then incubated for an additional hour with a 1:200 dilution of fluorescein isothiocyanate (FITC)-labelled goat anti-rabbit IgG. These cells were washed 3 times in PBS and observed in a Zeiss epifluorescence microscope (Axioplan). For flow cytometry analysis, the cells were examined in a fluorescence-activated cell sorter (FACS) FACSCalibur (BD Bioscience, USA) equipped with a 15 mW argon laser emitting at 488 nm. Untreated cells and those treated with the pre-immune rabbit antiserum and the secondary antibody only were assayed in parallel as controls. The mapped population (n=10 000) was then analysed for log green fluorescence by using a single-parameter histogram (Pereira et al. Reference Pereira, Elias, d'Avila-Levy, Branquinha and Santos2009).
Effects of proteolytic inhibitors on the growth rate
The experiments were performed in 10×100 mm glass tubes containing 1 ml of Schneider's medium supplemented with 10% FCS. The inoculum consisted of 10% of a 72-h culture containing about 1·8×106 promastigotes (of both avirulent and virulent strains). Drugs were dissolved as follows: EGTA and 1,10-phenanthroline were dissolved in methanol at 10 mM, while E-64 was dissolved in water at 1 mM. A dilution of methanol corresponding to that used to prepare the highest drug concentration was assessed in parallel. The parasites were grown at 26°C, for periods ranging from 24 to 120 h, in the absence or presence of proteolytic inhibitors at different concentrations (Santos et al. Reference Santos, d'Avila-Levy, Dias, Ribeiro, Pereira, Elias, Souto-Padrón, Lopes, Alviano, Branquinha and Soares2006).
Effect of proteolytic inhibitors and anti-peptidase antibodies on the interaction between L. braziliensis and macrophages
Thioglycolate-elicited peritoneal macrophages from female BALB/c mice (6–8 weeks of age) were collected in cold PBS and allowed to adhere onto cover-slips placed in 24-well culture plates, for 1 h at 37°C in a 5% CO2 atmosphere. Non-adherent cells were then removed and the adhered macrophages washed twice with PBS and cultured for 24 h in RPMI culture medium (Gibco), supplemented with 10% FCS. In order to obtain the peritoneal mouse macrophages, mice were previously killed, according to all federal guidelines and institutional policies. Before the interaction experiment assay, parasites were treated or not for 1 h with different concentrations of EGTA, 1,10-phenanthroline and E-64. In another set of experiments, the parasites were pre-incubated for 1 h individually with anti-cpb, anti-gp63 antibodies or rabbit pre-immune serum, at 1:1000 dilution. After that, the parasites were washed 3 times in RPMI and the viability was assessed by mobility and lack of staining after challenge with trypan blue. The parasite-macrophage interactions studies were performed at 37°C for 1 h in a ratio of 5 promastigotes to 1 macrophage cell. Following the interaction assays, the cover-slips were fixed and Giemsa-stained, and the percentage of infected macrophages was determined by counting 400 cells in triplicate cover-slips. The association index was determined by multiplying the percentage of infected macrophages by the mean number of parasites per infected cell. In parallel, the interaction assay between the avirulent strain of L. braziliensis and macrophages was modulated by the presence of untreated or 1,10-phenanthroline-treated spent culture medium obtained from the virulent strain.
Statistical analysis
All experiments were performed in triplicate, in 3 independent experimental sets. The mean and the standard error of at least 3 distinct experiments were determined. Statistical analysis was calculated by using EPI-INFO 6.04 (Database and Statistics Program for Public Health) computer software and Student's t-test.
RESULTS
Cell-associated peptidase profile in avirulent and virulent promastigotes of L. braziliensis
The peptidase profiles of avirulent and virulent promastigotes of L. braziliensis were analysed by gelatin-SDS-PAGE, after parasite disruption (Fig. 1A and B), and quantified through dentisometric analyses of the degradation halos (Table 1). At both acidic (Fig. 1A) and alkaline (Fig. 1B) pH values, a complex cellular peptidase profile was observed in virulent parasites in comparison with the avirulent strain. Avirulent parasites produced only 1 peptidase, active under acidic and alkaline conditions (Fig. 1A and B, lanes av), with an apparent molecular mass of 66 kDa that was completely inhibited by EGTA (data not shown) and 1,10-phenanthroline, identifying this enzyme as metallo-type peptidase (Fig. 1B). When the gel containing the virulent parasite extract was incubated at alkaline pH, 4 distinct peptidases of 60, 66, 90 and 120 kDa were observed (Fig. 1B, lane v), which were fully inhibited by metallopeptidase inhibitors such as EGTA (data not shown) and 1,10-phenanthroline (Fig. 1B, lane v+phen). Moreover, under acidic pH, 2 distinct classes of peptidases were observed in virulent parasites: metallopeptidases (66 and 90 kDa) and 2 major cysteine peptidases of 48 and 40 kDa (Fig. 1A, lane v) which were totally inhibited by E-64, a powerful cysteine peptidase inhibitor (Fig. 1A, lane v+e-64).

Fig. 1. Peptidase profiles in avirulent (av) and virulent (v) strains of Leishmania braziliensis. Promastigote forms were grown in Schneider's medium supplemented with 10% FCS at 28°C for 96 h. (A–C) Gelatin-SDS–PAGE showing the cellular (A, B) and extracellular (C) proteolytic activity profiles, in which the gel strips were incubated for 40 h at 37°C in 50 mM sodium phosphate buffer, pH 5·5, supplemented with 2 mM DTT (A, C) or in 10 mM glycine-NaOH buffer, pH 9·0 (B). The gel strips were incubated in the absence or in the presence of proteolytic inhibitors as follows: 1 μM E-64 (E64) or 1 mM 1,10-phenanthroline (Phen). In the enzymatic inhibition experiments, the virulent parasite extracts were used. (D) Western blotting showing the reactivity of extracellular polypeptides of both L. braziliensis strains with the anti-gp63 antibody. In all cases, the numbers on the left indicate the molecular masses of polypeptides, expressed in kilodaltons.
Table 1. Densitometric analyses of the peptidase synthesized in the avirulent and virulent strains of Leishmania braziliensis

The values represent means±standard deviation of three independent densitometric measurements, expressed in arbitrary units, of the digestion halos after gelatin-SDS–PAGE or reactive polypeptides after western blotting.
ND, not detected.
Secreted proteolytic activity by avirulent and virulent strains of L. braziliensis
The secretion of peptidases by virulent and avirulent strains of L. braziliensis was also evaluated submitting the spent culture medium to SDS-PAGE containing gelatin as substrate (Fig. 1C). The gel revealed the presence of 3 distinct proteolytic activities in the virulent strain with apparent molecular masses of 120, 66 and 45 kDa (Fig. 1C, lane v), and a single band of approximately 66 kDa in the avirulent strain (Fig. 1C, lane av). All the extracellular peptidases were inhibited by 1 mM 1,10-phenanthroline (Fig. 1C, lane v+phen), suggesting that they belong to the metallopeptidase class. Curiously, the same peptidase profiles were observed when the culture supernatant was placed in acidic or alkaline pH surroundings (data not shown).
Detection of parasite antigens that immunologically cross-reacted with gp63 and cpb
In order to evaluate the possible presence of antigens similar to gp63 and cpb in L. braziliensis, we carried out experiments of flow cytometry (Fig. 2A), fluorescence microscopy (Fig. 2B) and Western blotting (Fig. 2C) using polyclonal antibodies against these two well-characterized Leishmania peptidases. The result of Western blotting revealed the presence of gp63-like molecules showing a major band of about 63 kDa detected in both parasite cellular extracts (Fig. 2C, upper panel), while 2 major polypeptides of 30 and 20 kDa were recognized by the anti-cpb antibody (Fig. 2C, lower panel). In both cases, a high labelling level was observed in the virulent strain when compared with the avirulent strain (Fig. 2C, compare lanes v and av, Table 1). In contrast, the anti-α-tubulin antibody, used as a control in these experiments, showed similar reactivity with the lysates of both promastigotes (Fig. 2D), indicating that similar amounts of α-tubulin were present in these two cell types. Taken together, these Western blot results demonstrate that gp63-like and cpb-like proteins were down-expressed in the avirulent strain, as judged by densitometric analysis (Table 1).
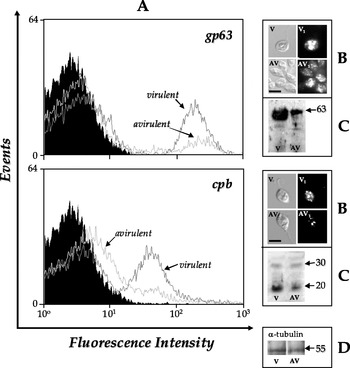
Fig. 2. Detection of gp63- and cpb-related molecules in avirulent (av) and virulent (v) strains of L. braziliensis. (A) Flow cytometry analysis showing the anti-gp63 and anti-cpb antibodies binding to the cell surface of L. braziliensis strains. Paraformaldehyde-fixed cells were incubated in the absence (black curves) or in the presence (gray curves) of anti-gp63 (upper panel) or anti-cpb (lower panel) antibodies as described in the Materials and Methods section, and then analyzed by fluorescence-activated cell sorter. For simplicity, only the autofluorescence of virulent strain is shown, since the avirulent strain is similar (data not shown). In the same way, parasites treated with secondary antibody or pre-serum presented the same fluorescence intensity depicted in the autofluorescence (data not shown). (B) Fluorescence microscopy analyses of the cellular distribution of gp63 and cpb homologues in L. braziliensis avirulent (av, av1) and virulent (v, v1) strains: the figures represent the differential interferential contrast images (av, v) and the corresponding immunofluorescence labeling (av1, v1) by anti-gp63 (upper panel) or anti-cpb (lower panel) antibodies. The bar represents 10 μm. (C) Western blotting showing the gp63- (upper panel) and cpb-like (lower panel) polypeptides detected in the whole cellular extract from L. braziliensis strains. (D) The anti-tubulin monoclonal antibody was used as a control for sample loading in the western blottings. The numbers on the right indicate the apparent molecular masses of the reactive polypeptides, expressed in kilodaltons (kDa).
The analysis by flow cytometry and fluorescence microscopy showed the presence of gp63 (Fig. 2A and B, upper panels) and cpb (Fig. 2A and B, lower panels) homologous molecules on the cell surface as well as in intracellular compartments of the L. braziliensis virulent and avirulent strains. Corroborating the immunoblotting findings, the fluorescence intensity was greater in the virulent strain for both anti-gp63 (Fig. 2A and B, upper panels) and anti-cpb (Fig. 2A and B, lower panels) antibodies than the avirulent strain. Western blotting assay also revealed a reactive secreted polypeptide of approximately 63 kDa in both strains studied (Fig. 1D, Table 1). When we used the anti-cpb antibody, no extracellular polypeptide was recognized under the employed experimental conditions (data not shown).
Effect of different passages in vitro on the peptidase activities from virulent strain of L. braziliensis
The peptidases have been described in several trypanosomatids as molecules important in the process of virulence (reviewed by Vermelho et al. Reference Vermelho, Giovanni-de-Simone, d'Avila-Levy, Santos, Nogueira de Melo, Silva-Junior, Bom and Branquinha2007). However, it is known that these enzymes may have their expression decreased over several passages of these parasites in vitro (Soares et al. Reference Soares, Santos, Bonaldo, Andrade, Alviano, Angluster and Goldenberg2003). To check this possibility, we evaluated the activities of proteolytic enzymes after successive different passages in vitro of the virulent strain of L. braziliensis. The results showed that the axenic growth of freshly isolated parasites from hamster lesions promoted an accentuated decrease in the peptidase production by L. braziliensis, in which the activity of metallopeptidases with high molecular masses and cysteine peptidases with low molecular masses were gradually repressed over the passages held every 5 days (Fig. 3).

Fig. 3. Gelatin-SDS-PAGE showing the proteolytic profiles of virulent promastigotes of L. braziliensis harvested from consecutive subpassages in vitro. The samples correspond to cellular extracts (25 μg) after disruption of parasites freshly isolated from hamster (0) or samples from subpassages 1, 2 and 3. The gel strips were incubated in 50 mM sodium phosphate buffer, pH 5·5, supplemented with 2 mM DTT for 40 h. The numbers on the left indicate the molecular masses of polypeptides, expressed in kilodaltons.
Effects of metallo- and cysteine peptidase inhibitors on cellular growth rate
Regarding the earlier results, we have decided to investigate the effect of metallo- (EGTA and 1,10-phenanthroline) and cysteine (E-64) peptidase inhibitors on the in vitro growth of virulent (Fig. 4A–C) and avirulent (Fig. 4D) L. braziliensis promastigotes. In respect to the virulent strain, all the proteolytic inhibitors used were able to decrease the promastigote growth after 24–120 h even in the lowest concentration (Fig. 4A–C). Furthermore, the metallo- and cysteine peptidase inhibitors promoted morphological alterations in the virulent strain, including parasites becoming short and round (Fig. 4E). On the other hand, these proteolytic inhibitors had no effect on the avirulent promastigote growth even in the highest concentration (Fig. 4D) and also did not promote alterations on their morphology, during a long period (up to 120 h) of parasite-drug contact (data not shown).

Fig. 4. Effect of metallo (EGTA or 1,10-phenanthroline [PHEN]) and cysteine (E-64) peptidase inhibitors on the growth rate of virulent (A–C) or avirulent (D) strains of L. braziliensis. Parasites were cultured in Schneider's medium plus 10% FCS in the absence (control cells) or presence of different concentrations of proteolytic inhibitors at 28°C up to 120 h. (E) Phase-contrast images of non-treated virulent (a) and avirulent (b) promastigotes or virulent strain treated with different proteolytic inhibitors at 10 μM as follows: (c) EGTA, (d) 1,10-phenanthroline or (e) E-64. For simplicity, the images of the avirulent parasite treated with the proteolytic inhibitors were not shown, since any morphological alterations were observed. In a similar way, the images of the virulent flagellates treated with the other proteolytic inhibitors concentrations were not shown, because the morphological alterations were similar to the parasites treated with peptidase inhibitors at 10 μM. Data shown are means±standard deviations of three independent experiments, which were performed in triplicate.
Effect of metallo- and cysteine peptidase inhibitors on the L. braziliensis–macrophage interaction
EGTA, 1,10-phenanthroline and E-64 promoted a decrease in the association index between virulent promastigotes and murine macrophages, in a dose-dependent manner (Fig. 5A). Conversely, these proteolytic inhibitors had no significant effect on the interaction of avirulent promastigotes and macrophages (Fig. 5B). Furthermore, the virulent strain showed an association index approximately 60% higher than the avirulent counterpart (compare black bars in Fig. 5A and B).

Fig. 5. Effect of proteolytic inhibitors and anti-peptidase antibodies on the interaction between L. braziliensis promastigote cells and macrophages. Both virulent (A) and avirulent (B) strains were treated for 1 h with metallo (EGTA and 1,10-phenanthroline [PHEN]) or cysteine (E-64) peptidase inhibitors at different concentrations. After that, parasites were washed in RPMI medium and the interaction process was performed as described in Materials and Methods section. Alternatively, the parasites were pre-incubated for 1 h with the anti-gp63, anti-cpb or pre-serum at 1:1000 dilution (C). The viability of the parasites was not affected by the proteolytic inhibitors or anti-peptidase antibodies treatments used in these experiments (data not shown). The parasite–macrophage interactions were performed at 37°C for 1 h in a ratio of 5 promastigotes to 1 macrophage cell. The values represent the mean±standard deviation of three independent experiments performed in triplicate. Symbols denote systems treated with proteolytic inhibitors or anti-peptidase antibodies that had an association index significantly different from the control (![]() , P<0·05; Student's t test).
, P<0·05; Student's t test).
Effect of anti-gp63 and anti-cpb antibodies on the L. braziliensis–macrophage interaction
The pre-treatment of virulent promastigotes with anti-gp63 or anti-cpb antibodies, both at a concentration that did not promote parasite agglutination (1:1000), caused a pronounced inhibition on the interaction process by approximately 55% and 36%, respectively (Fig. 5C). The effect of these two antibodies on the interaction process between avirulent promastigotes and macrophages was less prominent (Fig. 5C). Conversely, the treatment of both parasite strains with pre-serum did not alter the association indexes (Fig. 5C).
Effect of the spent culture medium from virulent promastigotes on the interaction between avirulent strain and macrophages
To corroborate the possible participation of metallo-type peptidases produced by L. braziliensis in the interaction with macrophage cells, we added the spent culture medium obtained from the virulent strain grown in BHI broth, which is rich in metallopeptidases (Fig. 1C, lane v), to the interaction mixture with avirulent strain and macrophages (Fig. 6). We noticed that the virulent-derived supernatant significantly enhanced the association index of avirulent strain of L. braziliensis and macrophage cells, in a dose-dependent manner, and this effect was reversed by 1 mM 1,10-phenanthroline (Fig. 6).

Fig. 6. Effect of the spent culture supernatant-derived from virulent strain of L. braziliensis on the interaction process between avirulent promastigotes and macrophage cells. The avirulent (av) strain was interacted with macrophage cells (MΦ) in the absence or in the presence of 25 and 50% culture supernatant-obtained from virulent strain (Sv), which was previously treated or not with 1 mM 1,10-phenanthroline (PHEN). The macrophage viability was not altered by the presence of spent culture medium neither of PHEN (data not shown). The parasite–macrophage interactions were performed at 37°C for 1 h in a ratio of 5 promastigotes to 1 macrophage cell. The values represent the mean±standard deviation of three independent experiments performed in triplicate. Symbols denote systems treated with proteolytic inhibitors or anti-peptidase antibodies that had an association index significantly different from the control (![]() , P<0·05; Student's t test).
, P<0·05; Student's t test).
DISCUSSION
Peptidases are important factors in the pathogenesis of several Leishmania species, with relevant participation in nutrition, cell invasion, intracellular survival, differentiation and proliferation (McKerrow et al. Reference McKerrow, Sun, Rosenthal and Bouvier1993; Vermelho et al. Reference Vermelho, Giovanni-de-Simone, d'Avila-Levy, Santos, Nogueira de Melo, Silva-Junior, Bom and Branquinha2007). In relation to L. braziliensis, very little is known about the real relevance of peptidases in crucial processes such as growth, nutrition and interaction with host components. Recently, Cuervo et al. (Reference Cuervo, Sabóia-Vahia, Silva-Filho, Fernandes, Cupolillo and Jesus2006, Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and Jesus2008) described the proteolytic activity profiles of 5 clinical strains of L. braziliensis isolated from Brazilian and Colombian patients with distinct clinical manifestations of American tegumentary leishmaniasis. The analysis by gelatin-SDS-PAGE of both cellular and extracellular extracts demonstrated different patterns of metallopeptidase activities ranging from 50 to 125 kDa. All those metallopeptidases were abrogated by 1,10-phenanthroline and presented activities in the pH range between 5·5 and 9·0; however, no cysteine peptidases were detected. In our present study, the virulent strain of L. braziliensis, originally isolated from a human case of cutaneous leishmaniasis, generated a distinct and complex proteolytic profile, being composed by both metallo- and cysteine peptidases whose hydrolytic activities were modulated by pH conditions. In the course of this manuscript preparation, Lanfranco et al. (Reference Lanfranco, Loayza-Muro, Clark, Núñez, Zavaleta, Jimenez, Meldal, Coombs, Mottram, Izidoro, Juliano, Juliano and Arévalo2008) reported the expression, isolation and biochemical characterization of a recombinant CPB from L. braziliensis. The mature region of the recombinant CPB shares a high percentage identity with its L. mexicana counterpart (named CPB2.8) and the mature active form presented optimum hydrolytic activity at pH 6·5, showing significant activity as low as pH 5·0, and molecular mass of 27 kDa.
Comparison of proteolytic activity in strains/lines with different biological behaviours may contribute to the recognition of the significance of this class of enzymes in the host and/or in the vector part of the Leishmania life cycle. In this context, we aimed to identify the active peptidases in 2 distinct strains of L. braziliensis that differ in their virulence for hamster, as well as the homology with the 2 major peptidase virulence factors expressed by several Leishmania species, named gp63 and cpb. Qualitative and quantitative differences in the cell-associated proteolytic activities of virulent and avirulent strains of L. braziliensis were observed by gelatin-SDS-PAGE, in which the virulent L. braziliensis strain presented a more complex peptidase pattern than the avirulent one. While the virulent strain present 4 proteolytic bands at pH 5·5 and 4 bands at pH 9·0, the avirulent strain always present just 1 band, with apparent molecular mass of 66 kDa, representing the major metallopeptidase, gp63, which is inhibited by the zinc-metallopeptidase inhibitor 1,10-phenanthroline. This metallo-type peptidase is also detected in the virulent strain; however, the hydrolytic activity is much more pronounced in this strain. The proteolytic halos with high apparent molecular masses produced by the virulent strain are considered metallopeptidases and the low molecular masses are characterized as cysteine peptidases due to complete blockage by E-64. Cysteine peptidases, in an active form, were only detected in cellular extracts of virulent forms. Such differences found in the production of peptidases are possibly associated with mechanisms of infection and virulence in L. braziliensis.
The extracellularly released peptidases can act as an important adaptive mechanism during the life cycle of protozoan parasites (McKerrow et al. Reference McKerrow, Sun, Rosenthal and Bouvier1993). The peptidases secreted by the two L. braziliensis strains were identified as metallopeptidases due to sensitivity to 1,10-phenanthroline. The virulent strain secreted 3 major distinct proteolytic enzymes of 120, 66 and 45 kDa. On the other hand, the avirulent strain secreted only a weak amount of the 66 kDa component.
In order to reveal whether L. braziliensis possesses proteins related to the well-known gp63 and cpb from L. mexicana, cellular protein extracts as well as the spent culture medium were probed with the anti-gp63 and anti-cpb antibodies. The anti-gp63 recognized a major polypeptide of 63 kDa in both avirulent and virulent strains of L. braziliensis. The gp63-like molecule was identified associated to the parasite cells as well as in the spent culture medium by means of Western blot assay. In this context, the virulent strain secreted an elevated level of gp63-like protein in comparison with the avirulent strain. The presence of gp63 has been detected in the extracellular medium of several Leishmania species, and secretion of this molecule can help in dissemination of the parasite since the gp63 protein is able to promote degradation of extracellular matrix components (Yao et al. Reference Yao, Donelson and Wilson2003). Furthermore, flow cytometry and fluorescence microscopy analyses revealed the cellular distribution of gp63-related molecules in promastigote forms of L. braziliensis, which were found in the parasite surface and in intracellular compartments. We also showed that L. braziliensis expressed 2 distinct cpb-like molecules of 30 and 20 kDa, the last polypeptide being drastically reduced in the avirulent strain. These results were reinforced by flow cytometry and fluorescent microscopy that demonstrated a significant increase in the fluorescence intensity in virulent parasites in comparison with avirulent ones when incubated with anti-cpb antibody. Intriguingly, 2 distinct populations with different affinities for the anti-gp63 and anti-cpb antibodies were clearly identified in avirulent and virulent strains of L. braziliensis, indicating that both gp63 and cpb molecules are not equally expressed on the surface of these strains. A first explanation for this observation would be that the lack of equal expression is correlated to the L. braziliensis cell cycle phase, since trypanosomatid cultures were not synchronized. Furthermore, the occurrence of distinct subpopulations could alternatively denote a different expression of surface gp63 and cpb molecules or even a diminished accessibility to external ligands in these cell subsets.
As is well known, the virulence of Leismania strains is determined by the concerted action of several parasite molecules. These cells lose their infectivity properties after prolonged cultivation in axenic growth media (Segovia et al. Reference Segovia, Artero, Mellado and Chance1992). This finding is in accordance with our presented results as well as with other reports in the same area. For instance, Wilson et al. (Reference Wilson, Hardin and Donelson1989) observed a reduced amount of gp63 protein in attenuated L. chagasi parasites in comparison with the virulent line. Brittingham et al. (Reference Brittingham, Miller, Donelson and Wilson2001) demonstrated that the different expression of gp63 in virulent promastigotes of L. chagasi when compared with avirulent counterpart is due to the regulation of gp63 mRNA stability. Sádlová et al. (Reference Sádlová, Volf, Victoir, Dujardin and Votýpka2006) showed that attenuated L. major LV561/AV line produced a low amount and a low enzymatic activity of gp63; however, serial passages of attenuated parasites through either Phlebotomus duboscqi or through mice led to a recovery of gp63 proteolytic activity to the level presented in virulent L. major LV561/V line. Chaudhuri and Chang (Reference Chaudhuri and Chang1988) have also shown that metallopeptidase activity and amount of gp63 decreases in avirulent L. amazonensis that had been passaged more than 100 times in vitro. Conversely, Soares et al. (Reference Soares, Santos, Bonaldo, Andrade, Alviano, Angluster and Goldenberg2003) found similar amounts of both gp63-related proteins and metallopeptidase activities in avirulent and virulent promastigotes of L. amazonensis Josefa strain. Similarly, Cuervo et al. (Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and Jesus2008) reported that different isolates of L. braziliensis remain unaltered after 1 year in continuous in vitro growth. These results probably reflect surprising species-specific as well as strain-specific differences in the role of gp63 in parasites belonging to the Leishmania genus.
Regarding cysteine peptidase activities, however, a consensus is observed in which reduced cysteine peptidase level is a frequent phenomenon in all Leishmania studied up to now that underwent a long-termed maintenance in vitro. Corroborating these findings, gene knockout studies of cysteine peptidases, many of which are stage-regulated, have demonstrated their relevance in the process of Leishmania spp. virulence in vivo and in vitro (Mottram et al. Reference Mottram, Souza, Hutchison, Carter, Frame and Coombs1996, Reference Mottram, Brooks and Coombs1998; Bart et al. Reference Bart, Frame, Carter, Coombs and Mottram1997; Alexander et al. Reference Alexander, Coombs and Mottram1998). For instance, L. mexicana mutants lacking cpb, a multicopy cysteine peptidase gene expressed in metacyclic promastigotes and amastigotes, not only have greatly reduced infectivity for mice but also generate a Th1 rather than a Th2 response (Mottram et al. Reference Mottram, Souza, Hutchison, Carter, Frame and Coombs1996; Alexander et al. Reference Alexander, Coombs and Mottram1998). These data provide strong encouragement that cysteine peptidase-deficient leishmania mutants are candidate attenuated live vaccines (Alexander et al. Reference Alexander, Coombs and Mottram1998). Under our employed methodology, no cysteine peptidase activities as well as a drastic reduction in the expression of cpb-like proteins in the avirulent strain of L. brasiliensis were noticed. Similar results were depicted from promastigote forms of L. amazonensis (Soares et al. Reference Soares, Santos, Bonaldo, Andrade, Alviano, Angluster and Goldenberg2003). Interestingly, a side-by-side comparison of a recently isolated virulent promastigotes of L. braziliensis from consecutive subpassages in vitro clearly showed a reduction of both metallo- and cysteine peptidase activities, in a similar fashion to that previously described for L. mexicana (Bates et al. Reference Bates, Robertson and Coombs1994).
Since virulent and avirulent strains of L. braziliensis produced different levels of both metallo- and cysteine peptidases, we tested the effect of classical inhibitors of these two peptidase classes on the parasite growth. Metallo- (EGTA and 1,10-phenanthroline) and cysteine (E-64) peptidase inhibitors were able to arrest only the growth rate of the virulent strain, having no effect on the avirulent strain proliferation behaviour. Furthermore, metallo- and cysteine peptidase inhibitors promoted some peculiar alterations in the cell morphology of L. braziliensis virulent strain, such as cells becoming round and short. Troeberg et al. (Reference Troeberg, Morty, Pike, Lonsdale-Eccles, Palmer, McKerrow and Coetzer1999) and Santos et al. (Reference Santos, d'Avila-Levy, Dias, Ribeiro, Pereira, Elias, Souto-Padrón, Lopes, Alviano, Branquinha and Soares2006) reported a similar phenomenon and postulated that it indicates osmotic stress caused by peptidase inhibition. The fundamental mechanism by which the metallopeptidase inhibitors act is by chelating essential divalent cations, such as magnesium, calcium, iron and zinc ions. For this reason, metallopeptidase inhibitors could also affect other metal-dependent biological processes besides the activity of metallopeptidases. E-64, a powerful cysteine peptidase inhibitor, is thought to be unable to cross membranes and so is likely to be denied access to many cell compartments (Bonaldo et al. Reference Bonaldo, D'Escoffier, Salles and Goldenberg1991). However, it may be taken up by fluid phase endocytosis and so enter lysosomes/phagosomes and inhibit the peptidases within those compartments (Irvine et al. Reference Irvine, North and Coombs1997).
The nature of the interaction between leishmania and its host macrophage-like cell, is critical to establish a new infection. The interaction between the macrophage and the promastigote form involves initial attachment to the macrophage surface and its subsequent internalization. Subsequently, the parasites need to resist the macrophage killing mechanisms. With no doubt, peptidases actively participate in these different phases of the leishmania-host cell relationship. Herein, we demonstrated that both avirulent and virulent strains of L. braziliensis interact with murine macrophage cells; however, in different ways, in which the association index was higher (around 1·6-fold) in the virulent strain than in the avirulent strain. The pre-treatment of virulent promastigotes with both metallo- and cysteine peptidase inhibitors reduced the association indexes in a dose-dependent manner, but did not alter the association indexes between avirulent strain and macrophages. Additionally, anti-cpb and anti-gp63 antibodies diminished the interaction of both L. braziliensis strains with macrophages, but this phenomenon was more pronounced when the virulent strain was pre-incubated with the antibodies. Taken together, it appears that avirulent and virulent strains of L. braziliensis probably utilize different receptors for their interaction with macrophages. In this context, Chakraborty et al. (Reference Chakraborty, Chakraborty and Basu1998) reported that avirulent promastigotes of L. donovani used the mannosyl fucosyl receptors more avidly for their initial attachment and subsequent internalization into the macrophages whereas the virulent counterpart exhibited limited use of this receptor. Interestingly, the incubation of L. braziliensis avirulent parasites with culture supernatant derived from the virulent strain, rich in metallopeptidases, enhanced the association with macrophages in a similar ratio found for the virulent strain counterpart. In addition, this effect was reversed by 1,10-phenanthroline, suggesting participation of metallo-type enzymes in the interaction process of L. braziliensis with macrophage cells.
The present paper reported significant alterations in the peptidase expression in avirulent and virulent strains of L. braziliensis as well as their distinct susceptibility to proteolytic inhibitors and interaction capability with macrophage cells, reaffirming the participation of peptidases in vital processes and virulence of parasites belonging to the Leishmania genus.
This work was supported by Fundação de Amparo à Pesquisa no Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Universitária José Bonifácio (FUJB), and Third World Academy of Science.









