INTRODUCTION
By draining resources from their hosts, ectoparasites negatively affect host fitness (Lehmann, Reference Lehmann1993; Clayton and Moore, Reference Clayton and Moore1997; Poulin, Reference Poulin2007), and hence may affect the dynamics of wildlife populations. Ectoparasite virulence – the damage an ectoparasite causes to its host – results from direct depletion of host resources, and from host energy resources being allocated away from normal maintenance, growth and reproduction, towards regenerative processes and counter measures against ectoparasite infestations. Furthermore, many ectoparasites are vectors of pathogens (viral, bacterial and protozoal) which may reduce the host's fitness (Sonenshine, Reference Sonenshine1991; Gray, Reference Gray1998; Garamszegi, Reference Garamszegi2006). The mechanisms of virulence are part of the proximate base to the understanding of host–parasite interactions, and therefore it is crucial to assess and interpret current damage caused by the parasites as an outcome of the co-evolution between parasite and host.
Although ectoparasites share the exploitation of the host's integument, the large diversity among different parasite taxa in morphology, host exploitation and life history makes the generalization of virulence by theoretical models challenging. Major factors included in models of ectoparasite virulence are: the dependence of the parasite on its host (Ewald, Reference Ewald1983; Lehmann, Reference Lehmann1993; Clayton and Tompkins, Reference Clayton and Tompkins1994), the genetic relatedness among co-exploiting parasites (Frank, Reference Frank1996), host specificity (Regoes et al. Reference Regoes, Nowak and Bonhoeffer2000; Garamszegi, Reference Garamszegi2006) and the co-evolutionary history between parasite and host (Combes, Reference Combes1997; Møller et al. Reference Møller, Arriero, Lobato and Merino2009). In spite of their importance in validating models of virulence, controlled experimental comparisons across ectoparasites and host species are rare. Most empirical studies are difficult to interpret since they are not phylogenetically controlled, which entails the risk that any difference in virulence just arose from different co-evolutionary histories (Harvey and Pagel, Reference Harvey and Pagel1991; Clayton and Tompkins, Reference Clayton and Tompkins1994).
In the present study we examined to what extent 2 congeneric blood-sucking ectoparasites, Ixodes arboricola (Schulze and Schlottke) and Ixodes ricinus (L.), differ in their impact on the health status of nestlings belonging to a single shared host species, the great tit (Parus major L.). Both tick species take a single bloodmeal from their host before detachment and moulting to the next developmental stage. Furthermore, they have a very low intrinsic mobility and therefore largely depend on hosts for dispersal. Ixodes arboricola is more prone to transmit vertically (i.e. transmission of the parasite from a parent host to its offspring) than I. ricinus, because of its life cycle, habitat specificity and host ecology, and therefore it likely has a tighter co-evolutionary history with its hosts. It is a nidicolous tick species, of which each parasitic stage (larva, nymph and adult females; males do not engorge) feeds on birds that breed or roost in tree cavities (Hudde and Walter, Reference Hudde and Walter1988; Hillyard, Reference Hillyard1996). During the birds’ breeding season this tick has short developmental durations, enabling larvae and nymphs to moult to the next developmental stage and parasitize again within a single breeding cycle (D. Heylen, personal observation). I. arboricola is prone to vertical transmission, since ticks infesting breeding parents may also infest nestlings later on in the breeding cycle, and since tree holes are used during autumn and winter as roosting sites by future breeding birds (Gosler, Reference Gosler1993). In contrast, I. ricinus is a field-dwelling ectoparasite and largely depends on horizontal transmission (i.e. transmission of the parasite to unrelated hosts), since each parasitic stage feeds on a different host from a wide range of species. The immature stages feed on small to medium-sized vertebrates, while the adult stages usually only attack large animals (Gray, Reference Gray1991). The large variety of potential hosts and the high number of potential tick-host contacts make it unlikely for I. ricinus’ development to be sustained by the same individual host and/or its offspring. According to current theories on evolution of virulence, due to the higher likelihood of vertical transmission, and the higher dependence on the fate of individual hosts and their offspring (Ewald, Reference Ewald1983; Lehmann, Reference Lehmann1993; Clayton and Tompkins, Reference Clayton and Tompkins1994) we expect I. arboricola to have lower virulence and to show more prudent host exploitation than I. ricinus. Furthermore, the low dispersal capability and isolated habitat of I. arboricola may lead to high levels of genetic relatedness among ticks inhabiting the same tree hole, increasing kin selection which should select for decreased virulence. This arises because of the lowered competition for resources among relatives (Frank, Reference Frank1996; Buckling and Brockhurst, Reference Buckling and Brockhurst2008), favouring their fitness by reducing the chance of a premature end of the ticks’ blood supply due to over-exploitation of the host.
In this study we experimentally infested nestlings with varying tick loads of both species, and compared their impact on host health (body condition, albumin/globulin ratios, tarsus length and haematocrit level). Furthermore, we compared levels of erythrocyte depletion caused by the two tick species by considering the total amount of blood ingested – estimated by tick engorgement weights – and the feeding duration. The tick's engorgement weight is known to be proportional to, but considerably less than, the absolute blood consumption by the feeding tick (Balashov, Reference Balashov1972). A higher level of erythrocyte depletion in terms of blood removed per day is characterized by a higher engorgement weight and/or shorter engorgement durations. By using a randomized parallel-group design within the same broods (split-unit design) we reduced the possible influences of genetic heterogeneity and rearing conditions of the host. We will discuss the results in the light of current theories of virulence in evolutionary parasitology.
MATERIALS AND METHODS
Study species
The great tit (Parus major L.) is a common woodland passerine, breeding and roosting inside tree holes, and is used as a model organism in many scientific fields (Gosler, Reference Gosler1993).
Ixodes ricinus is an ectoparasite of deciduous woodland, meadows and moorland, feeding on virtually any available terrestrial vertebrate hosts. Unfed I. ricinus ticks typically climb to some vantage point in the lower vegetation (‘questing’) from where they contact passing vertebrate hosts. All stages of I. ricinus are very sensitive to desiccation (Knülle and Rudolph, Reference Knülle, Rudolph, Obenchain and Galun1982; Kahl and Knülle, Reference Kahl and Knülle1988; Gray, Reference Gray1998), which limits their vertical distribution in the vegetation (Lees, Reference Lees1948; Mejlon and Jaenson, Reference Mejlon and Jaenson1997). The adults are believed to feed successfully only on larger animals. Immature developmental stages can parasitize almost any terrestrial vertebrate (Gray, Reference Gray1991). Songbirds (Heylen et al. Reference Heylen, Adriaensen, Dauwe, Eens and Matthysen2009 and references therein), such as the great tit, are often infested by I. ricinus nymphs and larvae, particularly during the breeding season and autumn. Although I. ricinus is considered to be a non-nidicolous ectoparasite, it occasionally infests songbirds nestlings (Grégoire et al. Reference Grégoire, Faivre, Heeb and Cezilly2002; Gallizzi et al. Reference Gallizzi, Bischoff, Gern and Richner2008). Ixodes ricinus has been recognized as a key vector of many human and/or animal pathogens in Europe, including bacteria (e.g. Borrelia burgdorferi sensu lato, Rickettsia spp.), viruses (e.g. tick-borne encephalitis virus) and protozoans (e.g. Babesia sp.) (Gray, Reference Gray1991; Jongejan and Uilenberg, Reference Jongejan and Uilenberg2004).
All parasitic stages of I. arboricola parasitize birds that breed or roost in tree cavities (Hudde and Walter, Reference Hudde and Walter1988; Hillyard, Reference Hillyard1996). This tick species completes its life cycle inside tree holes and shows several adaptations for this such as negative geotropism and nocturnal detachment from the host (Heylen et al. Reference Heylen, Adriaensen, Dauwe, Eens and Matthysen2009). As observed in other nidicolous ectoparasites (e.g. Ixodes plumbeus see Balashov, Reference Balashov1972) the high temperature (25°C) in inhabited nests may permit rapid metamorphosis during the birds’ breeding season (April–June), enabling larvae and nymphs to moult to the next developmental stage within a single breeding cycle (time to moult: 12·83±0·28 days (mean±standard error) and 13·34±0·13 days in larvae (N=24) and nymphs (N=90) respectively, under 25°C and 83% relative humidity). This contrasts with the much longer development durations (months) observed in I. ricinus (Balashov, Reference Balashov1972; Gray, Reference Gray1991). Laboratory experiments have shown that I. arboricola can successfully be infected with Borrelia burgdorferi sensu lato (Thorud, Reference Thorud1999), and may act as a vector of the tick-borne encephalitis virus (Lichard and Kozuch, Reference Lichard and Kozuch1967).
Study design
Effects of tick infestation on the health of great tit nestlings were studied with an experiment carried out according to a randomized, parallel group design. Nests were randomly assigned to an experimental infestation either with larvae (N=11 nests), nymphs (N=11 nests) or adult ticks (N=11). Two nests infested with larvae were excluded from further analyses, since in one nest 70% of the experimentally manipulated nestlings were predated by a weasel (Mustela nivalis L.), another nest was deserted for unknown reasons. Within each nest, 3 nestlings were randomly chosen for infestation (or control) with I. ricinus, and 3 others for infestation with I. arboricola. The individual nestlings within each group were randomly exposed either to high tick levels (30, 10, 2 ticks for experiments with larvae, nymphs and adult female ticks respectively), low tick levels (15, 5, 1 ticks for experiments with larvae, nymphs and adult female ticks respectively) or to a sham treatment without ticks. The remaining nestlings were left untreated and are not considered further in the experiment. We decided to accompany each adult female tick with an adult male tick, to be sure that the females would attach and engorge. In some ixodid tick species, adult females tend not to attach to animals unless males of the same species are present (Rechav et al. Reference Rechav, Goldberg and Fielden1997; Sonenshine, Reference Sonenshine2004). Furthermore, mating and fertilization are essential for completing the bloodmeal of female ticks, and may dictate the feeding rate and final weight of female ticks (Pappas and Oliver, Reference Pappas and Oliver1972; Weiss and Kaufman, Reference Weiss and Kaufman2004; Donohue et al. Reference Donohue, Khalil, Ross, Mitchell, Roe and Sonenshine2009). All nest-boxes used in this study were checked for naturally occurring ticks from the start of the breeding season (end of March) until hatching, and any ticks found were removed.
Experimental infestation
Experimental manipulations took place during the breeding season (May–June) of 2008 in a study site in northern Belgium (Matthysen et al. Reference Matthysen, Adriaensen and Dhondt2001). We collected I. ricinus nymphs and adults for use in the experiment 1 day before the start of the experiment, by dragging a flannel cloth over suitable vegetation in an oak woodlot known to contain high numbers of I. ricinus ticks. After identification and selection, I. ricinus individuals were kept in sterile tubes at 25 °C and >90% relative humidity until use. Ixodes ricinus larvae were obtained from eggs laid by 5 engorged females, isolated from 2 domestic cats (Felis catus L.). Females were kept individually in tubes at 25°C and >90% relative humidity until larvae emerged from the deposited eggs. Emerged larvae were kept in a climate room at 12 h:12 h light:dark photoperiod (20°C:10°C temperature cycle) until infestation. All I. arboricola ticks were obtained during the winter of 2007–2008 from nestboxes in which great tits and blue tits (Cyanistes caeruleus) breed and roost. Throughout the year, except for the bird breeding season, tick individuals were kept at the outside temperature and 85% relative humidity in the dark. However, the second half of April, when great tit females start breeding, ticks were maintained at 20°C until use. At the beginning of March, we assembled the adult tick developmental stages in a few vials (10 females with 10 males per vial).
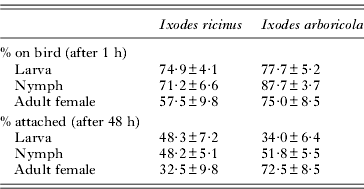
Nestlings were infested at 8 days old by placing them in prepared tubes containing ticks. For each nest, 6 plastic cylindrical tubes (radius: 15 mm; height: 70 mm) with ticks were prepared the morning before infestation: 2 with high levels, 2 with low levels of ticks, and 2 with no ticks (see above for details). Tubes were covered with an air-permeable mesh fabric preventing ticks from escaping. To minimize the impact of cold and food stress on nestlings, all manipulations took place in the afternoon on warm and dry days. The 6 tubes with nestlings and ticks were placed in a single thermo-insulated container during 60 min, after which the birds were returned to their nest. At this stage in the experiment, we estimated the number of ticks that were on the birds by counting the number left in the tubes (Table 1). We did not search for ticks on the birds, since unfed ticks are difficult to detect without prolonged manipulation which may disturb the attachment process. Before infestation, each nestling was ringed with a coloured ring according to its treatment. At 48 h after infestation, we counted the number of ticks attached per bird (5 min check per bird; see Table 1), which is used as explanatory variable in further statistical analyses.
Table 1. Mean infestation success per bird (±s.e.) of the developmental stages of Ixodes arboricola and Ixodes ricinus fed on 8-day-old Parus major nestlings
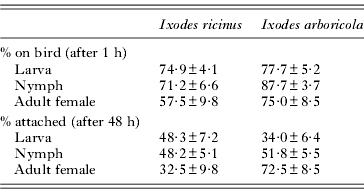
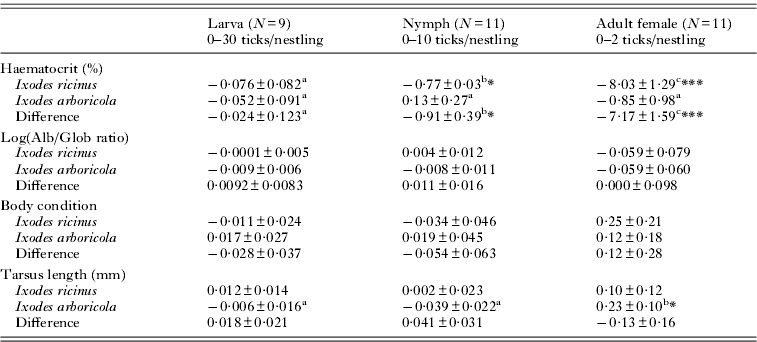
Table 2. Parameter estimates derived from generalized linear mixed models of the changes in Parus major health parameters with experimentally manipulated tick load
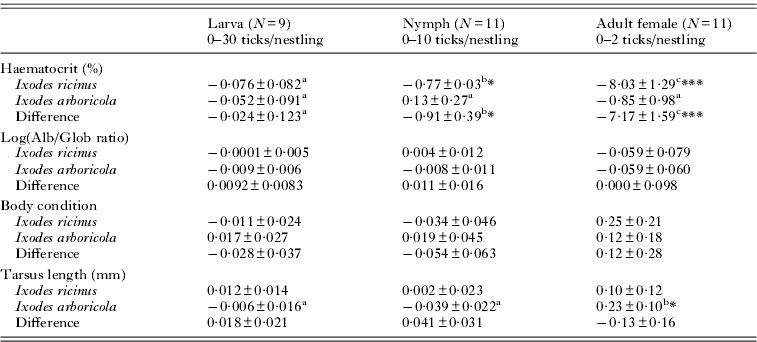
* P<0·05; **P <0·01; ***P <0·001; significance levels for testing whether the change with tick load differs from zero.
a,b,c Values with the same post-script do not differ significantly among developmental stages.
Tick collection and measuring
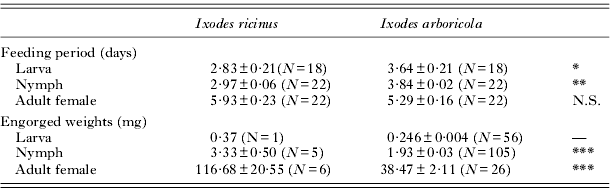
Starting 2 days after experimental infestation, and until 2 days after the last tick had detached, nests and nestboxes were checked daily for engorged ticks. One additional check was done immediately after fledging. After detachment, most I. arboricola ticks crawl towards the ceiling of the nest box, where they can be easily detected after removing the nestbox lid. In contrast, I. ricinus disappears in the nest material (positive geotropism), and consequently only a very small number of larvae and nymphs can be collected after feeding without sorting out the nest and disturbing the nestlings. After collection, ticks were transported to the laboratory and weighed to the nearest 0·001 mg (for larvae), or to the nearest 0·01 mg (for nymphs and adults). Since the number of collected immature I. ricinus ticks was low, we analysed additional data on engorgement weights from a previous experiment whereby full-grown juvenile great tits were experimentally infested under laboratory conditions. Although the additional I. ricinus weight data should be treated with caution, it is known that in I. ricinus and other 3-host field-parasitic ixodids that feed on a wide vertebrate host range, the weight differences in engorged nymphs and larvae are small among different host types (Balashov, Reference Balashov1972). We indeed found that the average weight of the few collected engorged nymphs fed on the nestlings in the current field experiment (3·33±0·50 mg, N=5, Table 3) was similar to the weight of nymphs fed on full-grown juvenile great tits infested under laboratory conditions (3·79±0·11 mg, N=85) (Heylen and Matthysen, Reference Heylen and Matthysen2010) and the weight of nymphs (3·34±0·15 mg, N=70) fed on 5-day-old Canary finches (Serinus canaria) (Jonas Vergauwen, D. Heylen and Wendt Müller; unpublished data).
Table 3. Feeding parameters of Ixodes ricinus and Ixodes arboricola ticks on 8-day-old Parus major nestlings
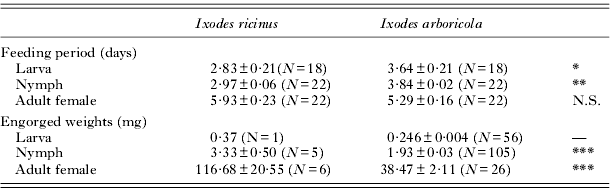
P >0·05 (N.S.)
* P<0·05; **P <0·01; ***P <0·001; significance levels for testing whether feeding parameters differ among the tick species. No tests were executed on the engorged weights of larvae, because of the low sample size in I. ricinus (—) (but see text for an analysis using data from a previous study).
Health and condition parameters of nestlings
Starting 2 days after the experimental infestation, manipulated nestlings were checked daily for attached ticks. For each nest, blood samples were taken from nestlings belonging to the same experimental group on the first day when at least 75% of the attached ticks had detached. This allowed us to evaluate their health status using 2 haematological parameters: haematocrit (Hct) and albumin/globulin ratio (Alb/Glob ratio). Acute or chronic anaemia, as indicated by low Hct, results in a reduced oxygen-carrying capacity of the blood and consequently restricts oxygen demanding processes (Dein, Reference Dein, Harrison and Harrison1986). Furthermore, Hct has been widely used to assess the condition of wild birds (Dubiec and Cichon, Reference Dubiec and Cichon2001 and references therein), as they are strongly influenced by nutritional status, with lower values associated with undernourishment (e.g. Latshaw, Reference Latshaw1991; Merino and Potti, Reference Merino and Potti1998). Challenges to humoral immune defence can be detected on the basis of albumin/globulin ratios. Both in acute disease and chronic infections or inflammatory disease, challenged individuals reveal higher total globulin concentrations and lower albumin concentrations than in healthy individuals (see Ots et al. Reference Ots, Murumagi and Hõrak1998 and references therein).
Capillaries containing the blood samples were stored and transported to the laboratory at 4°C. Hct was expressed as the volume of the part of the capillary occupied by red blood cells per total volume of blood in the capillary, after centrifugation for 10 min at 14 000 g. Subsequently, plasma was isolated and stored at −30°C until further analysis of the Alb/Glob ratio. Standard agarose gel electrophoresis (Helena Laboratories, UK) was used for detecting major protein groups. Gels were stained with an acid blue stain concentrate and densitometrically scanned at wavelength of 595 nm. Birds were sexed by using a polymerase chain reaction technique based on the CHD genes from the DNA extracted from red blood cells (for details see Griffiths et al. Reference Griffiths, Daan and Dijkstra1996). At 15 days of age, nestling body weight (to the nearest 0·1 g) and tarsus length (Gosler, Reference Gosler1993) were measured. The residuals of a regression of body mass on tarsus length were calculated to obtain a measure for body condition.
Statistical analysis
All data manipulations and statistical analyses were done in SAS v 9.2 (SAS Institute, Cary, NC, USA). For each tick developmental stage and for each health parameter with normally distributed residuals (Hct, log Alb/Glob ratio, nestling body condition) a general linear mixed effects model (GLMM; procedure PROC MIXED in SAS) was fitted to test if the two tick species show any differentiation in the impact on the nestlings’ health. By adding nest and the interaction between nest and tick load as random effects (which will model variation in intercept and change in the health parameter with a unit increase in tick load among nests) we correct for the non-independence in the response variables of the individuals within a nest. Fixed effects in the analysis of each experiment include: the tick species (I. ricinus/I. arboricola), the number of ticks that had attached (48 h after manipulation), their 2-way-interaction (tick species x number of attached ticks), the sex of the nestling and the nestling's age. Only significant random effects and subsequently significant fixed effects were left in the model in order to obtain the most parsimonious models. In all models a stepwise selection procedure was used in which the model was iteratively refitted after exclusion of the least significant effect. Terms were not removed from the model when they made part of a significant interaction. Random effects were tested by likelihood ratio tests (LRT). For the inference of maximum likelihood estimates of the fixed effects, Kenward Roger approximation was used to estimate the denominator degrees of freedom of the F-distributed test statistics (for details see Verbeke and Molenberghs, Reference Verbeke and Molenberghs2001). Tick engorgement weights were analysed by executing a GLMM with the tick species as a main effect, and the nest as a random effect. Because of the low sample size of engorgement weights of the nest-collected I. ricinus individuals, outcomes of an additional non-parametric Wilcoxon test are reported, since this test is less affected by outliers in sparse datasets. The duration until tick detachment, which is time-to-event data that is not normally distributed and therefore does not fulfil the assumption of the GLMM, was modelled by means of the marginal cox proportional hazards model for clustered data (procedure PROC PHREG in SAS). This is a survival analysis technique that takes into account the non-independence of repeated time-to-event measurements (in our study: the time until 75% tick detachment in the infested chicks) that belong to the same cluster (in our study: the brood). Tick species was added into the model of each tick developmental stage. For general information about modelling time-to-event data we refer to Cox and Oakes (Reference Cox and Oakes1984). For details about the marginal cox proportional hazards model for clustered data we refer to Lee et al. (Reference Lee, Wei, Amato, Klein and Goel1992). All estimates are reported as mean ± standard error, unless otherwise mentioned.
RESULTS
Effects of ticks on nestling health and condition
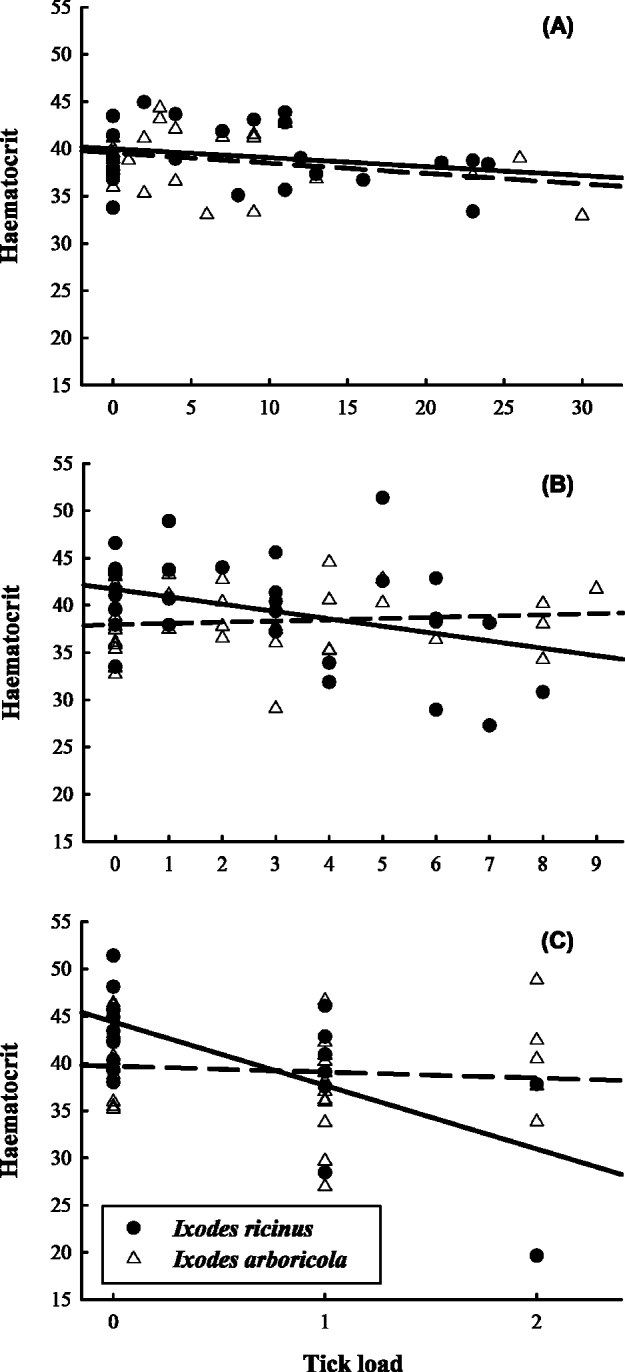
Haematocrit levels decreased with tick load for infestations with I. ricinus nymphs (T43·5=−2·61; P=0·013) and I. ricinus adults (T28·7=−4·95; P<0·0001; Fig. 1 and Table 2) while there was no trend in any of the developmental stages of I. arboricola (all P>0·39). Except for a slight increase in tarsus length with tick load in nestlings infested with adult I. arboricola females (T41·3=2·28; P=0·027), no relationships with tick load were observed in any of the other health or condition parameters. No significant 2-way-interactions between tick load and nestling age or sex were found in any of the models. Body condition (Δ male–female=0·04 g; F1·139=0·89; P<0·002) and tarsus length (Δ male–female=0·52 mm; F1·150=41·72; P<0·0001) were significantly lower in female nestlings than in male nestlings. The among-nest variation in intercepts (i.e. values when tick load equals zero) significantly differed from zero in the analyses of tarsus length (nymphs), Alb/Glob ratio (larvae and females), and body condition (females and nymphs) (all χ 2-values ⩾7·28; d.f.=1; P's ⩽0·007). We detected significant among-nest variation only in the change of body condition with tick load (χ 2=5·76; d.f.=1; P=0·016).
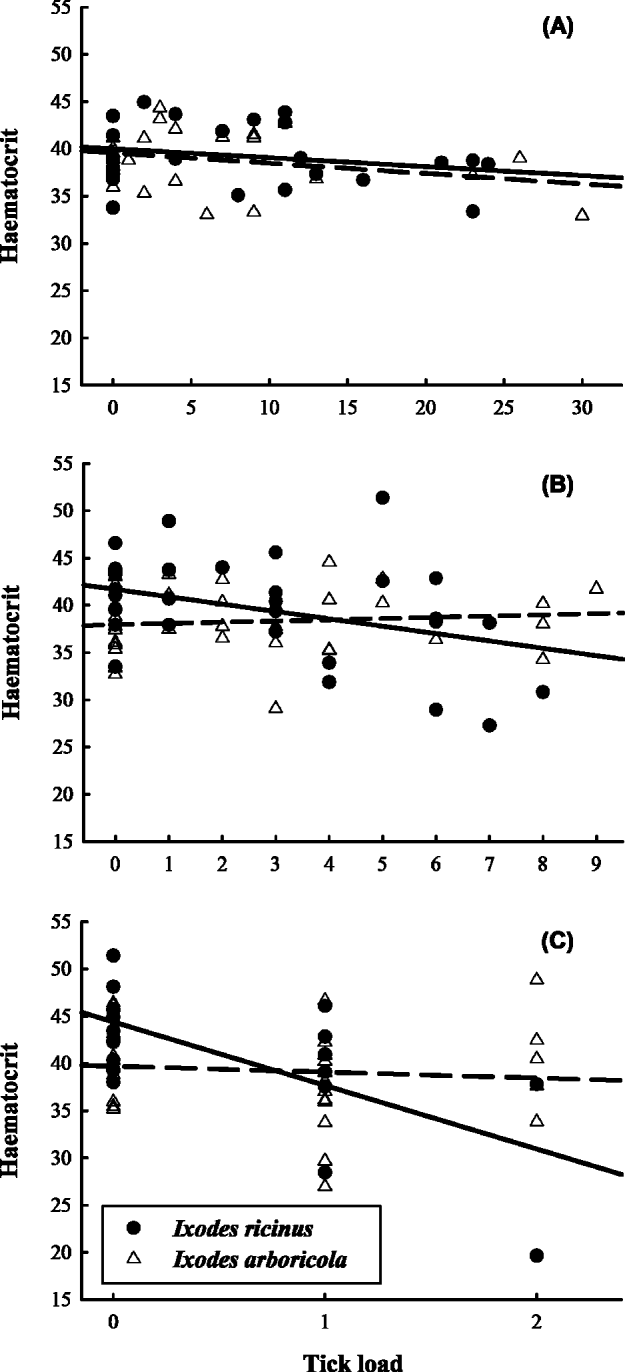
Fig. 1. Haematocrit levels of great tit nestlings in relation to tick loads. (A) Larvae, (B) nymphs and (C) adult female ticks. Least squares regression lines are fitted for birds infested with the tree hole tick Ixodes arboricola (dashed line) and sheep tick Ixodes ricinus (full line).
Feeding behaviour of ticks
In contrast to I. arboricola, only a very small number of engorged I. ricinus ticks were detected (see Table 3). Eleven engorged adult I. ricinus females were recovered, of which 3 were alive but had perforated ophistosomas and 2 were found dead at the bottom of the nest material. Only the 6 undamaged adults were weighed and used in further analyses. Since the number of collected immature I. ricinus ticks was low (see Materials and Methods section), we used additional data of I. ricinus from a previous laboratory experiment (average engorgement weights in larvae: 0·566±0·005 mg (N=202); average engorgement weights in nymphs: 3·79±0·11 mg (N=85)) for the statistical analyses on engorgement weights. The comparison with the I. ricinus laboratory data showed that in all developmental stages, I. arboricola had significantly lower engorgement weights than I. ricinus (all P⩽0·001). This finding was confirmed when we compared the engorgement weights of the few I. ricinus ticks obtained from the field experiment (Table 3) with I. arboricola adult females (Wilcoxon test: Z=3·45; P=0·0006; GLMM: T29·9=7·22; P<0·001; random nest effect: χ 2=0; d.f.=1; P=0·97) and nymphs (Wilcoxon test: Z=3·25; P=0·0012; GLMM: T97·3=8·25; P<0·0001; random nest effect: χ 2=14·9; d.f.=1; P<0·0001).
Furthermore, the duration until engorgement (analyses based on the estimated hazard to engorge, i.e. the likelihood that 75% of the ticks have detached from the nestling) was longer in I. arboricola larvae (hazard ratio I. arboricola vs I. ricinus: 2·72; 95% – confidence interval: 1·23–6·05; χ 2=6·09; d.f.=1; P=0·013) and nymphs (hazard ratio I. arboricola vs I. ricinus: 4·49; 95% – confidence interval: 1·77–11·42; χ 2=9·94; d.f.=1; P=0·002) compared to I. ricinus. No statistically significant difference between the tick species was found in the adult female ticks (hazard ratio I. arboricola vs I. ricinus: 0·45; 95% – confidence interval: 0·19–1·10; χ 2=3·08; d.f.=1; P=0·08).
DISCUSSION
To our knowledge this is the first study that experimentally compares the virulence and food exploitation of closely related ectoparasite species within the same broods of a common host, hence reducing the possible influences of host genetic heterogeneity and host rearing conditions. None of the tick developmental stages showed measurably negative effects on any of the nestlings’ health parameters and growth, with the exception of the significant decrease in haematocrit levels with increasing tick burdens in I. ricinus adult females and nymphs. Nevertheless, both tick species differ in several components of their feeding behaviour including bloodmeal size and time to detachment.
Effects on health status
Besides nutritional stress (Campbell, Reference Campbell, Harrison and Harrison1994; Piersma et al. Reference Piersma, Koolhaas, Dekinga and Gwinner2000) and erythrocyte destruction by haematozoa (Maley and Desser, Reference Maley and Desser1977; Dusek et al. Reference Dusek, Spalding, Forrester and Greiner2004), erythrocyte depletion via blood-sucking ectoparasites (Richner et al. Reference Richner, Oppliger and Christe1993; Potti et al. Reference Potti, Moreno, Merino, Frias and Rodriguez1999; Heylen and Matthysen, Reference Heylen and Matthysen2008) is one of the better-known causes of haematocrit reduction in birds. The stronger haematocrit reduction in I. ricinus compared to I. arboricola likely resulted from the more vigorous host exploitation by nymphs and adult female ticks, characterized by the higher volume of consumed erythrocytes in conjunction with comparable (in adult females) or shorter (in nymphs) engorgement durations. This exploitation behaviour leads to a higher blood depletion rate than is compensated for through the hosts’ erythropoiesis (Sonenshine, Reference Sonenshine1991; Heylen and Matthysen, Reference Heylen and Matthysen2008; Pfäffle et al. Reference Pfäffle, Petney, Elgas, Skuballa and Taraschewski2009). The feeding parameters of I. ricinus larvae also suggest less prudent host exploitation compared to I. arboricola, although this was not reflected in host haematocrit levels. From our data we cannot exclude the possibility that the negative association between haematocrit and tick loads is caused by a higher preference of I. ricinus for nestlings with the lowest haematocrit levels, since we did not measure the health status of the nestlings at infestations. However, this alternative explanation is unlikely, because previous experiments have shown that both I. ricinus and I. arboricola prefer the better developed and heavier nestlings (Gallizzi et al. Reference Gallizzi, Bischoff, Gern and Richner2008; Heylen and Matthysen, Reference Heylen and Matthysen2011) which are characterized by high haematocrit levels (Dubiec and Cichon, Reference Dubiec and Cichon2001; Heylen and Matthysen, Reference Heylen and Matthysen2011).
Since erythrocytes are the most important carriers of oxygen in birds, the majority of metabolic processes depend on them. Through blood loss, which in turn increases the chances of secondary infections (see further), high ectoparasite burdens may physically weaken the chicks. Metabolic processes for the compensation of the blood depletion are energetically demanding and, together with the repair of skin lesions and blood vessels and the mounting of an immune response, may lead to a reduced body condition and growth (Lehmann, Reference Lehmann1993 and references therein; Richner et al. Reference Richner, Oppliger and Christe1993; Kirby et al. Reference Kirby, Smith, Benton and Hudson2004). Extensive grooming (Glines and Samuel, Reference Glines and Samuel1989) or depressed food intake due to ticks (Okelly and Seifert, Reference O'Kelly and Seifert1970; Seebeck et al. Reference Seebeck, Springel and O'Kelly1971; Jonsson et al. Reference Jonsson, Mayer, Matschoss, Green and Ansell1998) may negatively affect the host's body condition as well. However, no measurably negative tick effects were found on the nestlings’ body condition. Similar results have been found in free-living great tits experimentally infested with I. ricinus nymphs (Heylen and Matthysen, Reference Heylen and Matthysen2008) and in great tit nestlings infested with I. ricinus larvae (Gallizzi et al. Reference Gallizzi, Bischoff, Gern and Richner2008), in which body condition seemed not to be affected by ixodid ticks at all. Host body condition is possibly only affected under less favourable conditions and/or higher tick burdens, when they are less able to fully compensate for the detrimental tick effects (Bouslama et al. Reference Bouslama, Chabi and Lambrechts2001). Parents also could have compensated for the resources drawn from the nestlings, by increasing the rate of food provisioning (Tripet and Richner, Reference Tripet and Richner1997).
No negative tick effects were found on the nestlings’ growth, when considering the full-grown tarsus length of the 15-day-old nestling. The only significant effect was opposite to our expectation, i.e. an increase in tarsus length with tick loads of I. arboricola females. This may have resulted from adaptations of parents and nestlings, which have overcompensated the expected negative tick effects (Eggert and Jodice, Reference Eggert and Jodice2008), e.g. increased begging behaviour may have increased the parental rate of food provisioning (Christe et al. Reference Christe, Richner and Oppliger1996; Tripet and Richner, Reference Tripet and Richner1997; Szép and Møller, Reference Szép and Møller1999). An alternative explanation is that the longer tarsus lengths in infested birds resulted from a physiological trade-off, whereby less energy is spent in costly immune responses (Szép and Møller, Reference Szép and Møller1999), and more energy is allocated to growth to fledge sooner and hence escape from parasitism (Saino et al. Reference Saino, Calza and Möller1998). However, this hypothesis is unlikely when considering the albumin/globulin ratio, since we did not find any association between the tarsus length and this measure of immune response (F1·167=0·03; P=0·86; controlled for nestling sex). A final explanation for the observed correlation is that adult I. arboricola females more successfully infest the larger nestlings. As shown in a previous experiment in which complete nests were inoculated at different moments in the nestlings’ development, I. arboricola females were more ready to attach to older nestlings (D. Heylen, manuscript in preparation). These nestlings are characterized by longer tarsi, better developed feathers and higher body mass, and hypothetically may increase tick fitness by providing more food resources and better shelter against host-induced mortality (Heylen and Matthysen, Reference Heylen and Matthysen2011).
We did not find any effect of tick infestation on the albumin/globulin ratio, an aspecific measure of inflammation. In contrast, in sand martin (Riparia riparia L.) nestlings that were close to fledging, experimental infestation with the nidicolous tick Ixodes lividus (Koch 1844) led to increased concentrations of immunoglobulins (Szép and Møller, Reference Szép and Møller1999). Furthermore an experiment in which free-living great tits were infested with I. ricinus nymphs suggested an inflammatory reaction by the host (Heylen and Matthysen, Reference Heylen and Matthysen2008). Since the immune function in growing birds is developing (Apanius, Reference Apanius and Starck1998), and reaches peak performance only when nestlings become fully independent (Toivanen and Toivanen, Reference Toivanen and Toivanen1987), it is plausible that in our study the tick manipulations as well as the blood sampling are too early in the nestlings’ developmental trajectory to detect any traces of inflammation.
Virulence and tick ecology
As shown in our study, the overall harm of both tick species to the host was moderate. The low intrinsic mobility of the tick, the lack of adaptations to facilitate its own direct transmission from one host to the other, and the need for fluid blood during a long-lasting feeding period make both tick species dependent on the fate of its individual host during blood intake. Therefore we suppose that the fitness loss due to host death would be substantial. This contrasts with other ectoparasites that depend less on host fate (see Lehmann, Reference Lehmann1993 for review). For example, animals subjected to argasid ticks, that feed intermittently from multiple hosts before completion of the bloodmeal, may experience large blood loss over short time periods and even lethal exsanguination (Sonenshine, Reference Sonenshine1991).
Although the differences in the virulence between the two tick species were relatively subtle, they can be interpreted in the light of several current theories of virulence in parasitology given the contrasting ecologies of both species. One of the possible explanations for differential virulence among tick species is based on the virulence-transmission hypothesis (Lehmann, Reference Lehmann1993; Clayton and Tompkins, Reference Clayton and Tompkins1994). This hypothesis states that in horizontally transmitted parasites, where transmission occurs independently of host survival and reproductive output, parasites hardly suffer a reduction of fitness by harming their host. In contrast, in vertically transmitted parasites, the fitness of the parasite depends partially on host future reproductive output, hence those parasites are expected to evolve lower virulence (Anderson and May, Reference Anderson and May1982; Ewald, Reference Ewald1983; Lehmann, Reference Lehmann1993; Tompkins et al. Reference Tompkins, Jones and Clayton1996). Therefore, increased opportunity for vertical parasite transmission, as observed in I. arboricola, will promote the evolution of decreased virulence.
The mode of transmission of both tick species is closely linked to their habitat and host specialization (Sonenshine, Reference Sonenshine1991; Klompen et al. Reference Klompen, Black, Keirans and Oliver1996). Ixodes ricinus is associated with the ground vegetation of woodland areas (Hillyard, Reference Hillyard1996) where the number of potential hosts is high, favouring horizontal transmission. Even if hosts die as a consequence of infestation (Lehmann, Reference Lehmann1993) ticks are likely to return to their habitat and may find other suitable hosts. Because of this higher predictability, we suggest that the cost of virulence in I. ricinus and other so-called field-dwelling ticks should be low. Other experimental studies on field ticks have also shown moderate to severe effects on bird health and fitness (Hoodless et al. Reference Hoodless, Kurtenbach, Nuttall and Randolph2002; Heylen and Matthysen, Reference Heylen and Matthysen2008; Heylen et al. Reference Heylen, Adriaensen, Dauwe, Eens and Matthysen2009). Even at low tick loads, some ixodid field ticks may have very high degrees of virulence. For example, tick paralysis – a syndrome characterized by birds showing acute depression or death due to toxins secreted by ticks (Monks et al. Reference Monks, Fisher and Forbes2006) – is frequently reported in birds infested with Ixodes frontalis Pantzer 1795 (Doby, Reference Doby1998) and Ixodes brunneus Koch 1844 (Luttrell et al. Reference Luttrell, Creekmore and Mertins1996), but has never been reported in I. ricinus. Contrary to I. ricinus, I. arboricola is specialized in tree cavities used by birds for breeding (Hillyard, Reference Hillyard1996; Heylen et al. Reference Heylen, Adriaensen, Dauwe, Eens and Matthysen2009). Given its low intrinsic mobility and the high site-fidelity of hosts to single cavities, horizontal transmission among tree holes and their hosts is probably rare. Thus this tick's fitness depends on the survival and re-use by tree holes by the same hosts and their offspring, implicating vertical rather than horizontal transmission.
Another but more speculative explanation for the observed difference in virulence is based on the theory of kin selection, stating that closely related parasites are favoured to cooperate and exploit their host prudently, whereas unrelated parasites are favoured to compete intensely (Frank, Reference Frank1996; Buckling and Brockhurst, Reference Buckling and Brockhurst2008). The low dispersal capability and isolated habitat of I. arboricola may lead to high levels of genetic relatedness among ticks inhabiting the same tree hole.
Similar low levels of virulence as in I. arboricola have been observed in other systems with specialized nidicolous ticks inhabiting isolated and dispersed niches. For example, in Ixodes hexagonus Leach 1815, a nidicolous tick that chiefly parasitizes hedgehogs (Erinaceus europaeus L.) (Pfäffle et al. Reference Pfäffle, Petney, Elgas, Skuballa and Taraschewski2009), adult female engorgement weights are lower (Toutoungi et al. Reference Toutoungi, Gern and Aeschlimann1995) and feeding exploitation in larvae and nymphs less vigorous (Toutoungi et al. Reference Toutoungi, Gern and Aeschlimann1993) than in I. ricinus. The low virulence of dispersed nidicolous ticks contrasts with studies that have examined the virulence of nidicolous ticks in colonially breeding birds. Several studies have illustrated the potentially devastating effects of nidicolous ticks on colony breeders. Particularly in the mobile argasid ticks, infestations may lead to massive nest desertion, nest failure and nestling mortality (King et al. Reference King, Keith, Mitchell and Keirans1977; Duffy, Reference Duffy1983; Chapman and George, Reference Chapman, George, Loye and Zuk1991; Lehmann, Reference Lehmann1993; Kaufman, Reference Kaufman1996; McKilligan, Reference McKilligan1996; but see also Eggert and Jodice, Reference Eggert and Jodice2008 for sublethal levels in pelicans). Ixodid ticks infesting colony breeders generally show smaller effects than argasids (Kaufman, Reference Kaufman1996; Szép and Møller, Reference Szép and Møller2000; Gauthier-Clerc et al. Reference Gauthier-Clerc, Mangin, Le Bohec and Gendner2003), although high infestation levels have been shown to trigger nest desertions and affect health and survival as well (Baerg, Reference Baerg1944; Hesse, Reference Hesse1985; Wanless et al. Reference Wanless, Barton and Harris1997; Bergström et al. Reference Bergström, Haemig and Olsen1999; Mangin et al. Reference Mangin, Gauthier-Clerc, Frenot, Gendner and Le Maho2003). We thus suggest that autonomous horizontal transmission is more frequent among birds breeding in close proximity, which would also lead to lower genetic relatedness among ticks sharing the same nest. Both factors would promote higher levels of virulence than observed in the nidicolous I. arboricola.
A third possible explanation for the difference between tick species, besides mode of transmission and genetic relatedness, may be host specificity. The generalist I. ricinus probably has a less tight co-evolutionary history with great tits than I. arboricola. It has been suggested that co-evolution should lead to more benign parasites (Combes, Reference Combes1997; Møller et al. Reference Møller, Arriero, Lobato and Merino2009). We note, however, that the above-mentioned studies on the high virulence of specialized ticks in colonial birds do not support this co-evolutionary hypothesis. We also note that this reasoning contrasts with an alternative hypothesis in which a trade-off is proposed between host specialization and virulence, resulting in a lower virulence for generalist parasites. For explaining this trade-off, it has been suggested that the development of the parasite's evasion mechanisms against the immune system of each additional host is costly. As a consequence, generalist parasites are expected to have a lower success in exploiting their different hosts, and therefore have lower reproductive success and virulence (Combes, Reference Combes1997; Regoes et al. Reference Regoes, Nowak and Bonhoeffer2000; Garamszegi, Reference Garamszegi2006). However, this trade-off seems not to hold in our study, in which the generalist tick has a slightly higher virulence than the specialist tick.
We cannot completely rule out the possibility that the differential health impact in the congeneric ticks is partly due to potential tick-borne pathogens transmitted by the ticks. Little is known about the vector capacity of I. arboricola. A recent study revealed that both I. arboricola and I. ricinus ticks collected in our study area may contain Borrelia burgdorferi sensu lato spirochetes (D. Heylen and Hein Sprong, unpublished data) of which the great tit is a reservoir host (Humair et al. Reference Humair, Turrian, Aeschlimann and Gern1993; Olsen et al. Reference Olsen, Jaenson and Bergström1995; Comstedt et al. Reference Comstedt, Bergstrom, Olsen, Garpmo, Marjavaara, Mejlon, Barbour and Bunikis2006). However, we lack information on the transmission, proliferation rate, and virulence of Borrelia spirochetes and other tick-borne micro-organisms in songbird nestlings. We note that limited signs of disease have been registered in full-grown songbirds when infected with Borrelia spirochetes (e.g. Olsen et al. Reference Olsen, Gylfe and Bergstrom1996; Kaiser et al. Reference Kaiser, Seitz and Strub2002), which suggests that a strong interfering role of these pathogens in our study is unlikely.
In conclusion, our study showed significant differences in the health impact of 2 congeneric ectoparasites on a common host, which can be interpreted in the light of different theories of virulence in evolutionary parasitology. To generalize and extrapolate our conclusions, experimental comparisons in other ectoparasite-host systems are needed whereby ectoparasite transmission, host specificity and virulence are quantified in related ectoparasite species. Furthermore, the analyses of genetic relatedness among ticks may yield indirect information on dispersal and transmission, and may give further support for the possibility of kin selection in vertically transmitted ticks.
ACKNOWLEDGMENTS
We are grateful to Roos Van Elzen and Peter Hõrak for helping us to execute the serum protein electrophoreses and interpreting the densitograms, and Natalie Van Houtte for the molecular sexing of the birds. We thank Joël White for his constructive comments on the article. Experiments were carried out under licence of the Flemish Ministry (Agentschap Natuur en Bos) and the experimental protocol was approved by the Ethical Committee of the University of Antwerp. This study was funded by FWO-project G.0049.10 awarded to E.M.