Published online by Cambridge University Press: 23 May 2005
Crude extract specific activities of thymidylate synthase, dUTPase, thymidine kinase and dihydrofolate reductase were high during the development of Caenorhabditis elegans, the dauer larva activities being similar to those previously determined in Trichinella spiralis and T. pseudospiralis muscle larvae (with the exception of thymidine kinase, not detected in Trichinella). High thymidylate synthase expression in developmentally arrested larvae, demonstrated also at the mRNA and protein levels, is in agreement with a global cell cycle arrest of dauer larvae and indicates this unusual cell cycle regulation pattern can be shared by developmentally arrested larvae of C. elegans and the two Trichnella species. Hence, the phenomenon may be characteristic for developmentally arrested larvae of different nematodes, rather than specific for the parasitic Trichinella muscle larvae. Endogenous C. elegans thymidylate synthase was purified and its molecular properties compared with those of the recombinant protein, expression of the latter in E. coli cells confirming the NCBI database sequence identity.
Enzymes involved in thymidylate (dTMP) biosynthesis, thymidylate synthase (EC 2.1.1.45), catalysing the N5,10-methylenetetrahydrofolate-dependent C(5) methylation of 2′-deoxyuridylate (dUMP), dihydrofolate reductase (EC 1.5.1.3), catalysing dihydrofolate reduction to tetrahydrofolate, dUTPase (EC 3.6.1.23), hydrolysing dUTP to dUMP, and thymidine kinase (EC 2.7.1.75), responsible for thymidine salvage via phosphorylation, are expressed in association with cell proliferation (Kit, 1976; Johnson, Fuhrman & Wiedemann, 1978; Vilpo, 1983; Blakley, 1984; Santi & Danenberg, 1984; Hokari et al. 1987; Békési et al. 2004). Therefore high specific activities of thymidylate synthase, dUTPase and dihydrofolate reductase, found in non-growing muscle larvae of Trichinella spiralis (Dąbrowska et al. 1996; Rode et al. 2000), a parasitic nematode causing trichinellosis, a serious disease involving colonization of striated skeletal muscle tissue by the parasite larvae (Despommier, 1998), were surprising. As thymidylate synthase induction in cells had been shown (Pestalozzi et al. 1995) to be due to leaving quiescence (cell cycle G0 phase), high thymidylate synthase expression in developmentally arrested muscle larvae, demonstrated also at the mRNA level (Dąbrowska et al. 2004), was hypothesized to be a consequence of cell cycle arrest (Rode et al. 2000; Dąbrowska et al. 2004).
Of obvious interest was whether the phenomenon, observed in T. spiralis muscle larvae and potentially exploitable as a target in anti-parasitic chemotherapy, is specific for intracellular parasitic life or is common for developmentally arrested life-cycle stages of other, including free-living, nematodes. In order to test the latter possibility, specific activity developmental pattern of enzymes involved in thymidylate biosynthesis was studied for Caenorhabditis elegans. The life-cycle of this free-living nematode, being a model for parasitic nematodes (Bürglin, Labos & Blaxter, 1998), also involves a developmentally arrested stage, dauer larva, corresponding to T. spiralis muscle larva (Bürglin et al. 1998). The results indicate the enzyme expression pattern in developmentally arrested larvae to be characteristic also for C. elegans, providing new evidence for previously hypothesized state of a global cell cycle arrest in dauer larvae (Euling & Ambros, 1996; Hong, Roy & Ambros, 1998). They also point to a possibility that this unusual regulatory pattern is specific for developmentally arrested life-cycle forms of other free-living/parasitic nematodes and indicate a convenient model for further studies of the underlying mechanism.
Protease Inhibitor Cocktail Tablets (Complete, Mini) were purchased from Roche Diagnostics GmbH (Mannheim, Germany), (6RS,αS)tetrahydrofolic acid, thymidine, dUMP and FdUMP from Sigma (St Louis, MO), DEAE-cellulose (DE-52) from Whatman (Maidstone, UK), [5-3H]dUMP (15 Ci/mmol) and [methyl–3H] thymidine (64,7 Ci/mmol) from Moravek Biochemicals (Brea, CA) and other reagents were of analytical grade. pBluescript SK− vector and E. coli DHFαF’ strain were from Stratagene, TX61−, a derivative of the E. coli BL21(DE3) strain with the endogenous thymidylate synthase gene inactivated by transposon-mediated insertion, was a gift from Dr W. S. Dallas (Glaxo Wellcome, UK).
The wild-type C. elegans strain N2, variety Bristol, was used, with stocks maintained at 25 °C on agar plates streaked with E. coli strain OP50 (Brenner, 1974). Worms to be used for determination of specific activity or purification of thymidylate synthase were grown with addition of E. coli strain TX61− (Cieśla et al. 2002) as the bacterial food. In other cultures the OP50 strain (a gift from the Caenorhabditis Genetic Center, University of Minnesota-St Paul) was used. In order to obtain a large number of worms, 1 litre of liquid culture in the complete S medium (Lewis & Fleming, 1995) at 25 °C with forced aeration was performed. C. elegans cultures were synchronized and worms at specific developmental stages obtained according to earlier described procedures (Lewis & Fleming, 1995). Briefly, dauer larvae were obtained by adding the stock culture to 1 litre of the S medium containing 50 g of bacteria and growing, unless otherwise indicated, for 2 weeks at 25 °C. Dauer larvae, constituting 67–100% of the resulting worm population, were cleaned by sedimentation at 1 g and either stored frozen at −30 °C or used (after culturing in the S medium containing bacteria) as a source of worms growing to adulthood. The adult worms were cleaned by sedimentation and flotation on 30% (w/v) sucrose in water and either stored at −30 °C or treated with alkaline hypochlorite solution to obtain embryos. Embryos were cleaned in the M9 buffer (Lewis & Fleming, 1995) by centrifugation at 150 g for 5 min and either stored frozen at −30 °C or suspended in buffer M9 and allowed to develop to starvation-arrested L1 larvae (17–24 h at 25 °C). The L1 larvae were cleaned in M9 buffer by centrifugation at 150 g for 5 min and either stored frozen at −30 °C or allowed to resume synchronous growth in S medium at 25 °C (after reintroduction to bacterial food) to develop to L3 larvae. These were cleaned by centrifugation at 100 g for 5 min and stored frozen at −30 °C.
The worms were homogenized by grinding with quartz sand in a mortar (placed on ice) with 3–5 volumes of homogenizing buffer and centrifuged (20000 g for 20 min), and the supernatant (crude extract) was saved. For assays of thymidylate synthase and dUTPase activities the worms were homogenized in ice-cold 0·05 M phosphate buffer, pH 7·5, containing 0·1 M KCl and 0·01 M 2-mercaptoethanol. For assays of thymidine kinase activity the worms were homogenized in 0·05 M Tris-HCl buffer, pH 7·5, containing 0·1 M KCl, 2 mM EDTA, protease inhibitor cocktail (Complete, Mini tablets from Roche; concentration corresponding to 1 tablet dissolved in 10 ml) and 0·01 M 2-mercaptoethanol. For assays of dihydrofolate reductase the worms were homogenized in 0·05 M Tris-HCl buffer, pH 7·0, containing 0·1 M KCl and 0·01 M 2-mercaptoethanol.
C. elegans crude extract activities of thymidylate synthase (Cieśla et al. 1995a), dihydrofolate reductase (Mathews, Scrimgeour & Huennekens, 1963), dUTPase (Gołos & Rode, 1999) and thymidine kinase (Nakata et al. 1985) were assayed according to previously published procedures. The recombinant thymidylate synthase activity was assayed spectrophotometrically (Wahba & Friedkin, 1961). The activity unit was defined as the enzyme amount required to convert 1 μmol of substrate per 1 min at 37 °C. Kinetic studies were done as previously described (Dąbrowska et al. 1996; Rode et al. 2000).
Protein concentrations were determined by the procedure of Spector (1978), with bovine serum albumin used as a standard.
Total RNA was isolated from C. elegans adult worms, and L1, L3 and dauer larvae, using Trizol ultra- pure reagent from GibcoBRL.
General methods used for DNA manipulations were as described (Sambrook, Fritsch & Maniatis, 1989). Reverse transcription was performed on total RNA with Moloney Murine Leukemia Virus Reverse Transcriptase (Promega) and oligo(dT) primer, with an additional round of reverse transcription reaction. After the first round the sample was denatured for 3 min at 95 °C, cooled on ice and, after addition of reverse transcriptase (50 units), incubated for 1h at 42 °C. In the PCR procedure the following primers, designed based on the NCBI database sequence (Accession no. NM 059131), were used: forward 5′-GGGAATTCATATGGAAGTCATGAACAAAGAA-3′, identical to the initial 21 nucleotide region of the coding sequence (bold), and reverse 5′-AAAGCAAGCTTTCAAACAGCCATATCCCAT-3′, complementing the last 19 bases of the coding region (bold). EcoRI and NdeI (in the forward primer) or HindIII (in the reverse primer) restriction sites (underlined) were included to permit cloning of the amplified sequence (a direct cloning into NdeI/HindIII sites was unsuccessful). This sequence, being the thymidylate synthase coding region, was ligated into the EcoRI/Hind III sites of the pBluescript SK− vector, the ligation mixture was used to transform E. coli DH5αF′ cells and the sequence of 1000 bp inserts in the isolated recombinant plasmids was confirmed. The coding region was restricted from pBluescript as an NdeI/Hind III fragment and ligated into the pPIGDM4+stop expression vector (Mikiewicz et al. 1997) as previously described (Cieśla et al. 2002). A pPIGDM4+stop/thymidylate synthase plasmid pool was used to transform the thymidylate synthase-deficient E. coli strain TX61−, in order to obtain a C. elegans thymidylate synthase overexpressing system.
Total RNA was size-fractionated on 1% agarose-formaldehyde gel then subjected to Northern transfer onto Hybond-N+ nylon membranes (Amersham) and hybridized with one of the following specific probes: (i) a 647 bp long cDNA fragment, corresponding to a part of C. elegans thymidylate synthase coding region or (ii) a reference probe – 612 bp long T. spiralis rDNA fragment (Kuratli et al. 1999; cfDąbrowska et al. 2004). All procedures were performed as previously described (Dąbrowska et al. 2004).
This was performed as previously described (Cieśla et al. 2002; Dąbrowska et al. 2004).
A portion (25 ml) of a pelleted mixed population of different developmental C. elegans forms, stored at −20 °C, was used to prepare crude extract that underwent ultracentrifugation (100000 g for 60 min) and the resulting supernatant served as a starting material to purify thymidylate synthase by the previously described (Cieśla et al. 1995a) affinity chromatography procedure, with Triton X-100 present only in the buffer eluting the enzyme from the concentrating DEAE-cellulose column. The recombinant enzyme was purified (Cieśla et al. 1995b), with protein contents determined and electrophoretic analyses performed (Cieśla et al. 1995a) as earlier reported. Thymidylate synthase capacity to form a ternary complex with [3H] FdUMP and CH2H4PteGlu allowed identification on the gel (Dąbrowska et al. 1996).
After PAGE-SDS (1 μg of purified recombinant thymidylate synthase or 50 μg of crude extract protein per lane), proteins were transferred into PVDF membrane. Thymidylate synthase was detected by treatment with polyclonal antibodies raised in rabbits against purified recombinant thymidylate synthase (antibodies were purified on the same recombinant thymidylate synthase preparation, immobilized on CNBr-Sepharose in the reaction using 2 mg of the recombinant thymidylate synthase protein per 1 ml of CNBr-Sepharose). Anti-rabbit IgGs coupled with HRP (Sigma) were used as secondary antibodies and colour was developed with the DAB/metal enhancer reagent (Bio-Rad). Density of bands was measured using the Scion Image program.
Unless otherwise indicated, these are presented as means ±S.E.M. or means ±% difference between the mean and each of the two results, followed by the number of experiments (N) in parentheses.
The specific activities of 4 enzymes involved in thymidylate biosynthesis, thymidylate synthase, dUTPase, dihydrofolate reductase and thymidine kinase, were high in adult forms, L1, L3 and dauer larvae (Table 1). Thymidylate synthase activity was not lowered when dauer larvae remained up to 6 weeks in the culture (Table 1). When measured in an extract from dauer larvae that were obtained from a starved stock culture based on their climbing behaviour (Riddle & Albert, 1997), thymidylate synthase activity was found to be similarly high (0·18 nmol/min/mg protein; N=1).

The results of Northern blot analyses of C. elegans total RNA are presented in Fig. 1(A,B). It should be noted that previously obtained 612 bp long T. spiralis rDNA fragment (Dąbrowska et al. 2004; cf GeneBank Accession no. AY497012) was used as a reference probe, as the corresponding C. elegans rDNA sequence (GeneBank Accession no. AY268117) is over 80% identical. Thymidylate synthase mRNA, relative to 18S rRNA, level was found to be constant in C. elegans adult worms, embryos and L1 and L3 larvae, only several-fold higher than that in dauer larvae. On the other hand, thymidylate synthase mRNA level in dauer larvae was not lowered when the larvae remained up to 6 weeks in the culture (Fig. 1A,B).

Fig. 1. (A) Thymidylate synthase (TS) mRNA and 18S rRNA bands in Caenorhabditis elegans adult forms (A), embryos (E), L1, L3 and dauer larvae (L1, L3 and D, respectively), and dauer larvae isolated from cultures prolonged up to 4 and 6 weeks at 25 °C (D4 and D6, respectively). (B) Thymidylate synthase mRNA level in C. elegans adult forms (A), embryos (E), L1, L3 and dauer larvae (L1, L3 and D, respectively), and dauer larvae isolated from cultures prolonged up to 4 and 6 weeks at 25 °C (D4 and D6, respectively), related to 18S rRNA level. Densitometric results are expressed as arbitrary units. Results for A, L3 and D are presented as mean result of N separate experiments ±S.D. (N=3 for A and L3, and N=4 for D) and those for E and L1 as mean result of 2 separate experiments, differing from each of the two results by no more than 2% (E) or 13% (L1). N=1 for D4 and D6. (C) Thymidylate synthase protein level in C. elegans adult forms (A), and L1, L3 and dauer larvae (L1, L3 and D, respectively). Immunoblot analysis with purified C. elegans recombinant thymidylate synthase preparation (Rec) used as a positive control. After PAGE-SDS (1 μg of RecTS or 50 μg of crude extract protein per lane), proteins were transferred into PVDF membrane, thymidylate synthase was detected by treatment with polyclonal antibodies raised against the recombinant enzyme. Anti-rabbit IgG coupled with HRP (Sigma) were used as secondary antibodies and colour was developed with the DAB/metal enhancer reagent (Bio-Rad). Density of bands was measured using the Scion Image program. The study was repeated twice with virtually identical results.
Purification of thymidylate synthase from C. elegans worms (asynchronous liquid culture), involving affinity chromatography based on dUMP-dependent enzyme binding on immobilized N10-formyl-5, 8-dideazafolate (Rode et al. 1979), resulted in highly enriched (2079-fold, 35% yield, specific activity of 0·4 unit/mg protein), albeit not homogeneous, preparation of the enzyme (Fig. 2). Of note is that successful application of the affinity chromatography system indicates an ordered addition of dUMP, before CH2H4PteGlu, to the enzyme. Thymidylate synthase monomer (identified based on bound [3H] FdUMP) molecular weight, determined by SDS polyacrylamide gel electrophoresis (Fig. 2), was 34800±13% (2).

Fig. 2. PAGE-SDS of Caenorhabditis elegans purified endogenous thymidylate synthase preparation (5 μg). (A) Distribution of radioactivity following separation of thymidylate synthase preparation pre-incubated with [3H] FdUMP in the presence of CH2H4PteGlu (Dąbrowska et al. 1996). (B) Separated thymidylate synthase preparation (upper lane) and molecular weight markers (lower lane; molecular weights 113000, 91000, 49900, 35100, 28400 and 20800 kDa).
Expression of the recombinant thymidylate synthase in E. coli cells confirmed the sequence identity. Its purification resulted in 38·5 mg of electrophoretically homogeneous preparation (not shown) with specific activity of 1·82 unit/mg and monomer molecular weight of 35100 (identified based on bound [3H] FdUMP).
Considerable differences in affinities for substrates were found with highly purified recombinant and endogenous enzyme preparations. The Kmapp values for dUMP and (6RS,αS) N5,10-methylenetetrahydrofolate (CH2H4PteGlu) of 1±20% μM (2) and 18±14% μM (2) with the endogenous thymidylate synthase, and 2·7±47% μM (2) and 107±6% μM (2) with the recombinant thymidylate synthase, respectively, pointing to possible influence of post-translational modification(s) on enzyme properties. FdUMP showed linear competitive inhibition of the endogenous C. elegans thymidylate synthase, described by the Kiapp value of 0·002±9% μM (2). When pre-incubated with the enzyme in the presence of CH2H4PteGlu, the analogue caused time-dependent inactivation of the enzyme, consistent with the behaviour of a slow-binding inhibitor (Morrison, 1982). The inactivation rate decreased after about 2 min pre-incubation (for similar pattern cfDąbrowska et al. 1996), reflected by a biphasic plot of log (remaining activity) versus time, suggesting differing interactions of FdUMP with the two known binding sites on the thymidylate synthase molecule. Inhibition constants and inactivation rate constants, calculated with the use of apparent inactivation rate constants during the initial (0·0–1·5 min) and later (4–10 min) periods of pre-incubation with various FdUMP concentrations, were for the endogenous enzyme Ki′=1·0±0·11 nM (3) and k2′=0·15±0·03 min−1 (3), and Ki″=0·57±0·01 nM (3) and k2″=0·03±0·02 min−1 (3), respectively. The corresponding values for the recombinant enzyme were Ki′=2·1±1·2 nM (3) and k2′=0·38±0·12 min−1 (3), and Ki″=0·61±0·08 nM (3) and k2″=0·05±0·01 min−1 (3).
Thymidylate synthase protein level was assessed by immunoblot analysis in crude extracts of C. elegans adult forms (A), and L1, L3 and dauer larvae by comparing with the level detected in the extract prepared from adult forms, run in each experiment on the same gel and considered 100%. Following electrophoretic analysis of each crude extract, the enzyme protein level was detected on blots with a polyclonal antibody raised against purified recombinant C. elegans thymidylate synthase (tested to react also with endogenous C. elegans, as well as recombinant T. spiralis, rat, mouse and human thymidylate synthases). Thymidylate synthase levels found in L1, L3 and dauer larvae, expressed as a percentage of the level detected in the extract from adult forms (Fig. 1C), were 25±7% (2), 114±20 (6) and 26±7 (6), respectively. It should be noted that the enzyme shows some heterogeneity, observed previously for thymidylate synthases from T. spiralis and other sources (Gołos et al. 2002), pointing to a possibility of a post-translational modification.
All enzyme activities studied were present at high levels throughout the life-cycle of C. elegans (Table 1). This includes thymidine kinase, which could not be detected in muscle larvae of Trichinella spiralis and T. psedospiralis (Rode et al. 2000), as well as in certain other parasitic nematodes (Farland & MacInnis, 1978; So, Wong & Ko, 1992), but was detected in Brugia pahangi and Dirofilaria immitis (Jaffe, Comley & Chrin, 1982). Expression of high activity of this enzyme in dauer larvae, in relation to other developmental stages, provides further support for the cell cycle arrest hypothesis, as thymidine kinase activity is known to be dramatically lower in G0 than in cycling cells (Pardee, 1989). Moreover, the three remaining enzyme activities studied were also high in developmentally arrested dauer larvae, in spite of the fact that the three enzymes, similar to thymidine kinase, are known to be associated with cell proliferation or cell cycling (Kit, 1976; Johnson, Fuhrman & Wiedemann, 1978; Vilpo, 1983; Blakley, 1984; Santi & Danenberg, 1984; Hokari et al. 1987; Pestalozzi et al. 1995; Békési et al. 2004) and no cell divisions occur in metabolically depressed dauer larvae (Riddle, 1988; Wadsworth & Riddle, 1988; Euling & Ambros, 1996). It is noteworthy that dauer larva specific activities of thymidylate synthase and dUTPase are comparable to those (0·1 nmol/min/mg protein and 0·7 nmol/min/mg protein, respectively) found in regenerating rat liver extracts (Cieśla et al. 1995a; Gołos & Rode, 1999).
The steady-state level of thymidylate synthase mRNA is known to be very low in quiescent mammalian cells, increasing 10 to 20-fold as such cells progress to the S phase and decreasing again with differentiation (discussed in Dąbrowska et al. 2004). When compared in a variety of cells and tissues, it reflects differences in cell proliferation rates (Lee, Shen & Johnson, 1999). The pattern of thymidylate synthase mRNA levels in C. elegans development is rather poorly correlated with proliferation, as dauer larvae, lacking cell divisions (Riddle, 1988; Wadsworth & Riddle, 1988; Euling & Ambros, 1996), show considerable levels of this mRNA. In accordance with thymidylate synthase specific activity, the enzyme mRNA level persists in dauer larvae for at least 6 weeks. Additionally, comparison of the enzyme activity, enzyme protein and mRNA levels in adult worms, and L1, L3 and dauer larvae, shows only the enzyme protein and mRNA patterns to be correlated. Interestingly, the enzyme activity pattern parallels the two former patterns only with L1, L3 and dauer larvae, with the enzyme activity level in adult forms being low, relative to both mRNA and enzyme protein levels. The latter points to some regulation of enzyme protein expression and activity during the development. Of note is that T. spiralis thymidylate synthase mRNA levels showed no differences between adults, newborns and muscle larvae, with the enzyme specific activities in adults and muscle larvae also being comparable (Rode et al. 2000; Dąbrowska et al. 2004).
Expression patterns of the three enzymes, thymidylate synthase, dihydrofolate reductase and dUTPase, indicate the unusual cell cycle regulation pattern to be shared by developmentally arrested larvae of C. elegans and the two Trichinella species (Rode et al. 2000; Dąbrowska et al. 2004). Thus, the cell cycle arrest hypothesis concerning Trichinella muscle larvae (Rode et al. 2000; Dąbrowska et al. 2004) is supported, as with C. elegans dauer larvae, a life-cycle stage corresponding to T. spiralis muscle larvae (Bürglin et al. 1998), a state of global cell cycle arrest has been hypothesized also based on other observations (Euling & Ambros, 1996; Hong et al. 1998). However, the apparent global cell cycle arrest in muscle larvae, reflected by high expression of the enzymes involved in thymidylate biosynthesis, does not seem to be specific for Trichinella parasites, as in view of the present results it may be characteristic also for developmentally arrested forms of other species of nematodes.
Notably, the possibility of global cell cycle arrest in non-developing T. spiralis muscle larvae poses a question of the relation of this unusual cell cycle regulation to changes induced by the larvae in muscle cells. Those changes, involving cell cycle re-entry and induction of DNA synthesis, followed by the apparent G2/M arrest in the cell cycle (Jasmer, 1995), point to muscle larvae as a source of cell cycle-specific signals.
High expression of thymidylate synthase in both C. elegans dauer and T. spiralis muscle larvae, where the enzyme's catalytic activity seems to be irrelevant, is interesting also in view of results suggesting possible non-catalytic functions of the enzyme protein in translational regulation (Liu et al. 2002) and neoplastic transformation (Rahman et al. 2004) of mammalian cells.
Supported by the State Committee for Scientific Research (Grant No. 6 P04C 003 21).

Table 1. Specific activities of selected enzymes involved in thymidylate biosynthesis in extracts of Caenorhabditis elegans adult forms (A), and L1, L3 and dauer larvae
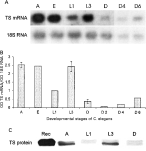
Fig. 1. (A) Thymidylate synthase (TS) mRNA and 18S rRNA bands in Caenorhabditis elegans adult forms (A), embryos (E), L1, L3 and dauer larvae (L1, L3 and D, respectively), and dauer larvae isolated from cultures prolonged up to 4 and 6 weeks at 25 °C (D4 and D6, respectively). (B) Thymidylate synthase mRNA level in C. elegans adult forms (A), embryos (E), L1, L3 and dauer larvae (L1, L3 and D, respectively), and dauer larvae isolated from cultures prolonged up to 4 and 6 weeks at 25 °C (D4 and D6, respectively), related to 18S rRNA level. Densitometric results are expressed as arbitrary units. Results for A, L3 and D are presented as mean result of N separate experiments ±S.D. (N=3 for A and L3, and N=4 for D) and those for E and L1 as mean result of 2 separate experiments, differing from each of the two results by no more than 2% (E) or 13% (L1). N=1 for D4 and D6. (C) Thymidylate synthase protein level in C. elegans adult forms (A), and L1, L3 and dauer larvae (L1, L3 and D, respectively). Immunoblot analysis with purified C. elegans recombinant thymidylate synthase preparation (Rec) used as a positive control. After PAGE-SDS (1 μg of RecTS or 50 μg of crude extract protein per lane), proteins were transferred into PVDF membrane, thymidylate synthase was detected by treatment with polyclonal antibodies raised against the recombinant enzyme. Anti-rabbit IgG coupled with HRP (Sigma) were used as secondary antibodies and colour was developed with the DAB/metal enhancer reagent (Bio-Rad). Density of bands was measured using the Scion Image program. The study was repeated twice with virtually identical results.
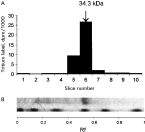
Fig. 2. PAGE-SDS of Caenorhabditis elegans purified endogenous thymidylate synthase preparation (5 μg). (A) Distribution of radioactivity following separation of thymidylate synthase preparation pre-incubated with [3H] FdUMP in the presence of CH2H4PteGlu (Dąbrowska et al. 1996). (B) Separated thymidylate synthase preparation (upper lane) and molecular weight markers (lower lane; molecular weights 113000, 91000, 49900, 35100, 28400 and 20800 kDa).