INTRODUCTION
Based on the latest statistics, the worldwide catch of marine fish was 8·35 million tons in 2011, only a 0·16% increase from 10 years ago (FAO, 2013). On the other hand, production of cultured marine fish is rapidly increasing, with production being 3·89 million tons in 2011, a 69·5% increase from 10 years ago (FAO, 2013). Among them, salmonid culture in Europe, North America and Chile comprises 55·9% of the total mariculture production, followed by those of carangids (0·28 million tons or 7·2% of the total production), sparids (0·26 million tons; 6·6%) and percichthyids and moronids (0·24 million tons; 6·2%). With respect to helminth parasitic diseases of cultured marine fishes, few serious helminth infections are known to occur among maricultured salmonids, whereas carangid, sparid, percichthyid and serranid fishes have important helminth diseases.
Traditionally marine fish were cultured in coastal ponds and natural coastal enclosures. These culture systems were practiced on a small scale, compared with the large-scale floating net cage system which appeared around 1960, which was first adopted for culture of Japanese amberjack Seriola quinqueradiata (Eng and Tec, Reference Eng, Tec, Woo, Bruno and Lim2002). Typically, cages are cubic with one side of 10 m or smaller in size. This system spread rapidly in western Japan, as it has many advantages over the traditional methods in terms of water exchange, cost for setup and maintenance, management of fishes in cages and applicability to other marine fish species. Recently, new types of larger net cages and offshore cages for bluefin tunas and submerged cages in rough areas have appeared (Beveridge, Reference Beveridge, Woo, Bruno and Lim2002). Net cages are not suitable for some fishes. Flat fishes like bastard halibut, Paralichthys olivaceus, are mainly cultured in land-based tanks with flow-through sea water.
In mariculture, fish are introduced into farm sites in the form of culture seed, either wild-caught or artificially produced. With the progress of artificial seed production techniques, the latter type is used more frequently. The former type is still the main source of seed for Japanese amberjack and greater amberjack, Seriola dumerili, because juveniles are caught in large numbers without any sign of overfishing and generally wild-caught juveniles have fewer health problems.
Traditionally marine fish are fed with chopped or minced raw trash fish. However, with expanding culture scales, this type of feeding caused serious environmental pollution problems now largely resolved by replacing it with moist pellets and dry extruded pellets.
Platyhelminth parasites, comprising monogeneans, digeneans and cestodes, are quite common in maricultured fish. They usually infect feral fish in low numbers, causing little pathology. However, mariculture farms, where fish are maintained in high densities, create favourable conditions for parasite proliferation because of the increased probability of encountering a host. Culture systems also impose considerable stress on farmed fish, which generally increases their susceptibility to parasitic infections. In spite of its many advantages as mentioned above, cage culture is an open system, where parasites are transmitted easily among fish in cages through free exchange of water. Some monogeneans are especially harmful in cage culture, as eggs entangle culture nets with their filamentous appendages, making fish in cages easy targets for re-infection. Blood flukes are dangerous parasites of vertebrates including teleost fish. Considerable numbers of fish blood flukes are known to infect farmed marine fish. Their intermediate hosts have rarely been identified, but they are present in and around many mariculture farm areas. Consequently, with rapid expansion and intensification of mariculture, economic losses caused by platyhelminthes have increased dramatically. Damage to mariculture industries ranges from mortality of farmed fish to growth retardation and sometimes loss of market value due to the unaesthetic appearance of infected fish.
Aquarium fish are not included here. Common names are based on FishBase (Froese and Pauly, Reference Froese and Pauly2014).
MONOGENEA
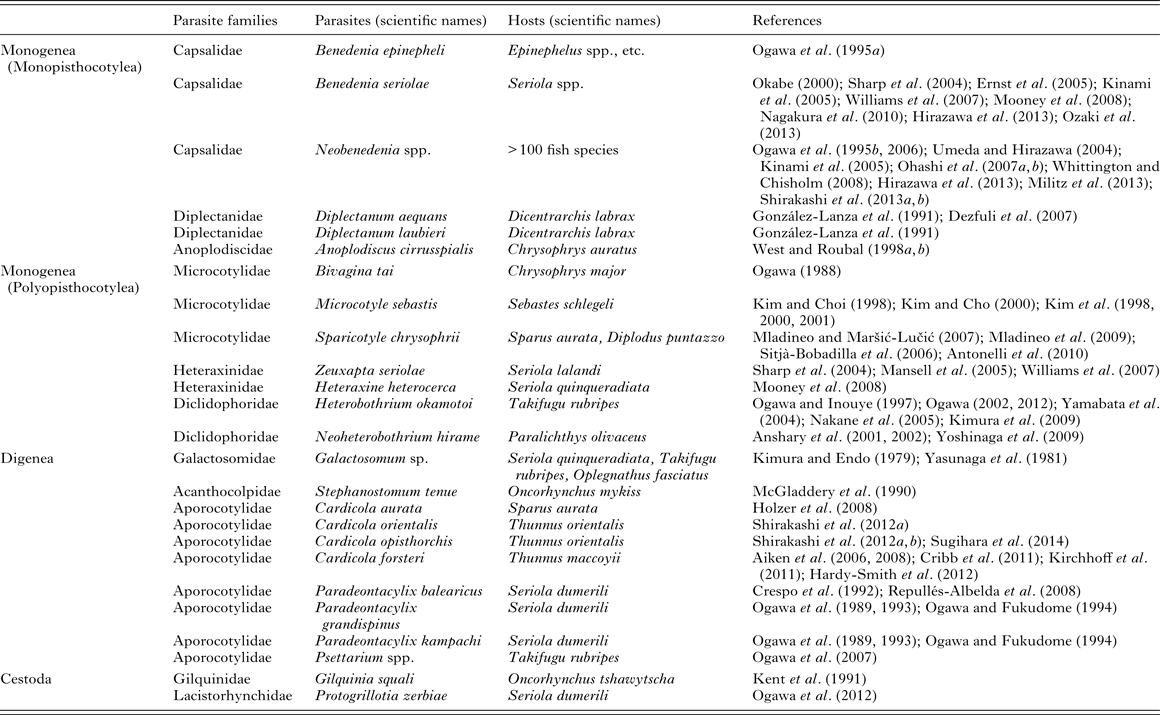
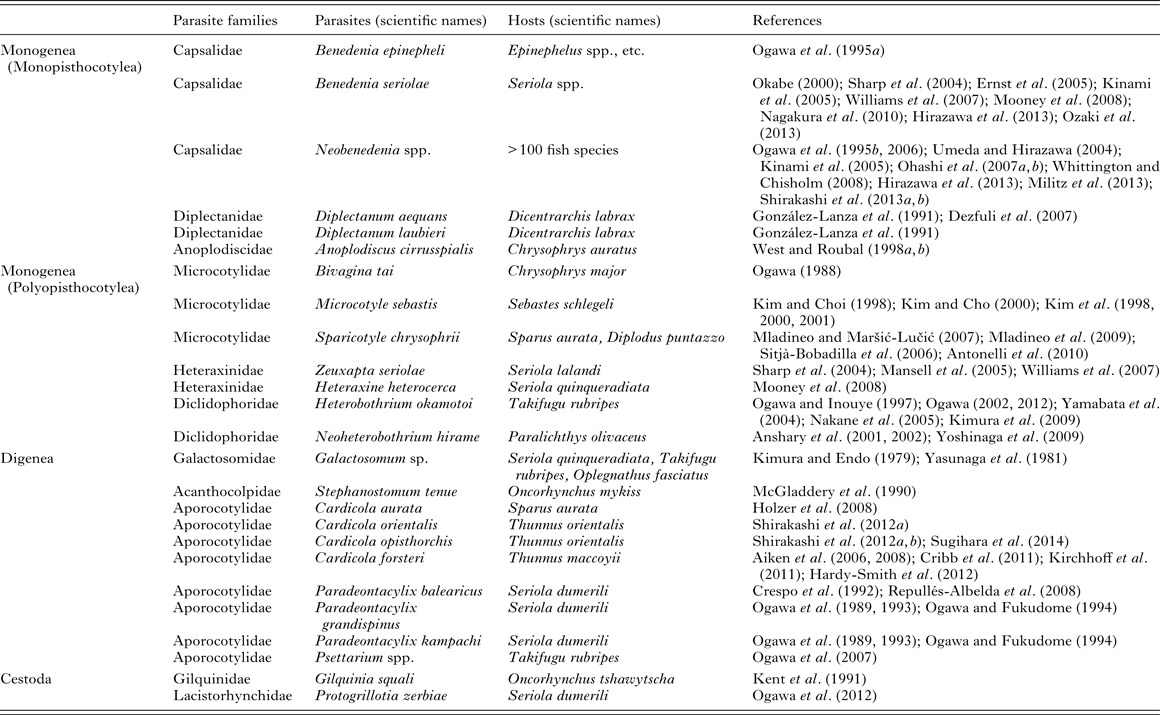
Monogeneans (Platyhelminthes: Monogenea) are hermaphrodites and mostly ectoparasites of fish, infecting the host's outer surfaces including the gills, skin and fins and less commonly buccal, branchial and nasal cavity linings. Except for the viviparous gyrodactylids, monogeneans are oviparous and typically, hatching larvae, or oncomiracidia, can swim in the water. Upon encounter with a suitable host, they either attach directly to the host surfaces or invade by inflowing currents to the gills, and start to grow. Between 4000 and 5000 species of monogeneans are currently described (Whittington and Chisholm, Reference Whittington, Chisholm, Eiras, Segner, Wahli and Kapoor2008). However, the actual number of species is much higher, as so many fish, including cultured ones, have not yet been fully examined for this group of parasites. Important monogeneans of maricultured fish are listed in Table 1.
Table 1. Platyhelminthes causing problems in mariculture
Monogeneans comprise the Monopisthocotylea and Polyopisthocotylea. Monopisthocotyleans are generally small, mostly up to 1 cm long. Most members of the families Dactylogyridae, Ancyrocephalidae, Diplectanidae and Gyrodactylidae are less than 1 mm long, and so are difficult to detect without the aid of a stereomicroscope. Polyopisthocotyleans are generally larger than monopisthocotyleans and some grow over 3 cm long. From species to species, clamps which grasp or suck gill tissues for attachment vary in shape and number (from eight to more than 100). In some polyopisthocotyleans, the body is asymmetrical due to the number and size of clamps being unequal between the two rows. Polyopisthocotyleans have a large uterus which can hold a large number of eggs.
Biology
Monopisthocotyleans basically feed on epidermis, whereas polyopisthocotyleans feed exclusively on host blood. In most cases monogeneans show a high degree of host specificity. However, some monogeneans show almost no host specificity, like the capsalids Neobenedenia melleni, having been recovered from more than 100 species of fish worldwide (Whittington and Chisholm, Reference Whittington, Chisholm, Eiras, Segner, Wahli and Kapoor2008) and Neobenedenia girellae (a synonym of N. melleni according to Whittington and Horton, Reference Whittington and Horton1996) and Benedenia epinepheli from 15 and seven species, respectively, of farmed fish in Japan (Ogawa et al. Reference Ogawa, Bondad-Reantaso, Fukudome and Wakabayashi1995a , Reference Ogawa, Bondad-Reantaso and Wakabayashi b ). This does not mean that all fish species show the same degree of susceptibility to these monogeneans. For example, susceptibility against N. girellae infection differed among greater amberjack, Japanese amberjack and bastard halibut (Ohno et al. Reference Ohno, Kawano and Hirazawa2008). When these three species of fish with approximately the same body size were exposed to N. girellae oncomiracidia in a tank, the parasite infected more intensely and grew faster in greater amberjack than in the other two fish species.
Biological parameters of monogeneans, such as generation time, fecundity and longevity, have been poorly studied or are completely unknown (Whittington and Chisholm, Reference Whittington, Chisholm, Eiras, Segner, Wahli and Kapoor2008). Farmed fish may provide a better source of information on monogenean biology than wild fish, as we can make a regular monitoring of infection under controlled conditions. Still, data obtained from farmed fish can be biased compared with those from wild fish, as they are kept under different conditions. Table 2 summarizes some biological data of monogeneans infecting marine fish cultured commercially or experimentally, which show variance with parasite species, host responses, water temperature (WT) and culture conditions. Most of the data in Table 2 are based on experiments where fish were kept in aquaria and exposed to oncomiracidia.
Table 2. Biological data on monogeneans from marine fish in culture conditions

* : estimated number based on daily egg output X duration of adult stage.
Concerning the generation time (time required for maturation on host fish), the monopisthocotyleans Anoplodiscus cirrusspiralis (Anoplodiscidae), Diplectanum aequans (Diplectanidae), Benedenia seriolae and N. girellae have a shorter pre-patent period of 2–4 weeks at 20–25 °C (Kearn et al. Reference Kearn, Ogawa and Maeno1992; Bondad-Reantaso et al. Reference Bondad-Reantaso, Ogawa, Fukudome and Wakabayashi1995a ; Cecchini et al. Reference Cecchini, Saroglia, Berni and Cognetti-Varriale1998; West and Roubal, Reference West and Roubal1998a ; Tubbs et al. Reference Tubbs, Poortenaar, Sewell and Diggles2005; Lackenby et al. Reference Lackenby, Chambers, Ernst and Whittington2007; Hirayama et al. Reference Hirayama, Kawano and Hirazawa2009) than the polyopisthocotyleans Zeuxapta seriolae (Heteraxinidae), Heterobothrium okamotoi (Diclidophoridae) and Neoheterobothrium hirame (Diclidophoridae) of 4–7 weeks in the same range of WT (Ogawa and Inouye, Reference Ogawa and Inouye1997; Tsutsumi et al. Reference Tsutsumi, Yoshinaga, Kamaishi, Nakayasu and Ogawa2003; Tubbs et al. Reference Tubbs, Poortenaar, Sewell and Diggles2005) (Table 2).
The lifespan is largely affected by WT, but no fundamental difference is seen between monopisthocotyleans and polyopisthocotyleans (Table 2). Longevity of Bivagina tai (Microcotylidae) was estimated from a 2-year field survey of a single population of red seabream, Chrysophrys major (as Pagrus major) maintained in an experimental net cage, in which the number of clamps was used as an indicator of parasite age (Ogawa, Reference Ogawa1988). It should be noted that A. cirrusspiralis on susceptible fish lived longer that those on resistant fish (West and Roubal, Reference West and Roubal1998b ).
Water temperature and experimental conditions aside, fecundity of monogeneans varies widely. For example, A. cirrusspiralis has a potential to deposit in excess of 3000 eggs throughout its lifespan (West and Roubal, Reference West and Roubal1998a ), whereas B. seriolae produced a mean value of 1398 eggs in one day (Mooney et al. Reference Mooney, Ernst and Whittington2008), suggesting a much higher fecundity by the latter parasite. Only a few data are available on the lifetime egg output by monogeneans. Heterobothrium okamotoi produced an average of 454 eggs per day at 25 °C (Yamabata et al. Reference Yamabata, Yoshinaga and Ogawa2004). Considering its generation time, longevity and a daily egg output, a single H. okamotoi could lay up to 32 000 eggs at 25 °C. At high WT, monogeneans produce more eggs but have shorter lifespans than at low WT. However, no data are available for any fish monogenean on how different WTs affect the lifetime egg production. According to the same method of calculation as that used for H. okamotoi above, the lifetime egg production by N. hirame was estimated to be up to 40 000 eggs at 15 °C, 24 000 eggs at 20 °C and 18 000 eggs at 25 °C, the number being negatively correlated with WT (Tsutsumi et al. Reference Tsutsumi, Mushiake, Mori, Yoshinaga and Ogawa2002, Reference Tsutsumi, Yoshinaga, Kamaishi, Nakayasu and Ogawa2003).
Monogeneans of farmed marine fish often show seasonality in infection. Generally, high WT induces high reproductive potential for parasites. However, unexpectedly, high levels of infection were also experienced at low WT. For example, prevalence of infection of the monopisthocotyleans D. aequans and Diplectanum laubieri on the gills of European seabass Dicentrarchus labrax and the polyopisthocotylean Sparicotyle chrysophrii (Microcotylidae) on the gills of gilthead sea bream Sparus aurata were high in winter (González-Lanza et al. Reference González-Lanza, Alvarez-Pellitero and Sitja-Bobadilla1991; Antonelli et al. Reference Antonelli, Quilichini and Marchand2010). A monthly monitoring of B. tai infection of red seabream cultured in a single net cage for 2 years showed infection peaks in early summer and winter, with WT ranging from 10 to 28 °C (Ogawa, Reference Ogawa1988). The 0-year-old seabream had the highest level of infection in winter, while infection was modest in the following winter, suggesting the peak infection resulted from lowered resistance of the small fish to infection at low temperatures. WT was a major factor regulating these seasonal fluctuations, affecting both the reproductive potential of the parasites and resistance to infection by the host fish.
A mixed infection of B. seriolae and N. girellae sharing the same habitat on greater amberjack was monitored for 1 year (WT between 20 and 29 °C) (Kinami et al. Reference Kinami, Miyamoto, Yoshinaga, Ogawa and Nagakura2005). Neobenedenia girellae was dominant in October to February. With increasing WT, B. seriolae appeared and its ratio to N. girellae increased up to above 90% in June. Then, in July, N. girellae began to increase again and almost replaced B. seriolae in September. It appears that the observed seasonal fluctuation was affected by different optimal temperatures for reproduction of each parasite and possibly by competition between the two parasites.
Pathogenicity and associated host responses
Mass mortalities associated with monogenean infection sometimes occur among maricultured fish. For example, 100% mortality was recorded in N. girellae infection of greater amberjack cultured in Okinawa, Japan (Ogawa et al. Reference Ogawa, Bondad-Reantaso, Fukudome and Wakabayashi1995a ). Mass mortality of yellowtail amberjack heavily infected with Z. seriolae cultured in cages was reported in the Mediterranean (Grau et al. Reference Grau, Crespo, Pastor, González and Carbonell2003).
Monopisthocotyleans including Capsalidae, Diplectanidae, Anoplodiscidae, Ancyrocephalidae and Gyrodactylidae are known to be harmful to maricultured fish. Pathological changes include excess secretion of mucus, haemorrhage, tissue loss due to feeding activities and inflammatory reactions to parasite attachment such as hyperplasia around their attachment organs (Buchmann and Bresciani, Reference Buchmann, Bresciani and Woo2006; Whittington and Chisholm, Reference Whittington, Chisholm, Eiras, Segner, Wahli and Kapoor2008). In D. aequans infection on the gills of European seabass, the attachment sites were marked by haemorrhages and a white mucoid exudate. Hyperplasia of the epithelium and inflammatory and haemorrhagic foci, especially around the areas of parasite attachment were observed (Fig. 2A). The opisthaptor penetrates deeply into the gills, inducing disruption and fusion of the secondary lamellae (González-Lanza et al. Reference González-Lanza, Alvarez-Pellitero and Sitja-Bobadilla1991; Dezfuli et al. Reference Dezfuli, Giari, Simoni, Menegatti, Shinn and Manera2007).
Capsalids generally cause considerable damage to the host skin through the attachment by the haptor and feeding activity. An infection experiment in a small aquarium demonstrated that N. girellae infection affected growth of greater amberjack with a parasite density of more than 0·285±0·042/cm2 fish surface (Hirayama et al. Reference Hirayama, Kawano and Hirazawa2009). The epidermis of infected fish became thinner as infection was prolonged. Furthermore, in B. seriolae and N. girellae infection of cultured Seriola spp., skin lesions such as ulcers and scale loss formed by the feeding and attaching activities of the parasites deteriorate after the fish rub their body against net cages to get rid of the parasites (Fig. 1). Neobenedenia girellae tended to gather on the eyes of cobia Rachycentron canadum cultured in Taiwan (Ogawa et al. Reference Ogawa, Miyamoto, Wang, Lo and Kou2006). The epithelial layer of the cornea was often partially lost, and the collagenous stroma was thickened, oedematous and associated with massive inflammatory cell infiltration (Ogawa et al. Reference Ogawa, Miyamoto, Wang, Lo and Kou2006).

Fig. 1. Extensive haemorrhage on the skin and ragged tail fin of greater amberjack, Seriola dumerili, caused by N. girellae infection (top) and rubbing its body to get rid of the parasite that followed (bottom) (photos kindly provided by Dr Sho Shirakashi).
Fish heavily infected with polyopisthocotyleans such as Microcotylidae, Heteraxinidae and Diclidophoridae show emaciation, dark body colour, slow swimming and anorexia (Ogawa, Reference Ogawa2002). These non-specific disease signs are mainly caused by anaemia (Fig. 2B). Negligible pathological changes are usually associated in the attachment sites (Fig. 2C). However, in Z. seriolae infection of yellowtail amberjack Seriola lalandi, hyperplasia and fusion of gill lamellae were observed away from the parasite attachment sites (Mansell et al. Reference Mansell, Powell, Ernst and Nowak2005). It was speculated that those changes were caused by feeding activity of the parasite. Heterobothrium okamotoi on Japanese pufferfish also causes anaemia and severe pathological changes in the branchial cavity wall. Severe epithelial hyperplasia and infiltration of inflammatory cells into the dermis and subcutaneous tissue were observed. As a result, its haptor and posterior body became embedded in host tissues (Fig. 2D, E). The epithelial lining around the parasite is degenerative and discontinuous due to actions generated by the clamps, leading to invasion of seawater around the parasite. The tissue surrounding the parasite is necrotic, giving off a putrid smell (Ogawa, Reference Ogawa2002). Neoheterobothrium hirame infects the buccal cavity wall of bastard halibut, with its posterior body and haptor embedded in host tissue, inducing significant inflammation and hyperplasia at the parasite attachment site (Anshary and Ogawa, Reference Anshary and Ogawa2001). Leucocytes constituting monocytes/macrophages, granulocytes and dense granular cells infiltrated and adhered to the parasite tegument (Nakayasu et al. Reference Nakayasu, Tsutsumi, Yoshitomi, Yoshinaga and Kumagai2003). The tegument was partially disrupted and phagocytized by infiltrating host cells, leading to the death and elimination of the parasite (Nakayasu et al. Reference Nakayasu, Tsutsumi, Oseko and Hasegawa2005).

Fig. 2. Pathogenicity of monogeneans. (A) anaemia caused by heavy Zeuxapta infection of greater amberjack, Seriola dumerili; (B) histological section of the gill of greater amberjack infected with Zeuxapta japonica (scale: 0·5 mm); (C) gills of European seabass, Dicentrarchus labrax infected with Diplectanum aequans (scale: 0·1 mm) (photo kindly provided by Dr Ariadna Sitjà-Bobadilla); (D) skin of the branchial cavity wall of Japanese pufferfish, Takifugu rubripes pinched by clamps of Heterobothrium okamotoi (scale: 0·5 mm); E: advanced pressure atrophy of host tissues caused by H. okamotoi in Japanese pufferfish (scale: 0·5 mm).
Generally, fish develop little or no immunity against ectoparasites. However, innate and acquired protection against monogenean infection have been suggested in many cases. Most studies on the mechanisms that underlie host reactions have been carried out in freshwater fish (see Buchmann and Bresciani, Reference Buchmann, Bresciani and Woo2006). Studies on the immune responses against monogenean infections of farmed marine fish are fragmentary, as discussed below.
Populations of silver seabream showed different degrees of innate resistance against the monogenean A. cirrusspiralis (West and Roubal, Reference West and Roubal1998b ). Fewer than 50% of oncomiracidia that attached successfully reached maturity. In spite of such innate resistance, intense infection did not confer subsequent protection (West and Roubal, Reference West and Roubal1998b ). Lectins may act as an innate element of resistance. A novel mannose-specific lectin named as pufflectin was detected in epithelial cells in the skin, gills and oral cavity of Japanese pufferfish (Tsutsui et al. Reference Tsutsui, Tasumi, Suetake and Suzuki2003), which binds to H. okamotoi under in vitro conditions (Tsutsui et al. Reference Tsutsui, Tasumi, Suetake, Kikuchi and Suzuki2005). This lectin may be involved in the innate protection against H. okamotoi.
There are several reports that marine fish infected with monogeneans acquire resistance or immunity against re-infection. Primed bastard halibut, which had been previously infected with N. girellae and treated by freshwater bath, had lower intensities of infection and smaller body size of parasites in secondary infections, compared with those of naïve control fish (Bondad-Reantaso et al. Reference Bondad-Reantaso, Ogawa, Yoshinaga and Wakabayashi1995b ). Alternatively, such apparent acquired resistance may actually be resource depletion and subsequent parasite decline (Lindenstøm and Buchmann, Reference Lindenstøm and Buchmann2000; Hirayama et al. Reference Hirayama, Kawano and Hirazawa2009). However, it seemed that antibodies against the monogenean were not involved in this reaction, as the level of antibody in the sera of primed fish was not raised. When fish were immunized by injection with sonicated parasite antigen, there was no significant difference in the parasite counts between antigen-injected fish and PBS-injected control after challenge with N. girellae oncomiracidia. These results indicate that the protection induced by the previous infection was not associated with the humoral antibody (Bondad-Reantaso et al. Reference Bondad-Reantaso, Ogawa, Yoshinaga and Wakabayashi1995b ).
Kim et al. (Reference Kim, Hwang, Cho and Park2000) reported a different kind of host immune response against monogenean infection. They suggested that Korean rockfish, Sebastes schlegeli, can acquire some degree of immunity against Microcotyle sebastis (Microcotylidae) infection through specific and non-specific immune stimuli. Rockfish injected with homogenized parasite antigen emulsified in an equal volume of Freund's complete adjuvant (FCA) or with FCA had significantly lower intensities of infection than controls which received PBS injection after challenge with parasite eggs (Kim et al. Reference Kim, Hwang, Cho and Park2000).
Farmed Japanese pufferfish persistently infected with H. okamotoi for longer than 1 year developed protection against infection. Infection experiments suggest they showed resistance against re-infection on the following three occasions: settlement of oncomiracidia on gills, early developmental stages when the mode of attachment changes from hooks to clamps, and migration of immature worms from the gills to the branchial cavity wall (Nakane et al. Reference Nakane, Ogawa, Fujita, Sameshima and Wakabayashi2005). Infection induced strong host inflammatory reactions and antibody production against the parasite (Wang et al. Reference Wang, Kim, Sameshima and Ogawa1997; Nakane et al. Reference Nakane, Ogawa, Fujita, Sameshima and Wakabayashi2005). However, such immune responses were not effective enough to eliminate infection. Neoheterobothrium hirame first infects the gills of bastard halibut and moves to the buccal cavity wall for maturation. In infection experiments, halibut produced antibody against the parasite after the movement to the buccal cavity wall. Antibody production was further enhanced after death of the parasite induced a strong host reaction (Tsutsumi et al. Reference Tsutsumi, Yoshinaga, Kamaishi, Nakayasu and Ogawa2003). How effectively the inflammatory response and subsequent antibody production by halibut induce immunity to re-infection remains to be studied.
Control methods
Control methods given below are not always applicable to every fish species and to all types of mariculture systems. Generally, application of a single method, if effective to some extent, will not be enough to control infection. Combinations of multiple methods are recommended.
Chemical treatment
Many chemical methods have been developed and used to control monogenean infections of maricultured fish. Although chemotherapy is applied on many occasions, it should be noted that development of resistance to selected chemical agents has been suggested in some freshwater monogeneans (Goven et al. Reference Goven, Gilbert and Gratzek1980; Buchmann et al. Reference Buchmann, Roepstorff and Waller1992). Traditionally, freshwater bath treatment for up to 10 min has been practiced against B. seriolae infection of Japanese amberjack. This method is effective against monopisthocotyleans such as B. seriolae and N. girellae and can eradicate worms completely from fish. However, care should be taken that after the freshwater bath treatment, both greater amberjack and Japanese amberjack became more susceptible to re-infection with N. girellae (Ohno et al. Reference Ohno, Kawano and Hirazawa2009). Besides, where transport of fresh water to offshore cages is difficult, it is not practical for commercial scale treatment. It is to be noted that freshwater bathing is generally ineffective against polyopisthocotyleans.
NaCl-supplemented seawater bathing in a small tank was used to eradicate N. hirame from bastard halibut (Yoshinaga et al. Reference Yoshinaga, Kamaishi, Segawa and Yamamoto2000). More than 90 and 100% of immature worms were detached from the gills after 30 min and 60 min treatment, respectively, but it was not effective against adults on the buccal cavity wall. All fish were normal after the bathing, but this method can only be applied as a small-scale treatment.
Freshwater bathing and NaCl-supplemented seawater bathing are effective and inexpensive methods. However, there are some limitations in practice in the field as mentioned above. In this sense, chemicals which can be given orally as in-feed therapy or can be mixed freely with seawater as a bathing treatment have advantages over freshwater and NaCl-supplemented seawater bathing. In Japan, hydrogen peroxide bathing is approved against infections with B. seriolae of Seriola spp. at 660 ppm for 3 min, N. girellae and H. okamotoi of Japanese pufferfish at 660 ppm for 20 min and 1320 ppm for 20–30 min, respectively, and B. tai of red seabream at 660 ppm for 3 min. The use of this chemical is only allowed for the above combinations of parasites and fish under Japanese legislation. In Australia, hydrogen peroxide bathing is used to remove Z. seriolae from the gills of yellowtail amberjack at 300 ppm for 10 min (Mansell et al. Reference Mansell, Powell, Ernst and Nowak2005). Care must be taken because hydrogen peroxide increases its toxicity to the host at a high temperature (25 °C and higher).
Effective in-feed chemicals against marine monogeneans include a synthetic anthelminthic, praziquantel and benzimidazole-based compounds, mebendazole and febantel. Results of treatment trials with in-feed chemicals (Table 3) generally yielded varying rates of eradication from infected fish. Among them, praziquantel (PZQ) is the most widely used chemical, effective for both monopisthocotyleans and polyopisthocotyleans. However, care should be taken since medicated pellets can affect palatability of feed (Sitjà-Bobadilla et al. Reference Sitjà-Bobadilla, Conde de Felipe and Alvarez-Pellitero2006; Williams et al. Reference Williams, Ernst, Chambers and Whittington2007). The efficacy of PZQ is significantly increased by administering cimetidine concurrently due to increased bioavailability of PZQ (Kim et al. Reference Kim, Lee, Kwon and Cho2001). Oral administration of mebendazole and bithionol were also used to remove M. sebastis from Korean rockfish (Kim and Choi, Reference Kim and Choi1998; Kim et al. Reference Kim, Park and Jee1998). In Japan, febantel is an approved chemical against H. okamotoi infection of Japanese pufferfish (Kimura et al. Reference Kimura, Nomura, Kawakami, Itano, Iwasaki, Morita and Enomoto2009). Its administration is effective to remove both immature worms on the gills and adults on the branchial cavity wall. Oral intubation and bath administration of the above compounds are usually quite limited in practice. However, it is to be noted that bathing of 100 ppm PZQ for 4 min almost eradicated M. sebastis from Korean rockfish kept in a net cage (Kim and Cho, Reference Kim and Cho2000).
Table 3. In-feed administration of chemicals to reduce monogeneans from host

Removal or inactivation of parasite eggs
Eggs of B. seriolae incubated at 30 °C did not hatch (Ernst et al. Reference Ernst, Whittington, Corneillie and Talbot2005). Hatching of the eggs of N. girellae kept in low salinities was suppressed (Umeda and Hirazawa, Reference Umeda and Hirazawa2004). However, such temperature and salinity manipulations are usually not practical in net cage culture systems.
Monogeneans such as capsalids and most polyopisthocotyleans deposit eggs with a long filament or in the form of long strings, which can entangle with the net meshing. Hatched oncomiracidia from eggs on the culture net can easily encounter suitable hosts kept in the net. Once such monogeneans establish infection on cage-cultured fish, infection can rapidly spread among fish in the same cage and adjacent cages. To prevent heavy infections, frequent net change is commonly practiced to remove entangled eggs, where the host fish of the above monogeneans are cultured. Drying of nets is effective in killing eggs of B. seriolae, as no hatching of eggs was observed after desiccation of eggs for 3 min (Ernst et al. Reference Ernst, Whittington, Corneillie and Talbot2005).
Bastard halibut cultured in land-based, circular tanks are often infected with N. hirame. Unlike other typical polyopisthocotyleans, its eggs are provided with only short processes on both ends, which are unable to entangle with substrates within the tanks. The eggs can be washed out by overflowing culture water from the drain pipe in the centre of the tanks before hatch-out (Ogawa, unpublished observation).
Avoidance of infection source
In off-shore cage culture of yellowtail amberjack in Australia, tidal currents allow eggs/oncomiracidia of B. seriolae to reach amberjack kept 8 km away (Chambers and Ernst, Reference Chambers and Ernst2005). This result indicates the importance of the cage location to effectively avoid infection. This also suggests that once amberjacks become infected in cages, it is extremely difficult to prevent infection from spreading. This is the main reason why B. seriolae infection is everywhere in amberjack farms of Japan.
Manipulation of culture methods, using knowledge of parasite biology, may provide, if not perfect, a solution for infection control. Juvenile greater amberjack placed in a shaded small experimental cage and exposed to N. girellae oncomiracidia had about 70% lower intensity of infection than those kept in a non-shaded cage (Shirakashi et al. Reference Shirakashi, Hirano, Asmara, Noor, Ishimaru and Miyashita2013a ). Juvenile greater amberjack were placed in a small cage and exposed to N. girellae oncomiracidia at depths of 0, 2 or 4 m. The intensity of infection was reduced by up to 80 and 95% in fish kept at depths of 2 and 4 m, respectively, compared with fish at 0 m (Shirakashi et al. Reference Shirakashi, Hirano, Ishitani and Ishimaru2013b ). These results may reflect positive phototaxis by the oncomiracidium. Modifications of culture techniques such as shading and submergence of culture nets may represent effective control measures (Shirakashi et al. Reference Shirakashi, Hirano, Asmara, Noor, Ishimaru and Miyashita2013a , Reference Shirakashi, Hirano, Ishitani and Ishimaru b ). Further studies will be needed to establish if these methods are as effective on a larger scale and if they are as effective to other monogenean species.
Selective breeding of fish strains with parasite resistance
Susceptibility of Japanese amberjack individuals to B. seriolae infection showed heritable variation, indicating that the host genes play a significant role in determining infection levels against the parasite (Nagakura et al. Reference Nagakura, Yoshinaga, Sakamoto, Hattori and Okamoto2010). Through genome-wide and chromosome-wide linkage analyses using F1 families of Japanese yellowtail based on a high-density linkage map with microsatellite and single nucleotide polymorphism markers, two major quantitative trait loci (QTL) regions were identified (Ozaki et al. Reference Ozaki, Yoshida, Fuji, Kubota, Kai, Koyama, Nakagawa, Hotta, Tsuzaki, Okamoto, Araki and Sakamoto2013). The results will help resolve the mechanism of resistance to B. seriolae infection of Japanese yellowtail and could be used to breed resistant strains. Yellowtail culture in Japan is dependent on seed from wild stock. Selective breeding of resistant strains of artificially produced seeds may eventually replace wild-caught seed.
Other new approaches
An in-feed preventative agent can be a practical alternative to bathing treatment in capsalid infections. Barramundi, Lates calcarifer, were fed pellets supplemented with garlic extract for 30 days. When exposed to Neobenedenia sp. oncomiracidia, fish became infected up to 70% less than controls (Militz et al. Reference Militz, Southgate, Carton and Hutson2013). Besides, no negative effect on palatability of the feed was recorded. Ohashi et al. (Reference Ohashi, Umeda, Hirazawa, Ozaki, Miura and Miura2007a ) purified glycoproteins from skin mucus of Japanese pufferfish, which could induce attachment of N. girellae oncomiracidia. Ohashi et al. (Reference Ohashi, Umeda, Hirazawa, Ozaki, Miura and Miura2007b ) successfully produced sterile N. girellae by introducing double-stranded RNA of vasa-related genes, essential for germ cell development. These results have not yet been applied on a larger scale for the control against this capsalid infection of maricultured fish.
DIGENEA
Digeneans (Platyhelminthes: Trematoda) infecting fish are hermaphrodites with the exceptions of some Didymozoidae, in which a male and female pair live together in a capsule. Life cycles of digeneans involve two or three hosts. Fish serve as intermediate hosts or final hosts. Adult worms are flat and leaf-shaped, have an oral sucker and a ventral one called an acetabulum, and infect various sites, mostly and typically alimentary tracts, of hosts. Blood flukes of fish, all belonging to Aporocotylidae, usually have no suckers; instead their lateral body is covered ventrally with rows of spines.
Most digeneans of fish are one to several mm in total length, with exceptionally large worms like some didymozoids of over 1 m (Bullard and Overstreet, Reference Bullard, Overstreet, Eiras, Segner, Wahli and Kapoor2008). The number of fish digeneans described is steadily increasing and will soon probably exceed the number of extant fish of about 28 000 species (Bullard and Overstreet, Reference Bullard, Overstreet, Eiras, Segner, Wahli and Kapoor2008). Important digeneans infecting maricultured fish are listed in Table 1.
Biology
As adult worms, fish digeneans are most common in the alimentary tracts of fish, and generally show negligible effects on their hosts, whereas aporocotylids representing blood flukes of fish infect the vascular system, sometimes causing mass mortalities of their hosts. Didymozoids form visible capsules in gills and visceral organs of marine fish (Fig. 4A), which are not harmful but may decrease or completely destroy the market value because of the unaesthetic appearance of infected fish.
Seasonality of digeneans of farmed marine fish is not always clear, as infection depends on the feeding of the fish on intermediate hosts harbouring metacercariae. Blood fluke infections are exceptions. Infection of farmed 0-year-old greater amberjack with Paradeontacylix grandispinus and Paradeontacylix kampachi (Aporocotylidae) was first detected in November, peaked in March and decreased toward July, judging by the number of accumulated eggs in the gills (Ogawa et al. Reference Ogawa, Andoh and Yamaguchi1993). A similar observation was made in Cardicola aurata (Aporocotylidae) infection of gilthead seabream, S. aurata cultured in Spain, in which the eggs in the gills were observed from November to May–June, with a peak in April (Holzer et al. Reference Holzer, Montero, Repullés, Nolan, Sitja-Bobadilla, Alvarez-Pellitero, Zarza and Raga2008). These cases suggest that the cercarial invasion peaked in winter. Fish as second intermediate hosts of digeneans harbour metacercariae in various tissues and organs. Cercarial emergence from the first intermediate host and subsequent invasion into the fish host has seasonality (Paperna and Dzikowski, Reference Paperna, Dzikowski and Woo2006), but seasonality in the metacercarial occurrence in fish becomes obscured, as they accumulate with fish age.
Our knowledge of the generation time and longevity of digeneans infecting maricultured fish is limited. The culture industry of southern bluefin tuna, Thunnus maccoyii, in South Australia is based on the capture of 2–3-year-old wild fish and growing-out in sea cages for 2–8 months (Aiken et al. Reference Aiken, Hayward, Crosbie, Watts and Nowak2008). The lifespan of the blood fluke Cardicola forsteri in cultured southern bluefin tuna is estimated to be a minimum of 33 days to a maximum of 95 days (Aiken et al. Reference Aiken, Hayward, Cameron and Nowak2009). Most culture seed of Pacific bluefin tuna are wild juveniles. Young Pacific bluefin tuna (300–930 g in body weight) caught in the Sea of Japan for culture seed were 100% infected with Didymocystis wedli (Didymozoidae) in the gills (Takebe et al. Reference Takebe, Saeki, Masuma, Nikaido, Ide, Shiozawa and Mano2013). Regular monitoring of the infected fish after introduction into farms showed that the prevalence of infection kept decreasing and that the parasite capsules disappeared from fish within 5 months. This suggests that the didymozoid in the wild seeds will not live beyond the culture period. Further, no transmission of this digenean to other Pacific bluefin tuna kept in the farm occurred, suggesting the infection cycle was not established within the farm, and thus no special treatment was needed for control.
Blood fluke infections have been reported in many farmed fish species of Carangidae, Sparidae and Tetraodontidae. Despite big economic losses caused by these parasites, infections are not under control mainly because our knowledge of their life cycles is limited. The life cycles of only three species have been elucidated: Aporocotyle simplex (Aporocotylidae) infecting wild pleuronectid fishes (Køie, Reference Køie1982; Køie and Petersen, Reference Køie and Petersen1988), C. forsteri infecting southern bluefin tuna and Cardicola opisthorchis infecting Pacific bluefin tuna (Sugihara et al. Reference Sugihara, Yamada, Tamaki, Yamanishi and Kanai2014). In all cases, the intermediate hosts are terebellid polychaetes, Artacama proboscidea and Lanassa nordenskioeldi for A. simplex, Longicarpus modestus for C. forsteri and Terebella sp. for C. opisthorchis (Køie, Reference Køie1982; Køie and Petersen, Reference Køie and Petersen1988; Cribb et al. Reference Cribb, Adlard, Hayward, Bott, Ellis, Evans and Nowak2011; Sugihara et al. Reference Sugihara, Yamada, Tamaki, Yamanishi and Kanai2014). For C. forsteri, the intermediate host, though a single specimen was found infected, was collected from a sediment sample in the immediate vicinity of tuna cages (Cribb et al. Reference Cribb, Adlard, Hayward, Bott, Ellis, Evans and Nowak2011). The infected terebellid had hundreds of sporocysts in the coelom. The cercariae were small, with a mean body length of 71 μm and had a short tail. It was speculated that the cercariae are not active swimmers and are thus heavily dependent on currents for dispersal (Cribb et al. Reference Cribb, Adlard, Hayward, Bott, Ellis, Evans and Nowak2011).
Pathogenicity and associated host responses
Relatively few digenean species harm farmed fish (Bullard and Overstreet, Reference Bullard, Overstreet, Eiras, Segner, Wahli and Kapoor2008). Among the few harmful digeneans, blood flukes can cause mass mortalities of maricultured fish. For example, 50% to more than 80% of juvenile greater amberjack were lost in one month due to P. grandispinus and P. kampachi infection in Japan (Ogawa and Fukudome, Reference Ogawa and Fukudome1994) and more than half of Japanese pufferfish were killed by an unidentified Psettarium (Aporocotylidae) (designated as Psettarium sp. TPC) within 3 months after puffers were introduced from China (Ogawa et al. Reference Ogawa, Nagano, Akai, Sugita and Hall2007).
In Japan, cultured 0-year-old greater amberjack were heavily infected with P. grandispinus and P. kampachi in the heart and gill blood vessels. Their eggs accumulated in the gills, and the number sometimes exceeded 1 million eggs per fish (Ogawa et al. Reference Ogawa, Andoh and Yamaguchi1993). Dead fish were characterized by opened mouth and opercula, showing typical signs of asphyxiation (Fig. 4B) (Ogawa and Fukudome, Reference Ogawa and Fukudome1994). Pathological changes were limited to the heart and gills with hyperplasia of gills, encapsulation of eggs in the gills and ventricle and papillate proliferation of the endothelium in the afferent branchial arteries (Fig. 3A) (Ogawa et al. Reference Ogawa, Hattori, Hatai and Kubota1989). Nodules were formed around the eggs, and extensive hyperplasia around them resulted in lamellar fusion, finally leading to hyperplastic clubbing of filaments (Fig. 3B). In spite of these host responses, most eggs in the gills developed normally to miracidia (Fig. 3B) and hatched out, whereas the eggs in the heart ventricle and most of the eggs at the base of the gill filaments were killed by the encapsulation and showed different stages of degeneration (Fig. 3C, D) (Ogawa et al. Reference Ogawa, Hattori, Hatai and Kubota1989). Crespo et al. (Reference Crespo, Grau and Padros1992) reported mass mortality of juvenile greater amberjack cultured in Spain associated with unidentified blood flukes. Two species of Paradeontacylix have been described from S. dumerili in the Mediterranean, of which the causative blood fluke of the mass mortality could be P. balearicus, due to the presence of parasite eggs in the gill lamellae (Repullés-Albelda et al. Reference Repullés-Albelda, Montero, Holzer, Ogawa, Hutson and Raga2008).

Fig. 3. Pathogenicity of digeneans – 1. (A) Cross section of an afferent branchial artery of greater amberjack, Seriola dumerili, heavily infected with Paradeontacylix spp. (scale: 0·1 mm); (B) Paradeontacylix eggs containing fully grown miracidia in the gill of greater amberjack (scale: 0·05 mm); (C) Paradeontacylix eggs encapsulated with the heart ventricle tissue of greater amberjack (scale: 0·1 mm); (D) Mass of encapsulated Paradeontacylix eggs at the basal part of gill filaments of greater amberjack (scale: 0·1 mm); (E) fresh brain tissue of red seabream, Chrysophrys major infected with Galactosomum sp. metacercaria (scale: 0·5 mm) (photo kindly provided by Mr Yukitaka Sugihara).

Fig. 4. Pathogenicity of digeneans – 2. (A) capsules of Didymocystis wedli on the gills of Pacific bluefin tuna, Thunnus orientalis; (B) suffocated juvenile greater amberjack, Seiola dumerili due to Paradeontacylix infection.
Similar pathological changes were observed in juvenile Pacific bluefin tuna infected with Cardicola orientalis and C. opisthorchis (Aporocotylidae) (Shirakashi et al. Reference Shirakashi, Kishimoto, Kinami, Katano, Ishimaru, Murata and Ogawa2012a ). Mixed infection with these two Cardicola species was common. The blood flukes could be identified by the morphology of the accumulated eggs in the gills; smaller, oval-shaped eggs, with a mean length of 44·0 μm, in the gill lamellae were produced by C. orientalis and larger, crescent-shaped eggs with a mean length of 52·1 μm that occurred primarily in the filamentary arteries, by C. opisthorchis (Shirakashi et al. Reference Shirakashi, Kishimoto, Kinami, Katano, Ishimaru, Murata and Ogawa2012a ). The number of eggs in the gills was highly variable among filaments. In a heavily infected 0-year-old fish, more than 9 million eggs were present.
Psettarium sp. TPC infected the visceral vascular system of farmed Japanese pufferfish, unlike the above cases of Paradeontacylix and Cardicola, which infect the heart and gill blood vessels of host fish (Ogawa et al. Reference Ogawa, Nagano, Akai, Sugita and Hall2007). Eggs accumulated in visceral organs such as the spleen, liver, testis, intestine and less frequently the gills. Egg masses in the visceral organs could sometimes be recognized as white spots in gross observations, suggestive of malfunction of these organs.
Very few metacercariae have been recorded as harmful parasites of maricultured fish, though intense metacercarial infections sometimes occur. Metacercariae of an acanthocolpid digenean Stephanostomum tenue (Acanthocolpidae) formed cysts in the bulbous arteriosus of maricultured rainbow trout, Oncorhynchus mykiss (as Salmo gairdneri). Degenerating larvae induced a severe inflammatory response and decreased respiration efficiency was responsible for mass mortalities of the host (McGladdery et al. Reference McGladdery, Murphy, Hicks, Wagner, Chen and Perkins1990). Metacercariae of the galactosomid Galactosomum sp. (Galactosomidae) formed cysts in the brain of marine fish including Japanese amberjack, Japanese pufferfish, barred knifejaw, Oplegnathus fasciatus (Yasunaga et al. Reference Yasunaga, Ogawa, Hirakawa, Hatai, Yasumoto and Yamamoto1981) and red seabream (Fig. 3E), causing trematode whirling disease. Infected fish showed whirling swimming near the water surface. Diseased fish usually had one, rarely two, encysted metacercariae in the interbrain. Neurons around the metacercaria were degenerative or necrotic due to mechanical pressure of the cyst (Kimura and Endo, Reference Kimura and Endo1979). It is assumed that this abnormal swimming behaviour facilitates the final host, the black-tailed gull, Larus crassirostris, to find and catch the infected fish (Kamegai et al. Reference Kamegai, Yasunaga, Ogawa and Yasumoto1982).
There are reports that fish infected with blood flukes acquire resistance or immunity against re-infection. One-year-old greater amberjack which had survived Paradeontacylix spp. infection in the previous year received milder infections than 0-year-old fish. The older fish may have acquired some immunity against blood fluke infection (Ogawa et al. Reference Ogawa, Hattori, Hatai and Kubota1989). Southern bluefin tuna cultured in Australia were able to control C. forsteri infection over a 6-month grow-out period (Aiken et al. Reference Aiken, Hayward and Nowak2006). Antibody response to C. forsteri was initiated after transfer of wild tunas to sea cages in April, and antibody titres reached a peak in December, when the infection drastically decreased from a peak in May (Aiken et al. Reference Aiken, Hayward, Crosbie, Watts and Nowak2008). Tuna farmed for 16 months had significantly lower prevalences and abundances of infection than those farmed for 5 months, showing the former group had acquired resistance against the blood fluke infection (Aiken et al. Reference Aiken, Hayward, Crosbie, Watts and Nowak2008).
Control methods
Chemotherapy
Preliminary experiments demonstrated that oral treatment of Pacific bluefin tuna with praziquantel (PZQ) was effective to control C. opisthorchis infection in the heart (Shirakashi et al. Reference Shirakashi, Andrews, Kishimoto, Ishimaru, Sawada, Murata and Ogawa2012b ). The minimal effective dose for complete eradication was determined to be 7·5 mg kg−1 BW or higher for 3 consecutive days (Ishimaru et al. Reference Ishimaru, Mine, Shirakashi, Kaneko, Kubono, Okada, Sawada and Ogawa2013). Repeated treatment may be required, as small numbers of adults re-appeared in experimental fish at 3 or 5 weeks post treatment (Shirakashi et al. Reference Shirakashi, Andrews, Kishimoto, Ishimaru, Sawada, Murata and Ogawa2012b ). As noted in the control of monogenean infections with in-feed PZQ, medicated pellets can affect palatability of feed. PZQ is also effective to control C. forsteri infection of southern bluefin tuna though the minimum effective dose remains to be determined. A single oral intubation of PZQ at 75 mg kg−1 BW resulted in a significant reduction of the number of flukes in the hearts and eggs in the gills and myocardium (Hardy-Smith et al. Reference Hardy-Smith, Ellis, Humphrey, Evans, Evans, Rough, Valdenegro and Nowak2012).
Avoidance of infection source
In the case of S. tenue metacercariae infection of rainbow trout (McGladdery et al. Reference McGladdery, Murphy, Hicks, Wagner, Chen and Perkins1990), the first intermediate host Nassarius obsoletus and the final host, American eel Anguilla rostrata, were present in the vicinity of trout cages and removal of these natural hosts was impractical to avoid infection. Instead, placing net cages in water with over 7 m clearance from bottom successfully avoided cercarial invasion, reducing infection to a more or less negligible level (McGladdery et al. Reference McGladdery, Murphy, Hicks, Wagner, Chen and Perkins1990).
Kirchhoff et al. (Reference Kirchhoff, Rough and Nowak2011) monitored C. forsteri infection of southern bluefin tuna in two culture cages, one set near shore (about 30 km from shore; depth: 20 m) and the other offshore (about 46 km from shore; depth: 40 m). Six weeks after transfer of the cages to the two sites, offshore tuna had no C. forsteri infection, whereas a prevalence of 85% for the blood fluke was recorded in the near-shore tuna. The intermediate host, the terebellid polychaete, L. modestus, was collected from a bottom sample of 22 m in depth (Cribb et al. Reference Cribb, Adlard, Hayward, Bott, Ellis, Evans and Nowak2011). This polychaete is reported from the lower intertidal zone to a depth of 30 m (Hutchings and Glasby, Reference Hutchings and Glasby1988). It is possible then to reduce the blood fluke infection by physical separation from the intermediate host. From this, it may be deduced that cages should be set in deeper water as shown by Kirchhoff et al. (Reference Kirchhoff, Rough and Nowak2011), though such offshore setting is inconvenient for maintenance of tuna. In contrast, Terebella sp. infected with sporocysts of C. opisthorchis, a blood fluke of Pacific bluefin tuna, was collected not only from the bottom sediments (46 m deep), but also from the ropes attached to the tuna cages (2 m deep) (Sugihara et al. Reference Sugihara, Yamada, Tamaki, Yamanishi and Kanai2014). In this case, relocating tuna cages away from the source of infection appears difficult.
CESTODA
Cestodes (Platyhelminthes: Cestoda) are hermaphrodites and endoparasites of vertebrates including fish. Typically the body is long and flat, consisting of the scolex, the attachment organ to host and the neck which generates segments that follow posteriorly. They have two or three host life cycles. Fish serve as second intermediate hosts, infected through ingesting first intermediate hosts, or as final hosts, infected through ingesting second intermediate hosts. Second intermediate hosts harbour larval stages called plerocercoids or plerocerci in various tissues, whereas final hosts harbour adults in the alimentary tract.
Only a few cases of cestode infections are reported from maricultured fish (Table 1). Mortality of young chinook salmon Oncorhynchus tshawytscha cultured in net pens in Canada was caused by infections in the eye by plerocercoids of Gilquinia squali (Trypanorhyncha: Gilquiniidae) (Kent et al. Reference Kent, Margolis and Fournie1991). The definitive host for G. squali is picked dogfish Squalus acanthias (as spiny dogfish Squalus acanthus). The life cycle is unknown, but chinook salmon presumably became infected by ingesting the infected first intermediate host, probably a crustacean. The lens of heavily infected fish was opaque, suggesting cataractous changes.
Some larval cestodes cause lowered market value due to infections in the edible part of the fish. A blastocyst of plerocercoid of Trypanorhyncha was found in the skeletal muscle of marketable-sized greater amberjack (Fig. 5), though the infection was very rare, with the prevalence of infection as low as one out of tens of thousands of fish processed (Ogawa et al. Reference Ogawa, Iwaki, Itoh and Nagano2012). The cestode itself inside the blastocyst was not found, but molecular analysis of the small subunit ribosomal RNA gene revealed that the cestode to be Protogrillotia zerbiae (Lacistorhynchidae) (Tamaru, Klinger-Bowen, Ogawa, Iwaki, Kurashima and Itoh, unpublished data).

Fig. 5. A blastocyst of Protogrillotia zerbiae plerocercoid in the skeletal muscle of greater amberjack, Seriola dumerili.
PROBLEMS ASSOCIATED WITH INTERNATIONAL TRADE OF MARINE FISH FOR AQUACULTURE
Some fish platyhelminthes have expanded their host ranges and geographical distributions through anthropological activities. A hitherto unknown N. girellae was first recorded from farmed marine fish of Japan in the 1990s (Ogawa et al. Reference Ogawa, Bondad-Reantaso, Fukudome and Wakabayashi1995a ). The fact that juvenile greater amberjack imported from China as aquaculture seed was infected with this monogenean upon shipment to Japan and upon arrival in Japanese waters showed it is an introduced parasite. Neobenedenia girellae has been recorded from 15 species of farmed fish in Japan, posing a serious threat to Japanese aquaculture.
The diclidophorid N. hirame suddenly appeared in wild and farmed bastard halibut in Japan in the 1990s. First confirmation of infection was in 1993 on juvenile halibut collected in the Sea of Japan (Anshary et al. Reference Anshary, Ogawa, Higuchi and Fujii2001). The parasite rapidly expanded its distribution to the Pacific side in 1997 (Ogawa, Reference Ogawa, Woo and Buchmann2012), probably due to transfer of live juvenile and/or spawner halibut from the Sea of Japan side to the Pacific side for propagation and aquaculture purposes. Morphological and molecular evidence indicated that the original host for this monogenean is southern flounder Paralichthys lethostigma, naturally distributed on the Atlantic side of the USA (Yoshinaga et al. Reference Yoshinaga, Tsutsumi, Hall and Ogawa2009). This suggests that N. hirame was introduced to the Far East with live southern flounder, though the route of introduction remains unspecified. This is an example of host switch by parasites induced by human activities.
Young Japanese pufferfish suffered mass mortalities caused by the blood fluke Psettarium sp. TPC, morphologically different from the indigenous Psettarium sp. TPJ (Ogawa et al. Reference Ogawa, Nagano, Akai, Sugita and Hall2007). The host fish, imported from China to Japan, had already been infected, as the fish started to die within 3 days after arrival in Japan. Fortunately, no evidence was found that the parasite transmitted to wild and cultured domestic puffers.
PARASITE TRANSMISSION BETWEEN FARMED AND WILD FISH
Most parasites recovered from farmed fish are originally those of wild fish, and introduction of pathogens from the wild to farms is an important risk for sustainable aquaculture. Conversely, farmed fish may become sources of pathogens to wild fish, and release of pathogens from farms could negatively affect wild fish stocks. There are many reports and discussions on the parasite transmission between farmed and wild fish stocks, but problems lie in incongruence in the methods adopted for analysis of these phenomena (see Mladineo et al. Reference Mladineo, Šegvić and Grubišić2009).
European seabass D. labrax and gilthead seabream farmed in the Mediterranean did not share platyhelminthes with farm-associated wild bogue Boops boops and Mediterranean horse mackerel Trachurus mediterraneus (Fernandez-Jover et al. Reference Fernandez-Jover, Faliex, Sanchez-Jerez, Sasal and Bayle-Sempere2010). Farming had no effect on the total parasite community between farm-associated and non-farm-associated wild bogue and mackerel, but may be detrimental for some parasite species, while these same conditions, such as diet modification, could enhance others (Fernandez-Jover et al. Reference Fernandez-Jover, Faliex, Sanchez-Jerez, Sasal and Bayle-Sempere2010).
Mariculture conditions can give host-specific parasites a chance to expand their host ranges. A heavy infection with the microcotylid Polylabris tubicirrus, known only from breams of the genus Diplodus, occurred among gilthead seabream kept in raceways using recirculating water (Silan et al. Reference Silan, Cabral and Maillard1985). They speculated that the main cause of this unnatural infection was introduction of parasite eggs from the next raceway, where fish of the genus Diplodus, a natural host of this parasite, had been maintained. This is a case of host switch of a parasite, which occurred in a farm condition for the parasite to surmount the barrier of host specificity. The microcotylid S. chrysophrii is host specific to gilthead seabream. However, host switch of S. chrysophrii occurred from gilthead seabream to sharpsnout bream Diplodus puntazzo between net cages (Mladineo and Maršić-Lučić, Reference Mladineo and Maršić-Lučić2007). Abundance of S. chrysophrii was greater in the new host than the original host. In contrast, comparing the mtDNA cytochrome oxidase I locus of S. chrysophrii collected from farmed gilthead seabream and those from wild bogue Boops boops (Mladineo et al. Reference Mladineo, Šegvić and Grubišić2009) suggested that there was no transmission of this monogenean between farmed and wild fish in the Mediterranean.
Neoheterobothrium hirame infection of bastard halibut in Japan is another example of host switch by parasites, in which a parasite of wild fish in one country has transmitted to a different species of fish in a different country. With the introduction of this foreign parasite, both wild and farmed bastard halibut in Japan suffered heavy infections (Anshary et al. Reference Anshary, Yamamoto, Miyanaga and Ogawa2002), suggesting the monogenean transmits between wild and farmed halibut.
CONCLUSIONS
With a rapid expansion of the mariculture industry, many parasitic diseases have emerged among farmed marine fish. Some parasites have established their infection cycles within farms and it is not practically possible to eradicate them from farms. For monogeneans, egg filamentous appendages have a big advantage for their proliferation, as they entangle with net meshing and hatched larvae or oncomiracidia have much higher chances to encounter host fish in cages. A variety of control measures have been developed. A single method will not be enough and a combination of multiple methods is recommended for effective control of monogenean infections. Blood flukes are serious pathogens of farmed fish. Prevention of cercarial invasion into fish is hampered, as for most cases, their intermediate hosts have not yet been specified. In-feed administration of praziquantel is the only reliable control method. Chemotherapy is effective against monogenean and blood fluke infections, but can affect the health of farmed fish themselves. More effective and less harmful methods of parasite control within an ecosystem context that includes fish mariculture sites needs to be developed. Clearly, we have to expand our knowledge on the biology of important parasites of farmed fish to apply effective control.
ACKNOWLEDGEMENTS
I would like to thank Dr Juan Timi, Universidad Nacional de Mar del Plata-CONICET, Argentina, and Dr Ken MacKenzie, The University of Aberdeen, UK, for their valuable comments and critical reading of the manuscript. Thanks are also due to Dr A. Sitjá-Bobadilla, Consejo Superior de Investigaciones Científicas, Spain, Dr Sho Shirakashi, Kinki University, Japan and Mr Yukitaka Sugihara, Nagasaki Prefectural Institute of Fisheries, Japan, for kindly providing the photos used in Figures 1–3.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.