INTRODUCTION
Protozoan pathogens of the Apicomplexan genus Cryptosporidium are ubiquitous as well as significant enteropathogens of a wide range of vertebrates (O'Donoghue, Reference O'Donoghue1995). While there are currently 23 recognized species, Cryptosporidium parvum and Cryptosporidium hominis are the 2 species most frequently associated with human cryptosporidiosis (Gatei et al. Reference Gatei, Wamae, Mbae, Waruru, Mulinge, Waithera, Gatika, Kamwati, Revathi and Hart2006; Ajjampur et al. Reference Ajjampur, Gladstone, Selvapandian, Muliyil, Ward and Kang2007; Wielinga et al. Reference Wielinga, De Vries, Van Der Goot, Mank, Mars, Kortbeek and Van Der Giessen2008; Chalmers et al. Reference Chalmers, Smith, Elwin, Clifton-Hadley and Giles2011; Ren et al. Reference Ren, Zhao, Zhang, Ning, Jian, Wang, Lv, Wang, Arrowood and Xiao2012). The infectious form, the oocyst, contains 4 potentially infectious sporozoites. Following ingestion, oocysts pass through the stomach and enter into the small intestine, where sporozoites excyst and, through a process of active motility and regulated secretion of apical organelles, infect the host enterocytes of the luminal surfaces of the digestive tract (Barnes et al. Reference Barnes, Bonnin, Huang, Gousset, Wu, Gut, Doyle, Dubremetz, Ward and Petersen1998; Cevallos et al. Reference Cevallos, Zhang, Waldor, Jaison, Zhou, Tzipori, Neutra and Ward2000; Chen et al. Reference Chen, O'Hara, Huang, Nelson, Lin, Zhu, Ward and Larusso2004; Smith et al. Reference Smith, Nichols and Grimason2005).
While a number of triggers have been identified for excystation, including metabolic temperature, pH fluctuations, bile salts, reducing agents and time, a lack of comprehension of the hierarchy or synergism of specific triggers involved in Cryptosporidium excystation still exists (Smith et al. Reference Smith, Nichols and Grimason2005; Karanis and Aldeyarbi, Reference Karanis and Aldeyarbi2011). While those triggers responsible for sporozoite apical organelle discharges are even more poorly understood, this discharge has been demonstrated to be temperature, intracellular calcium and cytoskeleton dependent (Chen et al. Reference Chen, O'Hara, Huang, Nelson, Lin, Zhu, Ward and Larusso2004). In addition to being identified as a trigger for excystation (Gold et al. Reference Gold, Stein and Tzipori2001; Kato et al. Reference Kato, Jenkins, Ghiorse and Bowman2001), bile salts have also been recognized to play a role in increasing the invasiveness of sporozoites (Gold et al. Reference Gold, Stein and Tzipori2001; Feng et al. Reference Feng, Nie, Sheoran, Zhang and Tzipori2006). While the mechanism through this enhanced invasion is not yet understood, it has been suggested that this may be via modification of the secretory pathway, possibly involving calcium signalling (Feng et al. Reference Feng, Nie, Sheoran, Zhang and Tzipori2006). Conversely, the incorporation of bile or bile salts into in vitro assays is not universal (Alum et al. Reference Alum, Rubino and Khalid Ijaz2011) and recent work has reported negative effects on in vitro parasite growth by its inclusion (Lalancette et al. Reference Lalancette, Di Giovanni and Prevost2010).
To further examine this dichotomy, we investigated the effect of bile and the bile salt sodium taurocholate on both excystation and infection in in vitro systems. We report here that bile and sodium taurocholate significantly affect both excystation rate and parasite growth in vitro. We demonstrate that this effect on increased excystation is dose, time and excystation pre-treatment temperature dependent, while increases in parasite replication appear to be associated not only with modulation of parasite intracellular calcium but also with increased host cell susceptibility to infection. Critically we illustrate that bile has a significant effect on host cells which can be cytotoxic at concentrations not much higher than those currently used for in vitro assays and if not used in the correct assay format could result in a negative effect on in vitro infectivity.
MATERIALS AND METHODS
Source of oocysts
The C. parvum cattle isolate (Iowa) was used in these studies. Oocysts were obtained from either Waterborne Inc. (New Orleans, LA, USA) or BTF (Sydney, New South Wales, Australia). On receipt, infectivity of each oocyst batch was determined using a cell culture-Taqman PCR assay (Keegan et al. Reference Keegan, Fanok, Monis and Saint2003) and all experiments using oocysts were carried out within 12 weeks of receipt. Flow cytometry (Vesey et al. Reference Vesey, Griffiths, Gauci, Deere, Williams and Veal1997) and fluorescence microscopy (Keegan et al. Reference Keegan, Fanok, Monis and Saint2003) were used to perform oocyst counts. An Olympus Vanox BX60 microscope fitted with a 10× eyepiece and either 40× or 100 × objectives were used for examination of samples.
Excystation protocols
To promote excystation, our standard excystation technique for C. parvum oocysts involves oocysts being incubated in 1 ml of acidified water (pH 2·4)-trypsin (0·025% [wt/vol]) for 20 min at 37 °C, centrifugation (10 min at 1800 g) and re-suspension in 1 ml of supplemented RPMI 1640 medium (infection medium) (37 °C) (Sigma) (Keegan et al. Reference Keegan, Fanok, Monis and Saint2003). Excystation rates were determined for each oocyst batch using flow cytometry (Vesey et al. Reference Vesey, Griffiths, Gauci, Deere, Williams and Veal1997). In a number of experiments modified excystation techniques were used, which involved oocysts being incubated in 1 ml of acidified water (pH 2·4)-trypsin (0·025% [wt/vol]) for 20 min at 30 °C or 22 °C, centrifugation (10 min at 1800 g) and re-suspension in 1 ml of room temperature (RT) infection medium which contained varying amounts of bile (Sigma) or sodium taurocholate (Sigma) before in vitro infection and flow cytometric studies.
Excystation measurements using flow cytometry
Oocysts pre-treated using excystation procedures were re-suspended in 1 ml of infection medium at 37 °C for defined time-periods. Oocysts were centrifuged (10 min at 1800 g), supernatant removed down to 100 μl and resuspended in 390 μl of PBS with 10 μl of monoclonal antibody EasyStain™ (monoclonal antibody highly specific to the oocyst wall [BTF, Sydney, New South Wales, Australia]). Samples were incubated for 15 min in the dark at room temperature. Stained oocysts were then analysed by flow cytometry for excystation using a procedure described previously (Vesey et al. Reference Vesey, Griffiths, Gauci, Deere, Williams and Veal1997; King et al. Reference King, Keegan, Robinson and Monis2011).
Measurement of sporozoite intracellular calcium
Following incubation in infection medium, excysted sporozoites were centrifuged for 10 min at 1800 g, the majority of the supernatant was removed and sporozoites were re-suspended in phosphate-buffered saline pH 7·5 (PBS) and stained with the acetoxymethyl (AM) ester Fluo-4 AM (Molecular Probes, Eugene, Oregon, USA), a cell-permeant calcium sensitive indicator that exhibits large increases in green fluorescence intensity upon binding intracellular calcium. Fluo-4 AM (2 μ m) was loaded into sporozoites at 18 °C for 20 min at the end of the incubation period/treatment, stained sporozoites were then centrifuged for 10 min at 1800 g, and the majority of the supernatant was removed and replaced with PBS. This wash step was repeated before analysis by flow cytometry as previously described (King et al. Reference King, Hoefel, Lim, Robinson and Monis2009).
Cell culture-Taqman infectivity assay
A cell culture-Taqman assay, which measures total Cryptosporidium lifecycle stages in the cell culture monolayer, was utilized due to its quantitative nature over a large dynamic range (i.e. a wide range of parasite cell numbers), allowing measurement of up to 4 log10 inactivation (Keegan et al. Reference Keegan, Fanok, Monis and Saint2003). Oocyst concentrations were determined by microscopy and flow cytometry. Ten thousand oocysts were used for infection of each cell monolayer. The HCT-8 cell line used for cell culture infections was in vitro cultured as previously described (Keegan et al. Reference Keegan, Fanok, Monis and Saint2003). The cell line was seeded into 24-well plates (Greiner Bio-One CELLSTAR® 662 160) at 5 × 105 cells/well and grown until confluent over a 48 h incubation period before infection. Oocysts were centrifuged onto cell monolayers at 408 g for 5 min after pre-treatment using the standard excystation protocol (37 °C acidified water pre-treatment). After a 48-h incubation period, the infected monolayer was harvested and a crude DNA lysis performed as previously described (Keegan et al. Reference Keegan, Fanok, Monis and Saint2003). Real-time PCR and the preparation of DNA standards for the quantification of the level of cell culture infection were as previously described (King et al. Reference King, Keegan, Monis and Saint2005). Relative infectivity (Relative infection %) was calculated using the equation; Infectivity of the sample = (lifecycle stages of the treatment/lifecycle stages of the control) × 100.
Cell culture focus detection method
While the focus detection method does not allow the same dynamic range for quantitation as the cell-culture Taqman assay, it was also utilized because it permits a more qualitative assessment of infection by an individual oocyst (i.e. it can discriminate between differences in the relative size of foci as well as differences in the number of foci) (King et al. Reference King, Keegan, Robinson and Monis2011). Oocysts were applied to the cell culture monolayer after pre-treatment using a modified excystation protocol where oocysts were pre-treated in acidified water for 20 min at 30 °C before re-suspension in 1 ml of room temperature (RT) infection medium and application to the monolayer by centrifugation at 408 g for 5 min. We employ the criteria previously defined by Rochelle et al. Reference Rochelle, Ferguson, Johnson and De Leon(2001) to delineate an infective focus, as the area of infection formed by a single oocyst. Therefore a 30 °C pre-treatment was used for this assay as it increases the precision in defining an infective focus (King et al. Reference King, Keegan, Robinson and Monis2011). Unless stated otherwise, 100 oocysts were applied to each individual well of a LAB-TEK II (Nalge Nunc International) chamber slide. The HCT-8 cell line was seeded at 4 × 105 cells/well and grown until confluent over a 48 h incubation period before infection. In vitro culture of the inoculated HCT-8 line was as for the cell culture-Taqman infectivity assay (Keegan et al. Reference Keegan, Fanok, Monis and Saint2003). Infected monolayers were then fixed, stained (Sporo-Glo™, sporozoite polyclonal antibody which binds strongly to merozoites and all other intracellular reproductive stages [Waterborne Inc, New Orleans, LA, USA]) and foci of infection enumerated as previously described (King et al. Reference King, Keegan, Robinson and Monis2011).
Bile time-course excystation experiments
Bile time-course experiments were undertaken to examine the effect of bile within the infection medium on excystation after 2 pre-treatment temperature regimes. The initial excystation treatment temperature for the acidified water incubation pre-treatment was either 37 °C or 30 °C. Pre-treated oocysts were then incubated at 37 °C in infection medium containing either bovine bile (0.02% wt/vol) or no bile for defined time-periods. Excystation rates were determined using flow cytometry as described above.
Bile and sodium taurocholate dosage excystation experiments
Bile and sodium taurocholate dosage experiments were conducted to examine the effect of dose and temperature on excystation. The initial excystation treatment temperature for the acidified water incubation pre-treatment was either 37 °C, 30 °C or 22 °C. Pre-treated oocysts were then incubated at 37 °C in infection medium containing a range of bovine bile (0·00, 0·00125, 0·0025, 0·005, 0·01, 0·02, 0·04, 0·08% [wt/vol]) and sodium taurocholate (0·00, 0·02, 0·04, 0·08, 0·16% [wt/vol]/0·00, 0·37, 0·74, 1·48, 2·98 [mM]) concentrations for 30 min at 37 °C. Excystation rates were determined using flow cytometry.
Bile and sodium taurocholate infectivity experiments
Bile and sodium taurocholate infectivity experiments were performed to examine their effect on parasite in vitro infectivity. Two infectivity assays were utilized, a cell culture-Taqman infectivity assay and the focus detection method as described above. For the focus detection method, oocysts were taken through a 30 °C excystation in acidified water pre-treatment before application to cell monolayers in infection medium containing either bile (0·02% [wt/vol]), sodium taurocholate (0·08% [wt/vol]) or neither. For the cell culture-Taqman assay, oocysts were taken through the standard excystation methodology (37 °C excystation in acidified water pre-treatment) before being applied to cell monolayers in infection medium containing a range of bile (0·00, 0·01, 0·02, 0·04,% [wt/vol]) and sodium taurocholate (0·00, 0·02, 0·04, 0·08% [wt/vol]/0·00, 0·37, 0·74, 1·48 [mM]) concentrations.
Bile and sodium tauorcholate cell monolayer cytotoxcity experiments
The HCT-8 cell line used for cell culture infections was in vitro cultured as previously described (Keegan et al. Reference Keegan, Fanok, Monis and Saint2003). The cell line was seeded into 24-well plates (Greiner Bio-One CELLSTAR® 662 160) at 5 × 105 cells/well and grown until confluent over a 48-h incubation period, after which bile and sodium taurocholate dosage experiments were conducted without oocyst inoculums. Bile and sodium taurocholate dosage experiments were performed to examine the effect of dose on cell cytotoxicity. A range of bile (0·00, 0·00125, 0·0025, 0·005, 0·01, 0·02, 0·04, 0·08% [wt/vol]) and sodium taurocholate (0·00, 0·02, 0·04, 0·08, 0·16% [wt/vol]/0·00, 0·37, 0·74, 1·48, 2·98 [mM]) concentrations were applied to cell monolayers in infection medium and incubated for a further 48 h. Cells were then screened by light microscopy for cell cytotoxicity (Olympus inverted IX51 microscope) before a resazurin reduction assay was performed.
Resazurin (Sigma-Aldrich, MO, USA) is a non-fluorescent dye that is metabolized to a highly fluorescent product, resorufin, by viable cells. The level of fluorescence produced is directly proportional to the number of viable cells (Czekanska, Reference Czekanska2011). To conduct the resazurin reduction assay, the infection medium was aspirated from the treated cell monolayers, monolayers washed with phosphate buffered saline before being replaced with fresh medium (without bile or sodium taurocholate supplementation) containing resazurin at a final concentration of 100 μg/ml resazurin and incubated at 37 °C for a further 1 h. Fluorescence at 530/580 (excitation/emission) was measured using a Wallac VICTOR3 1420 Multilabel Counter (PerkinElmer). Cytotoxicity was expressed as the percentage reduction of cell viability compared with the no-treatment control.
The effect of bile on sporozoite attachment and lifecycle development
Bile infectivity experiments were performed to examine the effect of bile on sporozoite attachment and subsequent lifecycle development. Oocysts were taken through the standard excystation methodology (37 °C excystation in acidified water pre-treatment) before centrifugation onto cell monolayers in infection medium containing either bile (0·02% [wt/vol]) or no bile followed by incubation for 48 h before analysis. A further set of treatments was also performed in parallel. These treatments consisted of pre-treated oocysts centrifuged onto the cell monolayers in infection medium containing bile (0·02% [wt/vol]) or no bile, inoculated monolayers were then incubated at 37 °C for 4 h before the infection medium was removed and monolayers washed with PBS. The infection medium was then replaced (bile (0·02% [wt/vol]) or no bile) and incubated for a total incubation time of 48 h. Infectivity was calculated using a cell culture-Taqman PCR assay.
Subsequently, a time-course experiment was performed to further dissect the significance of bile on parasite lifecycle development. Pre-treated oocysts were applied to monolayers in infection medium containing bile (0·02% [wt/vol]) along with a no bile control. All monolayers were then washed at 4 h post-infection before re-introduction of infection medium with bile (0·02% [wt/vol]). Lifecycle development in vitro was then allowed to proceed for various time-points post-inoculation (4, 8, 12, 16 and 24 h) before the infection medium was removed, the cell monolayer washed and infection medium (no bile) replaced. Parasite in vitro culture was then allowed to develop until 48 h post-initial inoculation and infectivity calculated using a cell culture-Taqman PCR assay.
Bile and sodium tauorcholate sporozoite intracellular calcium experiments
Bile and sodium taurocholate time-course experiments were undertaken to examine the effect of either bile or sodium tauorcholate within the infection medium on sporozoite intracellular calcium. Oocysts were taken through the standard excystation methodology (37 °C excystation in acidified water pre-treatment) before being incubated in infection medium containing either bile (0·02% [wt/vol]), sodium taurocholate (0·08% [wt/vol]) or neither, for defined time-periods at 37 °C. A minimum of 4 independent experiments were conducted. A further set of experiments was conducted to examine the effect of both bile (0·00, 0·02, 0·04, 0·08% [wt/vol]) and sodium taurocholate (0·00, 0·02, 0·08, 0·16% [wt/vol]/0·00, 0·37, 1·48, 2·98 [mM]) concentrations on intracellular calcium. A minimum of 3 independent experiments were conducted. Sporozoite intracellular calcium was quantified by flow cytometry as previously described (King et al. Reference King, Hoefel, Lim, Robinson and Monis2009).
The effect of pre-treating the cell monolayer with bile before oocyst inoculation
The HCT-8 cell line used for cell culture infections was in vitro cultured as described above with the exception that cells were grown for a 24-h incubation period, after which the growth medium was refreshed with new growth medium with or without bile as a supplement (0·02% [wt/vol]), then incubated for a further 24 h until confluent. Pre-treated monolayers were then inoculated with oocysts in the presence or absence of bile (0·02% wt/vol) within the infection medium. Both the cell culture-Taqman PCR assay and the focus detection method were used to investigate parasite in vitro growth.
Statistics
Data were analysed by a one- or two-way ANOVA with Bonferroni post-hoc analysis using GraphPad Prism 4. The level of significance was set at either P < 0·05, P < 0·01 or P < 0·001. The mean and standard deviation were also determined.
RESULTS
Bile time-course excystation experiments
Initial bile time-course experiments were undertaken to examine the effect of bile within the infection medium on excystation after pre-treatment of oocysts with acidified water at either 37 °C or 30 °C (Fig. 1). Without bile in the infection medium, greater than 80% of oocysts were able to excyst by the end of the 2-h time-course after pre-treatment at 37 °C (Fig. 1A). The presence of bile within the infection medium augmented this to beyond 95% by the end of the period. This positive effect on excystation was even more pronounced at both the earlier time-points within the time-course. When the acidified water pre-treatment was lowered to 30 °C and bile was absent from the infection medium, fewer than 50% of the oocysts had excysted by completion of the time-course (Fig. 1B). However, the inclusion of bile within the infection medium after pre-treatment at 30 °C resulted in an amplified positive effect on excystation over the time-course and the total percentage of ooysts excysted was greater than 85% by the end of the period.

Fig. 1. Time-course experiments demonstrate the effect of bile on excystation after pre-treatment of oocysts at 2 temperature regimes. Oocysts were taken through acidified water pre-treatment at either 37 °C (A) or 30 °C (B) before incubation in infection medium with or without bile (0·02% wt/vol) at 37 °C for defined time-periods (acidified water pre-treatment with no incubation in infection medium (AC), or incubation in infection medium for 30 min, 60 min, and 120 min), followed by flow cytometric analysis. Intact oocysts not taken through the standard excystation method were used as non-excysted controls (C). Each data point is the mean value from 4 independent experiments and the error bars indicate the standard deviations between experiments. *P < 0·05, **P < 0·01 and ***P < 0·001.
Bile and sodium taurocholate dosage excystation experiments
To scrutinize whether the concentration of bile in the infection medium was optimal we undertook a series of experiments to examine any synergism between bile concentration and pre-treatment temperature on excystation. In addition the bile salt sodium taurocholate was included, as it had been previously reported in the literature to achieve similar effects to bovine bile and therefore was thought it may prove suitable as a substitute (Gold et al. Reference Gold, Stein and Tzipori2001).
The effect of both bovine bile and sodium taurocholate concentration on excystation was positive across all pre-treatment temperatures (Fig. 2) (P < 0·001). By lowering the excystation pre-treatment temperature to 30 °C, an increased concentration of bile was required to achieve similar excystation rates for the higher pre-treatment temperature of 37 °C (Fig. 2A). When the pre-treatment excystation temperature was lowered to room temperature, the highest bile concentration tested resulted in only approximately 50% excystation by 30 min post-pre-treatment. The interaction between pre-treatment temperature and bile concentration was determined to be highly significant (P < 0·001). When lowering the excystation pre-treatment temperature for sodium taurocholate to 30 °C, increasing the concentration of sodium taurocholate above 0·02% (wt/vol) did not appear to further enhanceexcystation (Fig. 2B). Though when the pre-treatment excystation temperature was lowered to room temperature, increasing the sodium taurocholate concentration above 0·02% (wt/vol) further increased excystation (P < 0·01); however, as with bile treatments, the highest concentration resulted in only approximately 50% excystation by 30 min post-pre-treatment.

Fig. 2. Effect of bile and sodium taurocholate (NaTC) concentration on excystation after pre-treatment of oocysts at 3 different temperature regimes. Oocysts were taken through acidified water pre-treatment at 37 °C, 30 °C and 22 °C before incubation in infection medium with varying concentrations of bile (A) or NaTC (B) at 37 °C for 30 min, followed by flow cytometric analysis. Each data point is the mean value from 3 independent experiments and the error bars indicate the standard deviations between experiments.
The minimum concentration of bile required for optimal excystation at 37 °C was 0·00125% (wt/vol), the lowest concentration tested. For 30 °C it was 0·02% (wt/vol), the concentration currently used in our infection medium for our standard assay format where excystation pre-treatment is conducted at 37 °C. For the bile salt sodium taurocholate, the minimum concentration for optimal excystation was 0·02% (wt/vol) (lowest concentration analysed) at both 30 °C and 37 °C. For all sodium taurocholate-treated oocysts taken through the 30 °C pre-treatment, total excysted oocysts at 30 min ranged between 70 and 75%, while not significantly different, all bile concentrations at and above 0·02% (wt/vl) achieved excystation rates greater than 80%.
Bile and sodium taurocholate infectivity experiments
In addition to excystation, bile and bile salts have been suggested to be essential for sporozoite attachment and subsequent infection. The presence of both bile and sodium taurocholate in infection medium and their effect on in vitro infectivity was therefore investigated using both the focus detection method and a Taqman cell culture assay. The absence of either bile or sodium taurocholate from the infection medium resulted in significantly fewer foci as quantified by the focus detection method (Table 1) (P < 0·01). For the few foci that formed in the absence of either bile or sodium taurocholate, they were typically smaller with considerably less lifecycle stages (Fig. 3). While there was no significant difference in the number of foci between the bile and sodium taurocholate treatments (Table 1), foci that formed in the presence of sodium taurocholate were typically smaller with less lifecycle stages than those that formed in the presence of bile, but still larger than those formed in the absence of either supplement (data not shown). Further experimentation using the cell culture-Taqman assay confirmed the positive effect of bile and sodium taurocholate on in vitro infectivity compared to absence of either supplement in the infection medium (P < 0·001) (data not shown).

Fig. 3. Representative focus images for oocysts taken through acidified water pre-treatment before application to cell monolayers in infection medium with or without bile (0·02% wt/vol). Scale bar is 10 μm. (a) bile, (b–e) no bile.
Table 1. Infectious foci detected for bile and sodium tauorocholate (NaTC) treatments

* This treatment was not performed for Exp. 1.
The cell culture-Taqman assay was further utilized to examine whether increasing the concentration of either bile or sodium taurocholate had a positive effect on in vitro infectivity. While the effect of increased bile and sodium taurocholate concentration appeared to be positively associated with increased infectivity in some experiments (Fig. 4), additional experiments found inconsistent results for in vitro infection for bile concentrations greater than 0·02% (wt/vol). When infected cell monolayers were examined by microscopy it appeared that bile may be cytotoxic at concentrations greater than 0·02% (wt/vol), resulting in an observed increase in cell death, and occasionally loss of the cell monolayer. Increased concentrations of sodium taurocholate resulted in increased in vitro parasite growth but did not appear to have similar gross effects on the cell monolayer morphology as bovine bile, yet in vitro infectivity as determined by cell culture Taqman assay for all sodium taurocholate concentrations was consistently less than the standard bile treatment (0·02% wt/vol) for the concentrations tested (P < 0·01). This was consistent with the slightly smaller foci that were observed when using sodium taurocholate as a substitute for bovine bile.

Fig. 4. A representative experiment of the effect of bile and sodium taurocholate (NaTC) concentration on parasite infectivity for in vitro cell culture. Oocysts were taken through acidified water pre-treatment at 37 °C before application to cell monolayers in infection medium with varying concentrations of bile or NaTC. Relative Infection (%) was calculated using a cell culture-Taqman PCR assay and expressed as a percentage of the standard treatment (bile 0·02% wt/vol). Error bars indicate standard deviation of 6 replicates.
Bile and sodium tauorcholate cell monolayer cytotoxcity experiments
A series of experiments was therefore undertaken to examine the potential cytotoxicity of bile and sodium taurocholate on the HCT-8 cell line (Fig. 5). A significant effect of both bovine bile and sodium taurocholate concentration was evident on cell metabolic activity for the concentrations tested (P < 0·001). For bovine bile, a gradual cytotoxic response was observed at concentrations equal to and greater than 0·055% (wt/vol) (<70% metabolic activity of the control), with strong visual cytotoxicity also observed at these concentrations (Fig. 5A). For sodium taurocholate typical cytotoxic cell morphology was not observed over the range of concentrations tested, though the resazurin reduction assay indicated decreased metabolic activity below 70% of the control with concentrations of sodium taurocholate of 0·16% (wt/vol) (Fig. 5B).

Fig. 5. Effect of bile and sodium tauorcholate (NaTC) on cell cytotoxicity. Bile (A) and NaTC (B) dosage experiments were performed to examine the effect of dose on cell cytotoxicity. Cell viability was determined by a resazurin reduction assay and results normalized against the no-treatment controls. Each data point is the mean value from 3 independent experiments and the error bars indicate the standard deviations between experiments.
The effect of bile on sporozoite attachment and lifecycle development
A series of bile infectivity treatments were undertaken to examine the effect of bile on both sporozoite attachment and subsequent lifecycle development (Fig. 6A). Pre-treated oocysts applied to cell monolayers within infection medium without bile again showed poor in vitro infectivity, while those in infection medium with bile (standard treatment) had improved infection as previously demonstrated (P < 0·001). Pre-treated oocysts applied to cell monolayers in infection medium without bile, then washed 4 h post-inoculation (post-sporozoite attachment/invasion) and infection medium (without bile) replenished showed a small but not significant increase over the without bile treatment that did not include a monolayer wash. This further control was included as we have previously identified that washing the monolayer after 4 h post-inoculation improves in vitro infectivity (King et al. Reference King, Keegan, Robinson and Monis2011).
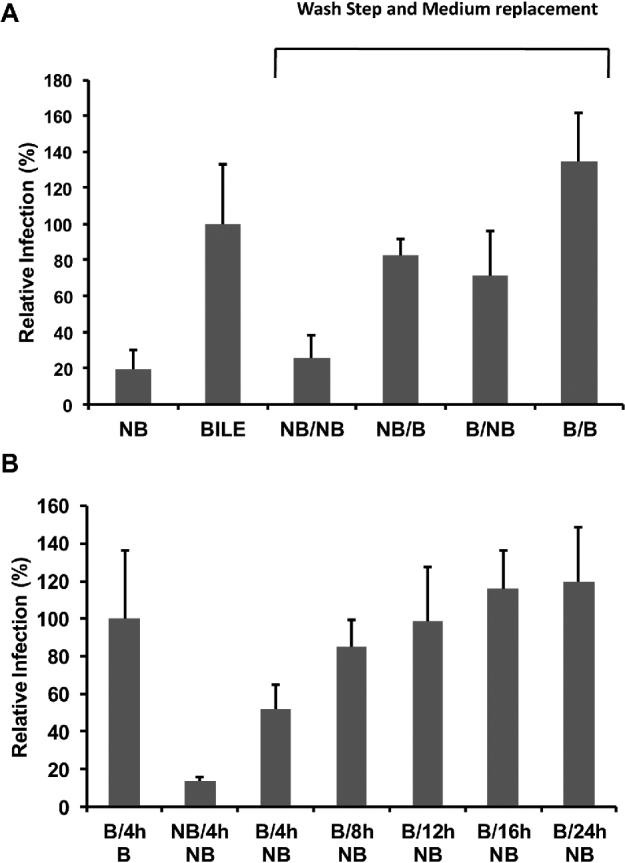
Fig. 6. Effect of bile on sporozoite attachment and subsequent lifecycle development. (A) Pre-treated oocysts were applied to cell monolayers in infection medium containing either no bile (NB) or bile (B) and incubated for 48 h. To separately assess the effect of bile on attachment and development, pre-treated oocysts were applied to monolayers in infection medium with or without bile, monolayers were then washed at 4 h post-infection and the infection medium replaced with fresh medium without bile (NB/NB and B/NB) or with bile (NB/B and B/B) and incubated until a total time of 48 h. Relative Infection (%) was calculated using a cell culture-Taqman PCR assay and expressed as a percentage of the standard bile treatment (B). Each data point is the mean value from at least 3 independent experiments and the error bars indicate the standard deviations between experiments. (B) An additional experiment was undertaken where pre-treated oocysts were applied to monolayers in infection medium containing bile (B) along with a no bile control (NB). All monolayers were then washed at 4 h post-infection before re-introduction of infection medium with bile. Life cycle development in vitro was then allowed to proceed for various time-points post-inoculation (4, 8, 12, 16 and 24 h) before the infection medium was removed and replaced with infection medium without bile and in vitro culture allowed to develop until 48 h post-initial inoculation. Relative Infection (%) was calculated using a cell culture-Taqman PCR assay and expressed as a percentage of the treatment were bile was continuously present in the in vitro culture of the parasite (B/4hB). Each data point is the mean value from 6 cell culture replicates and the error bars indicate the standard deviations of the mean.
The introduction of bile into the infection medium after the initial 4-h inoculation period significantly increased total in vitro infectivity over the no bile treatments (P < 0·001). For pre-treated oocysts applied to cell monolayers in infection medium containing bile and where monolayers were subsequently washed post-attachment/invasion and infection medium (without bile) replenished, significant improvements in infectivity over the no bile treatments was apparent (P < 0·01). In vitro infectivity was significantly less (P < 0·001) for these treatments where bile was omitted from the infection medium either before or after sporozoite attachment/invasion in comparison to the treatment where the medium was replenished but bile was included in the infection medium for the entire infection period.
Furthermore, a time-course experiment where bile was removed from the infection medium corresponding to particular parasite lifecycle developmental stages was undertaken (Fig. 6B). Bile was again removed from the infection medium at 4 h post-inoculation corresponding to the completion of sporozoite attachment/invasion, additionally bile was also removed at 8 and 12 h post-infection when trophozoite and merozoite development respectively have been reported. Moreover, bile was also removed from the infection medium at 16 and 24 h post-infection when there are substantial increases in parasite development in vitro. A general trend of increased parasite in vitro culture was evident the later in the time-course that bile was removed from the infection medium (fitted polynomial equation y = 5·1259x2 + 56·224x − 38·947, R2 = 0·9908).
The effect of bile and sodium tauorcholate on sporozoite intracellular calcium
A series of time-course experiments was undertaken to examine the effect of bile and sodium taurocholate on sporozoite intracellular calcium. The addition of either bile or sodium taurocholate was determined to be highly significant on both intracellular calcium and sporozoite internal granularity (P < 0·001). Sporozoites incubated in infection medium containing either bile or sodium taurocholate showed a gradual increase in sporozoite intracellular calcium over the time-course analysed (Fig. 7A). This was associated with a concomitant decrease in internal granularity of the sporozoite as indicated by reduction in the side-scatter of the sporozoite population (data not shown). For sporozoites incubated in infection medium without either supplement, sporozoite populations typically displayed high intracellular calcium at the start of the time course (Fig. 7A). This was associated with a more rapid reduction in initial internal granularity of the sporozoite (data not shown). Furthermore we investigated the effect of varying concentrations of both bile and sodium taurocholate on sporozoite intracellular calcium after incubation in infection medium at 37 °C for 60 min. Increasing the concentration of sodium taurocholate decreased the percentage of the sporozoite population with high intracellular calcium (Fig. 7B) (P < 0·01); however, increasing the concentration of bile above 0·02% (wt/vol) did not appear to further slow the rise in intracellular sporozoite calcium, with the population returning to a high intracellular calcium level (Fig. 7B).
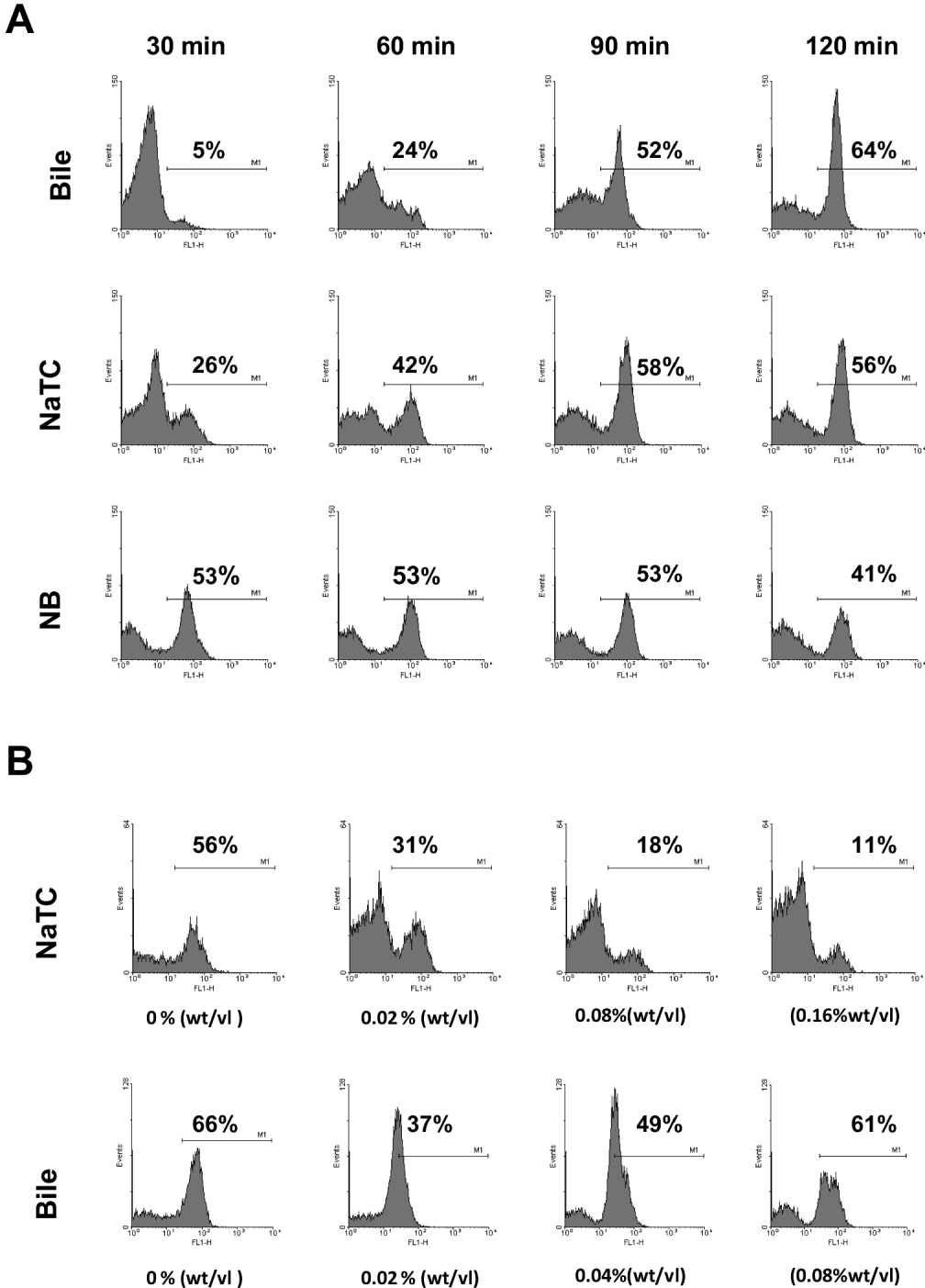
Fig. 7. A representative experiment of the effect of bile and sodium tauorcholate (NaTC) on sporozoite intracellular calcium. (A) Histograms of fluorescence intensity (FL1 channel) of sporozoite populations incubated in infection medium containing bile (0·02% [wt/vol]), sodium taurocholate (0·08% [wt/vol]) or neither supplement (NB) for 30, 60, 90 or 120 min post-excystation pre-treatment. (B) Histograms of fluorescence intensity (FL1 channel) of sporozoite populations incubated in infection medium for 60 min containing varying concentrations of sodium taurocholate (0·00, 0·02, 0·08, 0·16% [wt/vol]) or bile (0·00, 0·02, 0·04, 0·08% [wt/vol]). Changes in fluorescence intensity (FL1 channel) indicate changes intracellular calcium of the sporozoite population.
The effect of pre-treating the cell monolayer with bile before oocyst inoculation
The effect of bile pre-treatment of the cell monolayer was examined for its effect on Cryptosporidium in vitro infectivity using both the cell culture Taqman assay and focus detection method. For oocysts applied to monolayers in infection medium minus bile, pre-treatment of the monolayer with bile resulted in superior infection above those monolayers not pre-treated with bile, as indicated by both the cell culture Taqman assay (Fig. 8A) (P < 0·05) and focus detection method (Fig. 8B) (P < 0·05). For oocysts applied to monolayers in infection medium containing bile, pre-treatment of the monolayer with bile did not result in any additional gains in infectivity (not significant) as measured for either the Taqman assay (Fig. 8A) or focus detection method (Fig. 8B). Furthermore, additional experimentation (data not shown) confirmed that pre-treatment of the cell monolayer with sodium taurocholate (P < 0·001) also resulted in superior infection when sporozoites were subsequently inoculated onto cell monolayers without either supplement in the infection medium compared to monolayers that had not been pre-treated.

Fig. 8. Representative experiments of bile pre-treatment of the cell monolayer and its effect on Cryptosporidium in vitro infectivity. Cell monolayers were either pre-treated with or without bile (0·02% wt/vol) before inoculation of oocysts in the presence or absence of bile (0·02% wt/vol) within the infection medium. (B/B) Pre-treatment with bile/oocysts added in infection medium containing bile. (NB/B) Pre-treatment without bile/oocysts added in infection medium containing bile. (B/NB) Pre-treatment with bile/oocysts added in infection medium without bile. (NB/NB) Pre-treatment without bile/oocysts added in infection medium without bile. (A) Relative Infection (%) calculated using a cell culture-Taqman PCR assay and expressed as a percentage of the NB/B treatment. Error bars indicate standard deviations of 6 replicates. (B) Infectivity was also determined using the focus detection method and expressed in number of foci observed. Error bars indicate standard deviations of 8 replicates.
DISCUSSION
Our interest in bile and bile salts focuses on their incorporation as supplements into in vitro cell culture for the propagation of infectious Cryptosporidium parvum oocysts. Such supplements have been reported not only to enhance excystation (Gold et al. Reference Gold, Stein and Tzipori2001; Kato et al. Reference Kato, Jenkins, Ghiorse and Bowman2001) but also sporozoite invasiveness (Feng et al. Reference Feng, Nie, Sheoran, Zhang and Tzipori2006). Recent work, conversely, has reported negative effects on in vitro parasite growth and the inclusion of total bile or bile salt supplements for parasite culture has not been universally adopted by researchers (Lalancette et al. Reference Lalancette, Di Giovanni and Prevost2010; Alum et al. Reference Alum, Rubino and Khalid Ijaz2011). This prompted us to revisit the effect of bile and the bile salt sodium taurocholate on excystation in an attempt to dissect the hierarchy of this signal. Furthermore, we examined the effect on sporozoite infectivity and extended our investigations to the effect of bile and sodium taurocholate on the subsequent lifecycle stages, host cell susceptibility and sporozoite intracellular calcium.
Temperature elevation and pH change play important roles in the transduction of external signals across the oocyst wall to sporozoites, resulting in parasite-specific intracellular responses that initiate excystation (Robertson et al. Reference Robertson, Campbell and Smith1993; Forney et al. Reference Forney, Yang, Du and Healey1996a, Reference Forney, Yang and Healeyb; Okhuysen et al. Reference Okhuysen, Chappell, Kettner and Sterling1996; Smith et al. Reference Smith, Nichols and Grimason2005). Apart from temperature and pH, our knowledge is limited about the roles played by other triggers, including bile and any synergistic relationship it may exhibit with the aforementioned cues (Smith et al. Reference Smith, Nichols and Grimason2005). We therefore initially examined the effect of bile within our infection medium after acid pre-treatment.
While acid pre-treatment at 37 °C followed by incubation of pre-treated oocysts at 37 °C in infection medium appears to effectively activate excystation in the absence of bile stimuli, the addition of bile significantly improved the rate of excystation. Nevertheless, the magnitude of this effect diminished over the time-course analysed. Markedly, lowering the acid pre-treatment temperature to 30 °C with the removal of bile considerably retarded excystation over the time-course, but the incorporation of bile as a supplement almost entirely compensated for loss of the higher temperature stimulus. Previous work conducted in our laboratory (not presented herein) has demonstrated that without an acidified water pre-treatment, excystation after a 2-h incubation period at 37 °C in infection medium containing bile is greatly reduced (<40% excystation). This suggests that the acidified water pre-treatment is critical for the transduction of a bile derived signal/s across the oocyst wall. We therefore hypothesized that by reducing the temperature of the acidified water pre-treatment the transduction of the external bile signal across the oocyst wall would be less efficacious.
To test this hypothesis a series of dosage experiments over 3 acidified water pre-treatment temperatures were undertaken using total bovine bile and the bile salt sodium taurocholate. The bile salt sodium taurocholate was included as it had previously been demonstrated to have a positive effect on excystation; we therefore presumed it may be the component of bile that acts as the chief molecular signal responsible for the enhanced effect on excystation. Reducing the acidified water pre-treatment temperature required an increased concentration of bile to compensate for the reduction in the efficacy of this signal. For the bile salt sodium taurocholate, increasing the concentration above 0·02% (wt/vol) only enhanced excystation further when the pre-treatment was lowered to RT, suggesting that the concentration of sodium taurocholate at temperatures tested above RT was not limiting. Yet, for sodium taurocholate experiments performed after 30 °C or 37 °C pre-treatments, total excysted oocysts were less than for total bile at the same weight/volume ratios. As a component of bile, sodium taurocholate, one of a number of bile salts, is approximately less than 10% of the total bile. Therefore if it is the only compound within bile affecting excystation it would be predicted that the equivalent quantity (weight/volume [%]) of sodium tauorcholate would have greater efficacy than the equivalent quantity of total bile. This suggested that while sodium tauorcholate may be a bile-derived signal, a number of other components within bovine bile may be important for excystation for which sodium taurocholate cannot substitute. This is in accordance with recent work that has demonstrated that the type of bile may affect excystation rates (Kar et al. Reference Kar, Daugschies, Cakmak, Yilmazer, Dittmar and Bangoura2011) while it has also been demonstrated that a variety of bile salts enhanced sporozoite invasion of cultured cells (Feng et al. Reference Feng, Nie, Sheoran, Zhang and Tzipori2006). Although these differences may also be explained as differences in the function of the uptake of bile or sodium taurocholate by either the presence of specific bile acid transporters and/or the more effective passive diffusion of bile in aggregated forms such as micelles.
When the pre-treatment excystation temperature was lowered to room temperature, the highest bile/sodium taurocholate concentration only resulted in approximately 50% excystation 30 min post-pre-treatment, indicating that either total bile or sodium taurocholate was not a sufficient gastrointestinal signal in itself to overcome a reduction in the temperature stimulus of the acidified water pre-treatment to room temperature. This reinforces the importance of the synergistic relationship of high temperature in combination with acidified water pre-treatment in the efficient transduction of bile-derived external signals across the oocyst wall to sporozoites resulting in the maximum excystation of sporozoites.
While bile and, in particular, the bile salt sodium taurocholate augment excystation, it has also been reported that a variety of purified bile salts and total bovine bile enhance sporozoite invasion of cultured cells (Gold et al. Reference Gold, Stein and Tzipori2001; Feng et al. Reference Feng, Nie, Sheoran, Zhang and Tzipori2006). Recent work though, has reported inhibitory effects on Cryptosporidium focus development for in vitro infectivity by the inclusion of bile in the infection medium (Lalancette et al. Reference Lalancette, Di Giovanni and Prevost2010). Furthermore, it has also been suggested that a lower cell passage may compensate for the differences between excystation protocols with or without bile salts (Alum et al. Reference Alum, Rubino and Khalid Ijaz2011). We therefore decided to examine the effect of both bile and sodium taurocholate initially on the development of infectious foci. When either supplement was absent from the infection medium the numbers of foci were dramatically reduced. The large reduction in foci number could not be wholly reconciled with the decrease that was expected as a result of an expected reduction in excystation, suggesting a possible effect on both sporozoite attachment and subsequent invasion. For those foci that did form in the absence of either supplement, they were considerably smaller in size and the number of lifecycle stages present, suggesting that the development of subsequent lifecycle stages may also be affected by these supplements. While there was no significant difference in foci number between total bile and sodium taurocholate, those foci that formed from sodium taurocholate appeared to be slightly smaller in size with less lifecycle stages, suggesting that the development of these subsequent lifecycle stages may be differentially affected by these different bile supplements.
To further explore the effect of bile and sodium taurocholate on total in vitro infectivity we examined whether increasing the concentration of either supplement would enhance infectivity. The cell culture Taqman assay was utilized due to both its quantitative nature and the fact that the acidified water pre-treatment is undertaken at 37 °C, greatly reducing any negative effects on infectivity that may result from a reduction in excystation without a bile/bile salt supplement in the infection medium. Increases observed in infectivity further emphasized the importance of either bile or sodium taurocholate in the infection medium to maximize Cryptosporidium in vitro growth and not just excystation. This is concomitant with a previous report that enhancement of infection with sodium tauorcholate is dose dependent (Feng et al. Reference Feng, Nie, Sheoran, Zhang and Tzipori2006). Interestingly, bile appeared to be superior as a supplement over sodium tauorcholate, suggesting as with excystation that a number of other components within bovine bile, possibly other bile salts (Feng et al. Reference Feng, Nie, Sheoran, Zhang and Tzipori2006), may be necessary for infectivity and for which sodium taurocholate cannot completely substitute. Yet, as previously mentioned, this may also be explained by differences in the uptake between these two supplements.
While increasing both bile and sodium tauorcholate concentration in the infection medium resulted in improved infectivity, it became apparent from cytotoxicity experiments that a slight increase above our standard concentration of bile (0·02% wt/vol) could result in a cell cytotoxic effect, while an increased concentration of sodium taurocholate decreased cell metabolic activity. Previous work has reported no cytotoxic effects from either supplement on cell monolayers, but much of this work only examined incubation time-periods of 2 h with either supplement (Gold et al. Reference Gold, Stein and Tzipori2001; Feng et al. Reference Feng, Nie, Sheoran, Zhang and Tzipori2006), instead of the 48-h incubation undertaken in this work. Considering the nature of bile supplements and batch variation, it is possible that cell cytotoxicity may be a reason why some authors have reported negative effects on in vitro infectivity by the inclusion of a bile supplement. Though, it must also be considered that the inclusion of bile in the infection medium could have negative effects if authors have not centrifuged oocysts onto monolayers and they excyst more rapidly. Excysted sporozoites not in the vicinity of the monolayer may not be able to successfully establish infection before exhausting metabolic reserves (King et al. Reference King, Keegan, Robinson and Monis2011). This emphasizes the critical nature of understanding excystation rate in relation to the assay format employed and how this may influence Cryptosporidium in vitro growth.
While our work confirmed the positive effect of bile supplements on total in vitro infectivity, we suspected that these supplements may increase the infectivity of subsequent lifecycle stages apart from initial sporozoite invasiveness. To further dissect this hypothesis, a series of bile infectivity treatments involving removal of bile before and/or after the infection/attachment phase were undertaken to examine the effect of bile on both sporozoite attachment/invasion and following lifecycle development. Removing bile during the attachment invasion stage confirmed its significance to initial host cell invasion. Treatments aimed at replacing bile after this stage confirmed that total in vitro infectivity improved, indicating the importance of bile for either subsequent parasite lifecycle development and/or re-invasion of host cells by ensuing invasive stages. To further scrutinize this we conducted a time-course experiment where bile was removed from the infection medium at time-points corresponding to trophozoite and merozoite development along with time-points when large increases in lifecycle stages have been previously reported (King et al. Reference King, Keegan, Robinson and Monis2011). Removal of bile at time-points corresponding to these stages confirmed the importance of the bile signal to their efficient in vitro development. Based on these observations it would be highly beneficial to maintain bile within the in vitro culture of the parasite for the complete incubation period rather than just the initial excystation and infection/invasion processes in order to maximize in vitro culture.
The regulated secretion of the apical organelles of Cryptosporidium is essential for sporozoite motility, cell attachment and host cell invasion. This secretion has been shown to be temperature, cytoskeleton and intracellular calcium dependant (Chen et al. Reference Chen, O'Hara, Huang, Nelson, Lin, Zhu, Ward and Larusso2004). While the initial trigger/s for zoite activation and subsequent apical discharge remain unclear, it has been suggested that the ‘in vivo’ intestinal environmental triggers sporozoite activation through intracellular signalling pathways including intracellular calcium to stimulate a cytoskeleton-mediated exocytosis response to apical organelle discharge and thus mediate host cell invasion (Chen et al. Reference Chen, O'Hara, Huang, Nelson, Lin, Zhu, Ward and Larusso2004). Feng et al. (Reference Feng, Nie, Sheoran, Zhang and Tzipori2006) demonstrated that sodium tauorcholate treatment increased the secretion of organelles and the gliding motility of sporozoites, thus facilitating their initial attachment and invasion of cells. They suggested that this modification of the secretory pathway may be dependent on the induction of calcium signalling by bile salts.
While the induction of signalling by bile salts has become well-documented in mammalian cells over the past decade (Parks et al. Reference Parks, Blanchard, Bledsoe, Chandra, Consler, Kliewer, Stimmel, Willson, Zavacki, Moore and Lehmann1999; Nguyen and Bouscarel Reference Nguyen and Bouscarel2008), whether or not bile salts induce calcium signalling in C. parvum or other Apicomlexa has yet to be demonstrated (Feng et al. Reference Feng, Nie, Sheoran, Zhang and Tzipori2006). Our group has previously utilized flow cytometric techniques for the investigation of Cryptosporidium sporozoite apical organelle discharge and in particular the analysis of sporozoite intracellular calcium (King et al. Reference King, Hoefel, Lim, Robinson and Monis2009). We therefore employed these techniques to investigate whether bile and sodium tauorcholate can affect sporozoite intracellular calcium.
We demonstrated that without bile or sodium taurocholate within the infection medium, sporozoite intracellular calcium was high immediately post-excystation and this was associated with large reductions in the internal granularity of sporozoites suggestive of pre-mature exocytosis. While sporozoite intracellular calcium appeared to be modulated by the inclusion of either of the supplements and this was also associated with increased sporozoite internal granularity, suggestive of controlled (regulated) secretion, opposed to uncontrolled. Previously we have demonstrated that solar radiation can rapidly induce sporozoite de-granulation, significantly affecting the ability of sporozoites to successfully attach and infect a compatible host cell (King et al. Reference King, Hoefel, Wong and Monis2010). Interestingly, bile salts have previously been demonstrated to induce de-granulation (relating to secretion and exocytosis) in a range of mammalian cells (Hardcastle et al. Reference Hardcastle, Hardcastle, Chapman and Taylor2001; Yamazaki et al. Reference Yamazaki, Gleich and Kita2001). This work provides the first evidence that bile, and in particular a bile salt, can directly affect intracellular calcium signalling in an Apicomplexan, and to our knowledge provides only the second report of an external host signal associated with affecting intracellular calcium signalling and controlled secretion of apical organelles (Singh et al. Reference Singh, Alam, Pal-Bhowmick, Brzostowski and Chitnis2010). We suggest that modulation of regulated secretion by bile and bile salts effectively gives excysted sporozoites more time to find a compatible target cell before extensive apical organelle discharge ensues, and that unregulated discharge (in the absence of bile/bile salts) results in low infectivity. A further avenue of research may include examining whether bile and bile salts affect the functioning of the sarcoplasmic-endoplasmic reticulum calcium ATPase pump, thereby modulating internal calcium reserves.
Finally, we examined whether there was any contribution of the host cell to increased in vitro infectivity from the presence of bile and sodium taurocholate in the infection medium. While small, these effects were significant and consistently reproducible for both bile and sodium taurocholate. Previous work has not established any effects of bile or sodium taurocholate to increases in the host cell ‘susceptibility’ to infection. It is possible that for bile, the lipase action may increase host cell receptor availability, increasing sites for attachment by sporozoites and other invasive stages. Secondly, it is well documented that bile acids are responsible for the induction of signalling in mammalian cells, and it is therefore possible that activation of such signalling cascades may increase host cell receptiveness to invasion, especially considering the role of the host cell membrane in encapsulating the parasite. Such modes of action are only speculation and further research would be needed to dissect the foundation of increases to the host cell-derived component.
This work demonstrates the multifaceted nature of the effects of bile and bile salts in relation to in vitro excystation, invasion and subsequent lifecycle development for Cryptosporidium parvum. Significantly, we have also begun to understand the hierarchy of the bile-derived signal in relation to excystation and its synergism with both temperature and oocyst acid pre-treatment stimuli. Furthermore, while we have demonstrated for the first time that bile, and in particular a bile salt, can directly affect intracellular calcium signalling in an Apicomplexan, the mechanism behind this action remains to be elucidated, and whether specific sporozoite bile acid receptors are involved is unknown. Finally, the information generated within this work will help in understanding appropriate in vitro assay design in order to improve both sensitivity and interpretation.
ACKNOWLEDGEMENTS
We are grateful to Bret Robinson, Shailaja Pannersilvam, Daniel Inglis, Kimberly Sieburn, Michael Webber for excellent technical assistance. We thank the South Australian Water Corporation and the Victorian Smart Water Fund (project 72M-7103) for financial, technical and administrative assistance in funding this work.











