INTRODUCTION
The pig roundworm, Ascaris suum is the most common nematode parasite in pigs reared in any kind of management system (Roepstorff et al. Reference Roepstorff, Jørgensen, Nansen, Henriksen, Skovgaard, Pedersen and Andreasen1992; Roepstorff, Reference Roepstorff1997; Roepstorff et al. Reference Roepstorff, Nilsson, Oksanen, Gjerde, Richter, Örtenberg, Christensson, Martinsson, Bartlett and Nansen1998). The eggs of A. suum are highly resistant to adverse environmental conditions and to a range of chemicals (Krishnaswami and Post, Reference Krishnaswami and Post1968; Barrett et al. Reference Barrett1976; Roepstorff and Murrell, Reference Roepstorff and Murrell1997). This makes A. suum infections difficult to control, but allows the eggs to be used as indicators of environmental self-limitation (hygienization) that may take place in compost material, including deep litter bedding.
Concern for animal welfare has resulted in new regulations for housing of pigs. EU Council Directive 2001/93/EU suggests the provision of suitable rooting and manipulation materials such as straw, sawdust, wood or peat in order to satisfy the exploratory behaviour of pigs raised indoors, the lack of which may lead to adverse activities such as tail biting and aggression (Guy and Edwards, Reference Guy, Edwards, Geersand and Madec2006). As a result, there is an increased focus on alternative housing and production systems, including systems with deep litter pens (Petit and van der Werf, Reference Petit and van der Werf2003; Honeyman, Reference Honeyman2005). Deep litter pens may vary in their design, e.g. a typical deep litter pen on a Danish organic pig fattening farm is characterized by an indoor area covered with barley, wheat or oat straw and an outdoor area with a partially slatted concrete floor. If pigs defecate indoors, the bedding material is continuously mixed with faeces and urine. Fresh straw is added regularly resulting in the build-up of a litter-bed. Removal of deep litter generally depends upon season, number of pigs and the convenience to the farmer. During this period, the organic matter in the litter may, depending on the availability of oxygen, biodegrade through composting, resulting in generation of heat as well as production of ammonia and volatile fatty acids (Kirchmann and Witter, Reference Kirchmann and Witter1989) which are toxic to most pathogens (Kunte et al. Reference Kunte, Yeole and Ranade2004; Nordin et al. Reference Nordin, Nyberg and Vinnerås2009). To what extent this takes place under farming conditions is largely unknown. Most of the studies on deep litter housing address issues on animal welfare, pig management, pig production and ammonia emission (Groenestein and Van Faassen, Reference Groenestein and Van Faassen1996; Hill et al. Reference Hill, McGlone, Fullwood and Miller1998; Gentry et al. Reference Gentry, McGlone, Miller and Blanton2004; Morrison et al. Reference Morrison, Johnston and Hilbrands2007) while few studies have investigated risk of pathogen survival and transmission, e.g. parasites, in animals kept in deep litter bedding (Holmgren and Nilsson, Reference Holmgren and Nilsson1998). None of the studies hitherto have focused on pathogen survival and described the actual habitat conditions such as temperature, moisture level and ammonia concentration in deep litter in different parts of pens. Thus, it is not known whether deep litter bedding constitutes a risk factor in the epidemiology of A. suum infections.
The aim of the present study was to determine the distribution of A. suum eggs and the impact of the different physico-chemical conditions prevailing in deep litter bedding material on an organic pig farm on development and viability of eggs, and through this to evaluate the potential indoor transmission of A. suum in deep litter.
MATERIALS AND METHODS
Study design
The study was conducted as a repeated cross-sectional investigation on a Danish organic pig farm with 200 sows where finishers were kept on deep litter in a barn. It was evident from preliminary farm visits that parts of the pens were used for different purposes by the pigs and could be designated as a clean resting area, a dirty defecation (latrine) area and an intermediate area (transition zone) covering around 40, 40 and 20%, respectively, of the surface of the pen. For each of the three areas, a total of four sample replicates, i.e. one from each of two sites in each of two pens with finishers of c. 5–6 months of age, were collected from each of several different layers of the deep litter at the end of a production cycle. This was done in September 2011, December 2011, March 2012 and June 2012. Litter was not removed from the pens during a production cycle (finishing pigs from 3 to 5–6 months of age). The deep litter accumulated over time and the profile thus represented a full time series up until the time of sampling. However, the entire deep litter was removed after pigs were delivered to the slaughterhouse, i.e. between samplings. On an average, litter was removed once in every 3 months and sampling took place in pens nearest to cleaning which indicates the average of age litter (c. below 3 months). The bedding material was examined for the total number, development and viability of A. suum eggs in relation to temperature, moisture content, pH and ammonia content of the bedding material.
Farm and pen description
Farrowing took place outside on pasture and piglets were moved indoors after weaning at 7–8 weeks of age to a stable unit with shallow litter (10–20 cm deep). At the approximate age of 12–16 weeks the pigs were then moved into the finishing stable with deep litter. This stable contained a total of 28 pens of equal size, 14 pens on each side of a wide aisle. Each pen housed 20–35 finishers and consisted of an indoor area (10 m × 3·9 m) with a concrete floor which was covered with barley straw, and access to an outdoor area (10 m × 3·9 m) with a partially slatted concrete floor. Automatic feeders, drinkers and a sprinkler system were all located in the outdoor area. The indoor and outdoor areas were connected by a small opening covered with rubber sheets, which allowed pigs to move freely while reducing influx of cold air to the indoor area. Pigs defecated both inside and outside. Inside the pens, the pigs defecated in the part of the pen facing the aisle, creating a latrine area where the straw was heavily mixed with urine and faeces. The adjacent area was characterized by some contamination with urine and faeces, but to a lesser extent compared with the latrine, and was considered as the intermediate area. The part of the pen closest to the outdoor area contained straw that appeared dry and clean, and thus with no apparent contamination with urine and faeces, was designated as the resting area. Fresh straw was spread on top of the latrine area once or twice a week. This led to a high build-up of material in the latrine area with a gradual decline in thickness towards the resting area.
Faecal samples
At each visit, rectal faecal samples were collected from 10 finisher pigs in each of the two pens. Faecal egg counts were estimated by a concentration McMaster technique (Roepstorff and Nansen, Reference Roepstorff and Nansen1998) with a sensitivity of 20 eggs per g (epg) faeces using a flotation fluid of a saturated NaCl solution with 500 g glucose L−1 (specific gravity 1·27 g mL−1).
Collection of bedding material
In each area of two pens, a rectangular pit (c. 20×40 cm) was dug by hand all the way down to the concrete floor. The depth of the pit varied for different areas, pens and seasons. The litter material was firm/intact and it was possible to gently remove it without mixing the material. Starting from the surface, the deep litter was divided into layer 1 (0–10 cm), layer 2 (20–25 cm), layer 3 (30–45 cm), layer 4 (50–65 cm) and layer 5 (70–85 cm) using a ruler. The temperature was then measured in each layer at either side of the pit by inserting a sensor (NavTMP, Forston Labs, USA) horizontally c. 10 cm into the solid litter. Air temperature of the pens was measured once at a height of about 2 m from the floor using the same sensor at each of the four sampling occasions. Litter samples (c. 500 g) were then collected from either side of the pit at regular intervals by inserting a hand well into the side of the pit to get material that had not yet been exposed to the external environment. A representative sub-sample of each sample was immediately placed in an airtight plastic bag and stored at −20 °C for later estimation of ammonia content. The remaining bedding material was homogenized by cutting it into pieces of c. 1–2 cm length that was thoroughly mixed before sub-sampling. Sub-samples were then stored at 5 °C for later measurement of moisture content, measurement of pH and isolation of eggs.
Measurement of physico-chemical parameters
The frozen sub-samples were thawed and homogenized by cutting them into pieces of up to 5 cm length and mixing thoroughly. Total ammonia nitrogen (TAN) was extracted from 5 g of the sample suspended in 1 m KCl to a total volume of 100 mL and subjected to end-over-end shaking for 45 min. The extracts were then filtered through filter papers (Advantec TM No. 5A) and stored at −20 °C. The TAN concentration was later measured in the thawed extracts by a flow injector analyser system (Lachat Instruments Division, Milwaukee, WI, USA). NH3(aq) concentrations were calculated using TAN, pH and dissociation constant (pKa) values as described by Armstrong et al. (Reference Armstrong, Chippendale, Knight and Colt1978).
Moisture content was measured by drying a 5 g refrigerated sample at 105 °C for 24 h. pH was measured with a pH sensor (NavPHA, Forston Labs, USA) in a 5 g sample diluted 1:15 in deionized water (modified from Jorgensen and Jensen (Reference Jorgensen and Jensen2009) for slurry samples).
Isolation of eggs and quantification of different developmental stages
Eggs from 5 g of a refrigerated litter sample were isolated by soaking the material in 1 m NaOH for 16–18 h. The material was then washed through a 212 μm sieve (to remove straw which might otherwise interfere with flotation of eggs in the later stages) on to a 20 μm sieve (to retain the eggs) (modified from Larsen and Roepstorff, Reference Larsen and Roepstorff1999). The collected residue was then transferred to a 50 mL centrifuge tube using tap water (total volume of sediment 10 mL) and flotation fluid (see above) was added to a total volume of 50 mL and centrifuged for 7 min at 253 g. The supernatant was transferred to a container and the pellet was re-suspended with flotation fluid after which centrifugation and collection of the supernatant were repeated. The supernatant was washed with tap water on a 20 μm sieve and the residue was collected in a centrifuge tube. The sample was centrifuged for 7 min at 253 g, the supernatant was removed and the pellet was re-suspended by adding flotation fluid (1:8). For samples with low numbers of eggs, the entire sample was transferred to McMaster chambers and all the eggs were counted. For samples containing large quantities of eggs, only 20% of the sample material was examined to estimate the total number of eggs. For each sample, up to 50 eggs were examined microscopically (200×) to differentiate different developmental stages and the findings were extrapolated to obtain total counts. Eggs with no development were considered as undeveloped (unembryonated) eggs; eggs which had displayed early development (a single condensed cell in the middle to multicellular stages) as pre-larval stage eggs; eggs at late development (thick early larva to a slender and fully developed larva) as larvated eggs; and eggs with vacuolization of the cytoplasm and irregular shape or structure as non-viable eggs.
Viability of un-embryonated A. suum eggs
Additional eggs were isolated from each straw sample by soaking 10–60 g of litter material in tap water for 16–18 h, followed by washing and flotation as described above. Viability of seemingly normal but yet un-embryonated or partially embryonated eggs was assessed by their ability to embryonate. The isolated eggs were kept in H2SO4 buffer (pH 1) for 100 days at 22 °C. Eggs with fully developed larvae were considered as viable and the percentage of viable eggs was estimated by evaluating up to 50 eggs microscopically. All eggs were examined in samples where the recovered number of eggs was below 50.
Statistical analysis and calculations
Different physico-chemical parameters (temperature, NH3(aq), pH and moisture content), level of litter contamination with A. suum eggs (total number of eggs g−1 dry litter) across different pen areas, depths and study periods were compared by analysis of variance (ANOVA) using PROC GLM (SAS version 9.2, SAS Institute Inc., 2000–2008). Area, depths and study period were categorical explanatory variables and each model had one of the measurements of the different physico-chemical parameters or number of eggs g−1 dry litter as response variables. Due to non-normal distribution of data, square-root or log transformations were carried out to temperature, NH3(aq) and number of eggs (total eggs and viable eggs) data for a better fit to model assumption. For analysing development and viability of eggs, temperature measurements were classified as low or high if the temperature was ⩽28 °C or >28 °C, respectively. This cut-off was chosen as infectivity of eggs that develop at >28 °C is poorer compared with eggs that develop at ⩽28 °C (Arene, Reference Arene1986). Moisture measurements were classified as low (⩽40%), medium (40–80%) or high (>80%) as composting activity decreases at high and low moisture levels (Regan et al. Reference Regan, Jeris, Basser, McCann and Hudek1973; EPA, 1994) and viability of eggs is reduced at low moisture levels (<40%) (Sanguinetti et al. Reference Sanguinetti, Tortul, Garcia, Ferrer, Montangero and Strauss2005). pH was classified as low (⩽8) or high (>8) and NH3(aq) measurements were classified as low (⩽2 mm) or high (>2 mm) based on the median of each parameter. All interactions were included in the initial model followed by step-wise elimination of non-significant interaction terms.
The effect of different conditions on percentage of developing eggs, number of viable eggs g−1 litter and percentage of viable eggs was analysed by ANOVA using the lm package in R (R Development Core Team (2010), R Foundation for Statistical Computing, Vienna, Austria) by using area, depth, season and all the physico-chemical parameters as explanatory variables. The number of viable eggs was normalized by log (x+1) transformation whereas per cent eggs with different development stages and per cent viable eggs were normalized by square root transformation. The parameter estimate within each level of the categorical explanatory variables was compared with the reference level by the t-statistic (R-lm and SAS-GLM).
The number of larvated eggs (before laboratory embryonation) was compared between different pen areas, layers of litter and study periods by the Kruskal–Wallis test using PROC NPAR1WAY in SAS. Spearman correlation for percentage egg viability with NH3(aq) and temperature was calculated using GraphPad Prism version 6.02 (GraphPad Software, San Diego, CA, USA).
The total number of eggs of different types (total eggs, larvated eggs, eggs with fully developed larva and viable eggs) per g straw (wet weight) was determined and used for estimating the approximate content in the total bedding material in each area. The density of bedding material was estimated by measuring the volume occupied by 5 g of bedding material from each area. The total volume of bedding material in each area was determined by measuring the length, width of respective areas and the height of the bedding material in that area. The total mass (m) of bedding material was calculated by using the volume occupied by it in a given area and density (d) of respective bedding material (m = v*d).
RESULTS
General observations
The height of the bedding material ranged from 30–65 cm in the resting areas, 30–55 cm in the intermediate areas and 40–85 cm in the latrine areas during different study periods. The straw in the latrine areas was moist, strongly interlaced, highly compacted and therefore difficult to dig into and heavily mixed with faeces and urine. The straw in the resting area was drier (except the bottom layer sampled in December 2011 and March 2012), looser, easier to sample and the pigs rooted in it more often than the other areas. The conditions of the straw found in the intermediate areas were similar to the latrine areas, but there was less faeces and urine.
The prevalence of A. suum in the finisher pigs was 100, 95, 75 and 75% and the mean egg excretion was 5000, 2026, 1116 and 787 epg in September 2011, December 2011, March 2012 and June 2012, respectively. Pigs excreted very few Trichuris suis and Strongyle eggs.
Temperature
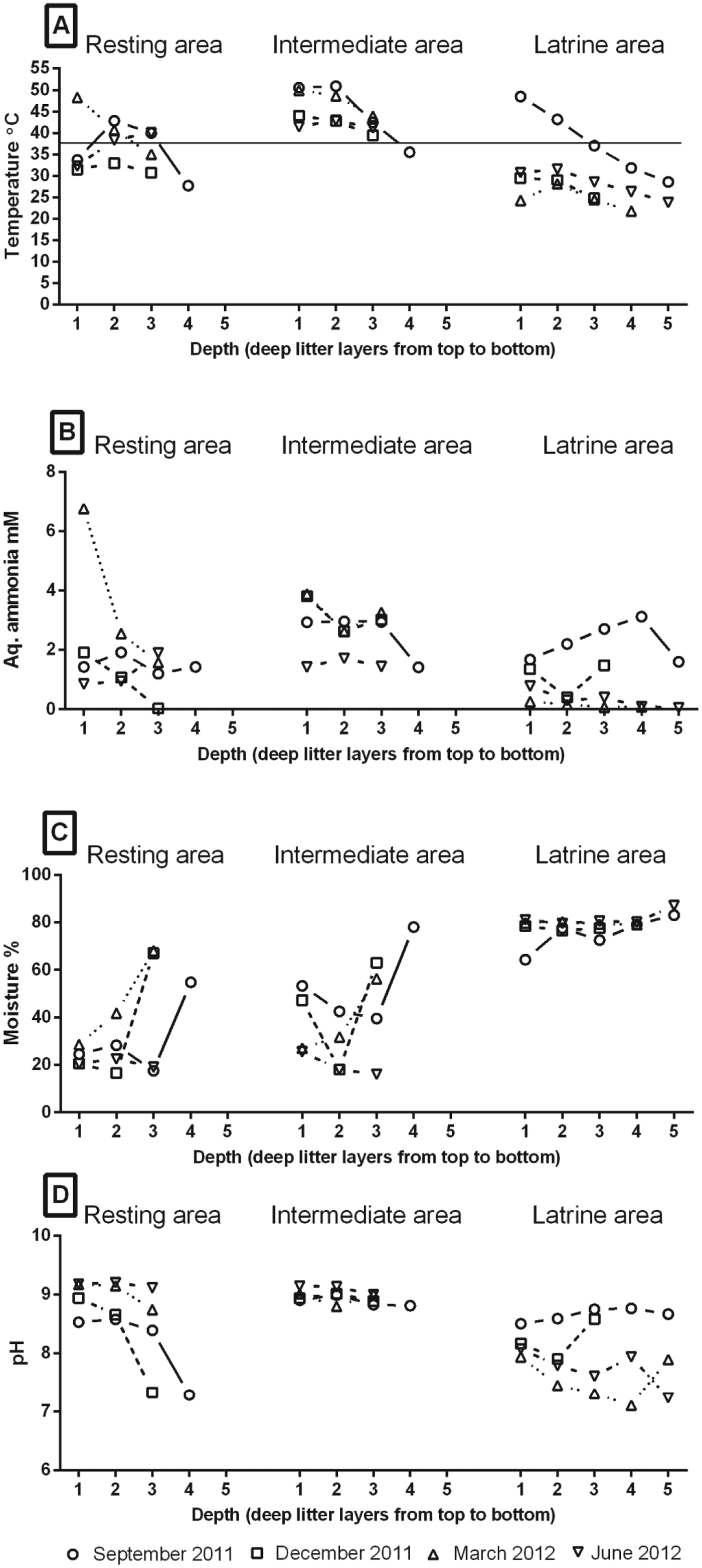
The air temperature of the pens was 18·8 °C, 1·2 °C, 5·2 °C and 16·8 °C during September 2011, December 2011, March 2012 and June 2012, respectively. The mean temperature (min–max) of the litter across depths was 35·7 °C (23·2–69·0 °C), 43·6 °C (33·8–57·0 °C) and 30 °C (18·9–53·8 °C) in the resting, intermediate and latrine areas, respectively, and there was an overall significant difference in temperature between the areas (P<0·0001) (Fig. 1A). In general, the September 2011 temperatures were higher in all areas compared with the other time points (P<0·0001). As the depth of the deep litter increased, the temperature declined in all the areas (P<0·0001), but the decline was very sharp in September 2011 compared with the other time points indicating a significant interaction between study period and depth (P = 0·034). In general, the temperature in the different layers of the three areas did not vary much between the different time points except in the latrine area where the deep litter in September 2011 was warmer at all the depths indicating a significant interaction of study period and area (P<0·0001). The majority of samples (92%) in the intermediate areas and some of the samples in the resting areas (25%) and latrine areas (13%) showed temperatures ⩾38 °C which is considered as a higher threshold for development of eggs (Seamster, Reference Seamster1950) at the time of sampling.

Fig. 1. (A) Mean temperature levels (°C). (B) Mean concentration of aqueous ammonia (NH3(aq), mm). (C) Mean moisture content (%) (D) Mean pH levels in different layers of litter in resting, intermediate and latrine areas of two finisher pig pens with deep litter at different seasons. Sample depths were: layer 1: 0–10 cm, layer 2: 20–25 cm, layer 3: 30–45 cm, layer 4: 50–65 cm and layer 5: 70–85 cm. Number of replicates = 4. Horizontal line in (A) indicates the maximum threshold temperature (38 °C) for development of eggs.
Aqueous ammonia (NH3(aq))
The NH3(aq) concentration in the bedding material varied significantly with pen area. Of the three areas, samples from the intermediate areas had significantly higher (P<0·0001) NH3(aq) concentrations followed by those from the resting and latrine areas (Fig. 1B) with mean (min–max) NH3(aq) concentrations of 2·6 mm (0·04–5·8), 1·5 mm (0·006–17·3) and 1·0 mm (0·01–6·7), respectively. The NH3(aq) concentration also varied with study period (P<0·0001) and was highest in September 2011 and lowest in June 2012. Overall, the depth of the deep litter also had an effect on the NH3(aq) concentration (P<0·0001) as the concentration decreased with increased depth. This was prominent in resting areas compared with the other two areas indicating a significant interaction of depth and area type (P = 0·002).
Moisture content
The bedding material in the latrine area had the highest moisture content, irrespective of depth, as pigs defecated and urinated in this area, followed by the intermediate and resting areas with means (min–max) of 79 (54–88%), 43 (14–86%) and 36 (14–84%), respectively (P<0·0001) (Fig. 1C). Samples from March 2012 had significantly higher moisture content compared with those in other time points (P<0·0001). Urine had accumulated in the bottom bedding material layers of all three pen areas in December 2011 and March 2012 and hence samples from these layers had significantly higher moisture content compared with the top layers (P<0·0001).
pH
The pH varied significantly with area (P<0·0001) (Fig. 1D), study period (P = 0·0347) and depth (P<0·0001). Samples from the intermediate areas (mean (min–max): 8·88 (6·82–9·31)) had the overall highest pH followed by the resting (8·60 (6·57–9·36)) and latrine areas (8·04 (6·50–8·95)). Though statistical analysis revealed significant effect of study period and depth on pH, no clear pattern was observed.
Contamination levels of A. suum in litter
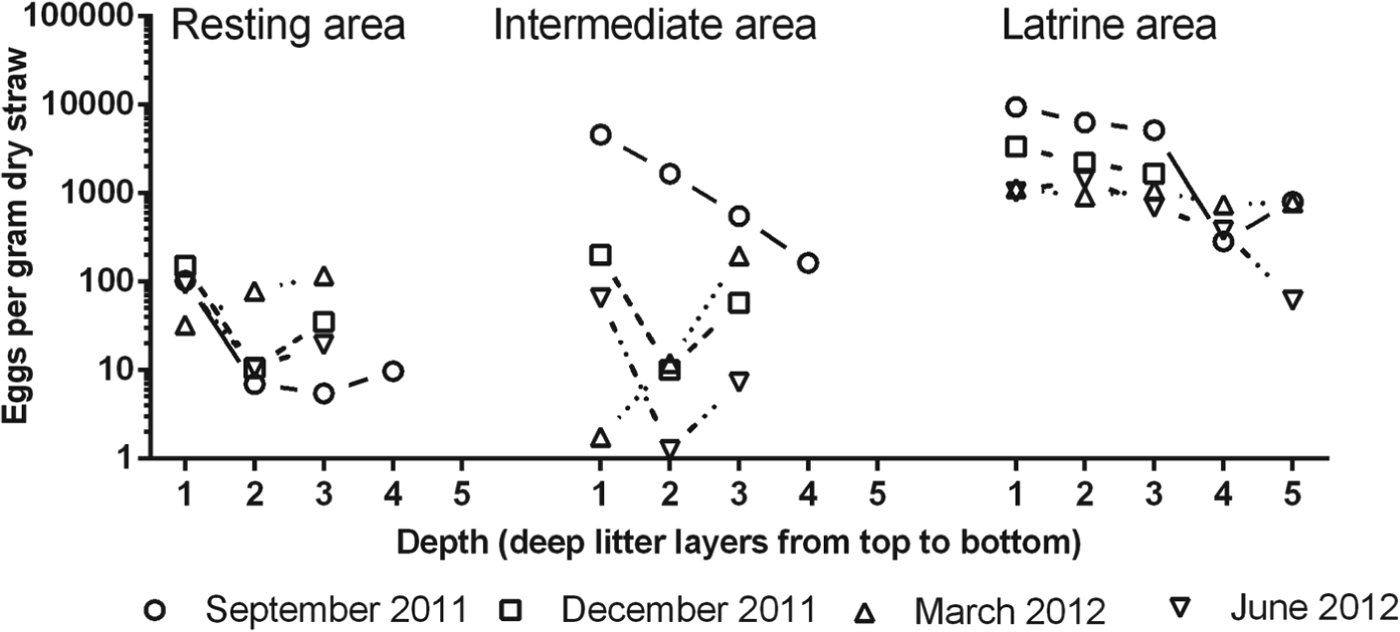
The total number of eggs per g dry straw varied significantly with area (P<0·0001), study period (P<0·0001) and depth (P<0·0001). Latrine areas contained the highest number of eggs followed by the intermediate and resting areas (Fig. 2) which was reflected in the estimated total numbers of eggs in the entire volume of bedding material in the various areas (Table 1). The mean (min–max) number of eggs per g dry straw in latrine, intermediate and resting areas was 1444 (37–11 317), 523 (0–7791) and 50 (1–434), respectively. Except for the resting areas, the highest numbers of eggs were found in September 2011 followed by December 2011 whereas egg counts were lowest, but comparable, in March 2012 and June 2012. As the depth of the litter increased, the number of eggs decreased and the decrease was overall more prominent in latrine areas indicating an interaction of depth and area on egg numbers (P = 0·012). As very few eggs of T. suis were observed in litter and as Strongyle eggs hatch very quickly and isolation of larvae needs different techniques, these parasites were not included in the present study.

Fig. 2. Mean number of Ascaris suum eggs g−1 dry straw in different layers of litter in the resting, intermediate and latrine areas of two finisher pig pens with deep litter at different seasons. Sample depths were: layer 1: 0–10 cm, layer 2: 20–25 cm, layer 3: 30–45 cm, layer 4: 50–65 cm and layer 5: 70–85 cm. Number of replicates = 4.
Table 1. Mean (min–max) number of total eggs, larvated eggs, fully developed eggs and viable eggs (in millions) in bedding material of different areas of two pens during four seasons. Min–max denotes variation between seasons. N denotes total number of samples collected throughout the study

Development of eggs within the litter
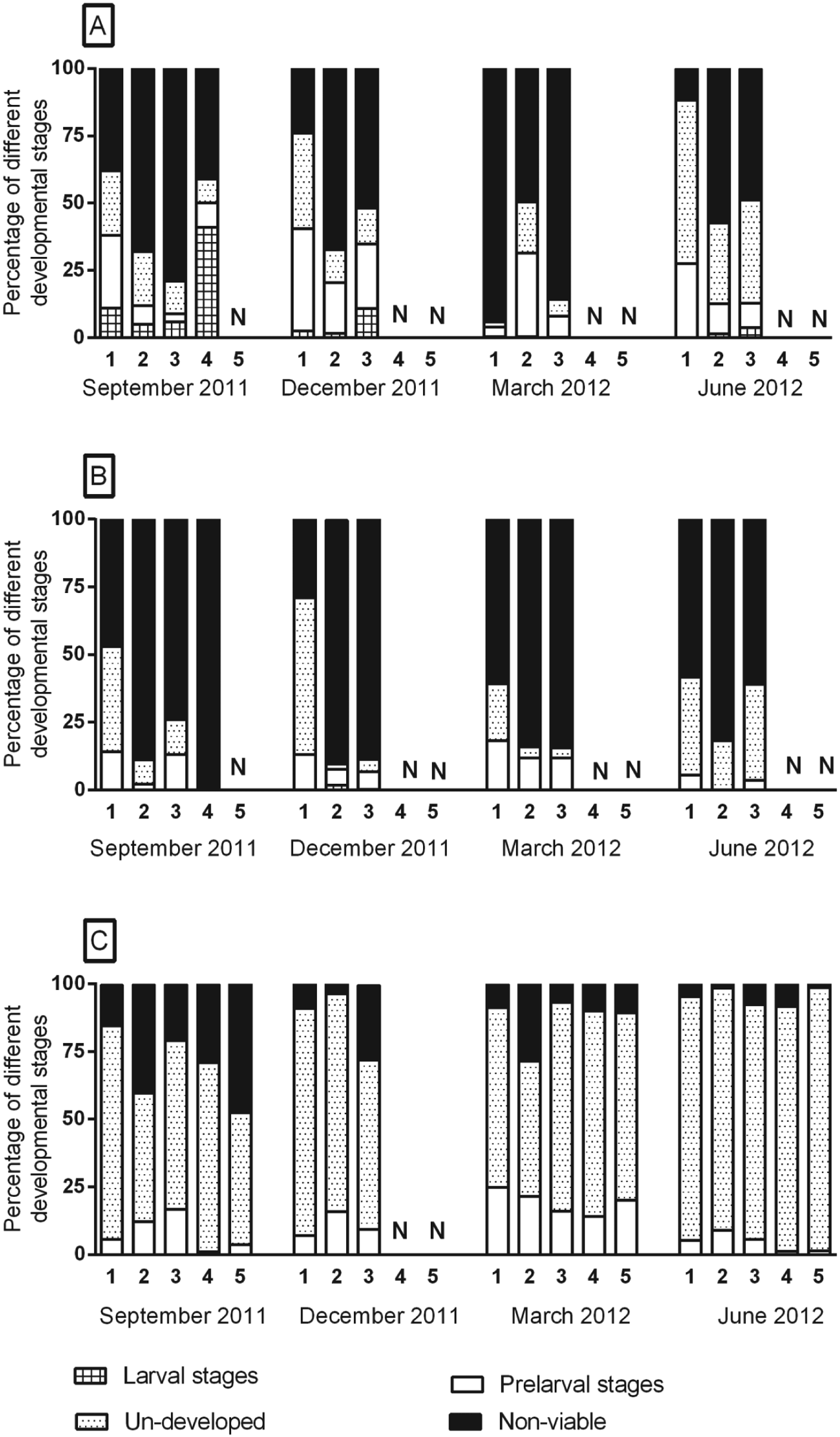
Sampling time had a highly significant effect on the development of eggs while they were in the litter (P<0·0001) and the highest proportion of eggs which had started developing was detected in December 2011 (22%) followed by March 2012 (17%), September 2011 (12%) and June 2012 (8%). The pen area also had a significant effect on development (P = 0·036) in that the resting area seemed to be the most favourable (Fig. 3) with around 21% of the eggs at some stage of development compared with the intermediate (15%) and latrine areas (9%). Depth of the deep litter also had a significant affect (P = 0·037) and 20% of the eggs had started developing in layer 1 as compared with 13–15% in the underlying layers. Of the physico-chemical parameters measured, only pH significantly influenced egg development (P = 0·006). At the low pH level (⩽8), 20% of eggs had started to develop whereas this was the case for only 10% of the eggs at the high pH level (>8). The proportion of larvated eggs (before laboratory embryonation) were significantly higher (P<0·0001) in resting areas (4%) compared with intermediate (0·004%) and latrine areas (0%). Similarly, the proportion of fully developed (presumably infective) eggs was higher in resting areas (0·7%) compared with intermediate (0·002%) and latrine areas (0%). This corresponds to the estimated total number of larvated and fully developed eggs in Table 1. Combined across areas, a significantly higher (P = 0·03) proportion of larvated eggs (0·1%) was observed during December 2011 than other study periods (September 2011 = 0·05%, June 2012 = 0·04% and March 2012 = 0·02%).

Fig. 3. Mean percentage of different developmental stage of Ascaris suum eggs in different layers of litter in (A) resting, (B) intermediate and (C) latrine areas of two finisher pig pens with deep litter at different seasons. Sample depths were: layer 1: 0–10 cm, layer 2: 20–25 cm, layer 3: 30–45 cm, layer 4: 50–65 cm and layer 5: 70–85 cm. N denotes absence of specific layer in that particular area and season. Number of replicates = 4.
Total number of viable eggs
Following embryonation in the laboratory, the total number of viable eggs varied significantly with pen area (P = 0·0001), depth (P = 0·0001), study period (P = 0·0001), temperature (P = 0·0014), pH (P = 0·0001) and moisture (P = 0·019). The highest numbers of viable eggs per g dry straw were found in the latrine areas followed by the resting and intermediate pen areas which correspond to the estimated total number of viable eggs in the entire bedding material (Table 1). Layer 1 generally contained the most viable eggs as the number of viable eggs decreased as the depth increased. Overall viability was highest in March 2012 followed by December 2011, June 2012 and September 2011. Low temperatures and low pH seemed to enhance viability, and though high moisture seemed to have the same effect, the effect of medium and low moisture levels was less clear. There was an interaction of season and temperature (P = 0·0089) and the lowest viability was observed in March 2012 and at high temperature (>28 °C).
Percentage of viable eggs
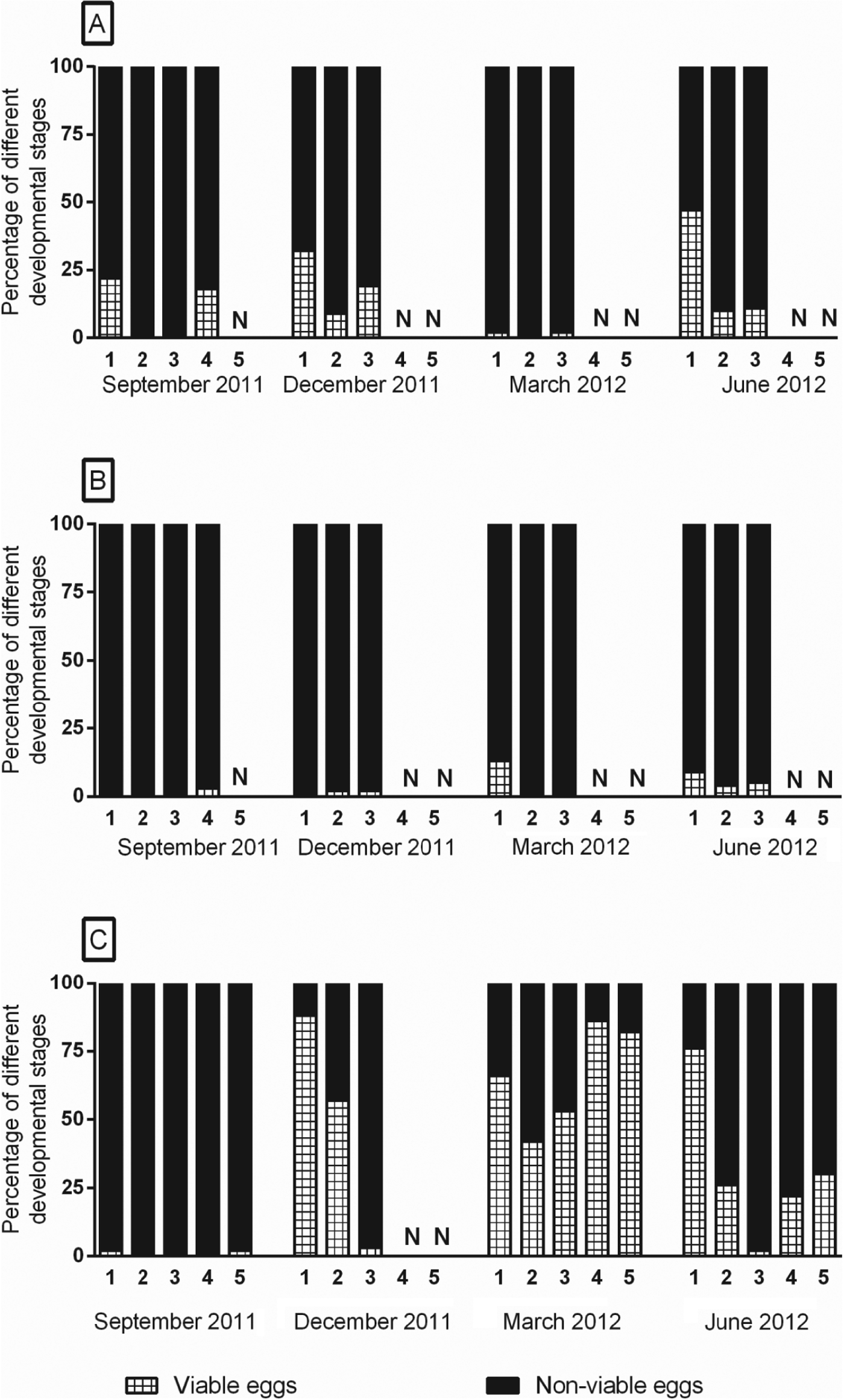
The overall proportion of viable A. suum eggs was significantly influenced by pen area (P<0·0001), depth (P = 0·0002) and study period (P = 0·0002) (Fig. 4). The overall percentage of viable A. suum eggs was thus highest in latrine areas (32%) followed by resting (17%) and intermediate pen areas (5%). Viability of eggs was overall highest in layer 1 (30%) as 11–28% of eggs in the underlying layers were found to be viable. Viability of eggs was highest in December 2011 (25%) followed by March 2012 (23%), June 2012 (18%) and September 2011 (11%). Viability was also significantly influenced by temperature (P<0·0001), pH (P<0·0001) and NH3(aq) (P = 0·008) in that high levels negatively influenced the viability. At low temperatures overall viability was 42% whereas at high temperatures it was 13%. Similarly, viability was 37 and 22% at low levels of pH and NH3(aq) and only 13 and 7% at higher levels of pH and NH3(aq), respectively. Effect of moisture was significant (P = 0·049) and lower viabilities were observed at low (15%) and medium (18%) levels of moisture when compared with that of high (31%) levels of moisture. The Spearman's correlation (r) for viability of eggs with temperatures was −0·53 whereas with NH3(aq) was −0·55.

Fig. 4. Mean percentage of viable Ascaris suum eggs in different layers of litter in resting (A), intermediate (B) and latrine areas (C) of two finisher pig pens with deep litter at different seasons. Viability was a measure of ability of previously un-embryonated eggs to develop in to fully larvated eggs upon in-vitro embryonation. Sample depths were: layer 1: 0–10 cm, layer 2: 20–25 cm, layer 3: 30–45 cm, layer 4: 50–65 cm and layer 5: 70–85 cm. N denotes absence of specific layer in that particular area and season. Number of replicates = 4.
DISCUSSION
The present study determined the development and viability of A. suum eggs in relation to selected physico-chemical conditions in deep litter bedding material on an organic pig farm. The study also documented that different areas of a given pen can provide different environments and that the development and viability of A. suum eggs may differ accordingly. The majority (99·99%) of eggs did not fully embryonate while in the litter in the pen, however, a sizeable proportion of eggs (19%) remained viable and could complete embryonation (i.e. to fully developed larvae) once removed from the litter and manure. The study further revealed that though A. suum eggs could be detected throughout the deep litter, the concentration of eggs in a given area generally reflected the level of faecal contamination of the straw. The eggs recovered from the apparently clean resting areas were probably due to contamination with eggs through passive transfer via the movement and rooting behaviour of the pigs.
The seasonality of the A. suum infections in the current study are in agreement with earlier studies (Martini et al. Reference Martini, Poglayen and Mancini1988; Menzies et al. Reference Menzies, Goodall and Taylor1994) with high prevalence and egg excretion levels seen in late summer–early winter (September 2011 and December 2011). This probably indicates a higher level of transmission of A. suum during the summer and early autumn due to high temperatures favourable for the development of eggs both on pastures and in pens (Connan, Reference Connan1977; Stevenson, Reference Stevenson1979; Roepstorff and Murrel, Reference Roepstorff and Murrell1997). It can only be speculated if the patent infections were picked up in the farrowing pastures, weaner pens or the finisher pens. Both weaner and finisher pens might have been contaminated by previous batches of pigs and not cleaned properly or the finisher pens could have been contaminated directly after introduction of the examined finisher pigs. Embryonation of A. suum eggs requires at least 4–5 weeks at constant ideal temperature (Oksanen et al. Reference Oksanen, Eriksen, Roepstorff, Ilsoe, Nansen and Lind1990) and the pre-patent period is 6–8 weeks (Roepstorff et al. Reference Roepstorff, Eriksen, Slotved and Nansen1997). As most pigs had been in the finisher pens for 2–3 months before the sampling, it is potentially possible that the first eggs excreted in the pen could have become infective and transmission might have taken place. However, the population dynamics of A. suum are complex in that protective immunity against migrating larvae (pre-hepatic barrier) and reduced establishment of new incoming larvae due to already established adult worms due to previous exposure (e.g. on farrowing pastures) may have reduced the chance of acquiring patent infections while in the finisher pens (Eriksen et al. Reference Eriksen, Nansen, Roepstorff, Lind and Nilsson1992; Jungersen et al. Reference Jungersen, Eriksen, Roepstorff, Lind, Meeusen, Rasmussen and Nansen1999; Mejer and Roepstorff, Reference Mejer and Roepstorff2006). Despite finding approximately 10 billion eggs in the bedding material of the deep litter pens, only 0·1 million eggs were fully developed. Thus, the level of transmission of A. suum may have been low in the pens due to a low probability of picking up these few eggs spread across the deep litter combined with probable acquired immunity due to previous exposure on farrowing pastures. However, the presence of infective eggs, though very few, indicates that transmission of A. suum could have taken place in the pens.
Though the temperature varied substantially among the different pen areas, it was generally high compared with ambient temperatures, e.g. maximum temperatures above 50 °C irrespective of the height of the litter. Similar findings were reported by Sommer and Moller (Reference Sommer and Moller2000) who observed temperatures of up to 70 °C in deep litter of fattening pigs. The high temperatures in the current deep litter indicate that composting took place due to aerobic microbial activity, which may result in the production of heat, NH3(aq), CO2, H2O and organic acids (Bernal et al. Reference Bernal, Alburquerque and Moral2009). The observed variation in temperature among the three pen areas indicates differences in composting activity, which was found to be higher in the intermediate areas followed by the resting and latrine areas. Several factors like temperature, pH, moisture, porosity (oxygen), C/N ratio and nutrient availability may affect the composting process (Bernal et al. Reference Bernal, Alburquerque and Moral2009). For effective composting, the initial temperature of the material should ideally be 20 °C or more (Mosher and Anderson, Reference Mosher and Anderson1977), the pH 5·5–8 and moisture content 50–70% (de Bertoldi et al. Reference de Bertoldi, Vallini and Pera1983; Kalyuzhnyi et al. Reference Kalyuzhnyi, Sklyar, Fedorovich, Kovalev, Nozhevnikova and Klapwijk1999; Petric et al. Reference Petric, Šestan and Šestan2009). The composting process is slowed down if the moisture content either drops below 20% due to reduced microbial activity (EPA, 1994) or exceeds 70% due to reduced aeration (Bernal et al. Reference Bernal, Alburquerque and Moral2009).
Both excess and limited porosity has a negative effect on the composting process due to loss of heat and creation of anaerobic conditions, respectively (Bernal et al. Reference Bernal, Alburquerque and Moral2009). Though we did not measure porosity of the bedding material, one can assume a high porosity in resting areas as the bedding material was loose and contained no or hardly any faeces and urine. In contrast, porosity was low in latrine areas as the bedding material was highly compacted and heavily mixed with faeces and urine, leaving no pockets of air and thus creating anaerobic conditions. The porosity of bedding material in the intermediate areas was likely to have been more optimal due to the moderate levels of faeces and urine, thus favouring microbial composting which caused the temperature to rise. The combination of different sub-optimal conditions such as low temperature, high moisture and low aeration in latrine areas and similarly low moisture and limited availability of nutrients in resting areas might have resulted in low levels of composting as indicated by the low observed temperatures. Higher levels of composting activity in latrine areas in September 2011 might have been due to higher ambient temperatures up to the time of sampling. The decline in temperature with increasing depth might be due to reduced composting activity due to anaerobic conditions as a result of compaction of bedding material and/or high moisture.
Recovery of eggs from latrine areas in September 2011 was different from the other three time points as practically no viable eggs were present, which might be due to high temperatures. Lack of or a low level of development while in the litter and presence of larvated eggs upon in vitro embryonation of eggs from latrine areas at the other three time points revealed that the general conditions prevailing in latrine areas may temporarily arrest the development of eggs. But once removed from the latrine 23% of the previously un-embryonated eggs were still viable and able to complete development. In contrast, the conditions prevailing (e.g. high temperatures) in the intermediate areas inactivated almost all eggs (99·6%). When compared with the other two areas, the resting area created favourable conditions for the development of eggs as it had the highest overall percentage of eggs which had started developing (21%) and a considerable proportion of eggs managed to reach early to late larval stages (4%) in this area. Nevertheless, only 0·7% of the eggs contained fully developed larva. The source of heat to promote egg development in this area might have been body heat of the resting pigs or transfer of heat from the adjoining intermediate area or a combination of both. As only 50 eggs were differentiated in each sample irrespective of the number of eggs present, the number of fully developed eggs may have been underestimated in samples with large numbers of eggs.
Viability of A. suum eggs decreases with time and unfavourable conditions may hasten inactivation (O'Donnell et al. Reference O'Donnell, Meyer, Jones, Benton, Kaneshiro, Nichols and Schaefer1984; Pecson et al. Reference Pecson, Barrios, Jiménez and Nelson2007; Nordin et al. Reference Nordin, Nyberg and Vinnerås2009; Katakam et al. Reference Katakam, Roepstorff, Popovic, Kyvsgaard, Thamsborg and Dalsgaard2013). In the current study, viability of eggs generally was highest in the top layer. In the underlying layers, eggs found at the same depth (and presumably of similar age) showed different viability patterns in different seasons and areas. This indicates that conditions (e.g. temperature, ammonia, pH and moisture as mentioned below) to which eggs are exposed will have more influence on viability than the age of eggs alone.
Development of A. suum eggs depends upon temperature (Seamster, Reference Seamster1950; Arene, Reference Arene1986; Katakam et al. Reference Katakam, Mejer, Dalsgaard, Kyvsgaard and Thamsborg2014) and below 14·5 °C they stop developing but remain viable for extended periods of time (Seamster, Reference Seamster1950). Higher temperatures adversely affect the eggs either by inhibition of physiological processes, desiccation in the absence of moisture or by increasing the permeability of eggs to toxic substances in the presence of moisture (Seamster, Reference Seamster1950; Barrett, Reference Barrett1976; Barnard et al. Reference Barnard, Bier, Jackson and McClure1987). The eggs thus stop developing at 38 °C and can remain viable for only 8 days at this temperature, after which they permanently lose the capacity to develop (Seamster, Reference Seamster1950). At 50 °C, Barnard et al. (Reference Barnard, Bier, Jackson and McClure1987) reported a t99 (time needed for inactivation of 99% of eggs) of about 6 h in water for A. suum eggs whereas Pecson et al. (Reference Pecson, Barrios, Jiménez and Nelson2007) reported a t99 of about 110 min in sludge. The very high proportion of samples with high temperatures ⩾38 °C in the intermediate areas might explain the high degree of inactivation of eggs in those areas. In the latrine areas, the temperatures were mostly much lower than 38 °C and still only a few per cent of the eggs started developing and very few per cent reached larval stages. This supports that the presumed very low porosity (thus low oxygen) may have been a major inhibiting factor in preventing eggs from developing.
Above the minimum threshold temperature, the rate of development increases with an increase in temperature; at 22 °C it takes 28 days to fully develop whereas at 31 °C it takes only 8 days (Seamster, Reference Seamster1950). Though higher temperatures increases the rate of development, infectivity of the eggs may be reduced as Arene (Reference Arene1986) reported that eggs embryonated at ⩾28 °C resulted in larvae with limited ability to migrate in vitro compared with those embryonated at lower temperatures. In the present study, it was mainly eggs in the resting areas that contained larval stages and the majority of samples (90%) in the resting areas had a temperature of ⩾28 °C indicating that infectivity to pigs may have been low even for fully developed eggs.
NH3(aq) levels were highest in intermediate areas and resting areas. This might have been due to overall higher temperatures and pH in these areas shifting the equilibrium between NH4+ and NH3 towards NH3 (Philippe et al. Reference Philippe, Cabaraux and Nicks2011). The decrease in NH3(aq) concentration as the depth of the litter in the latrine areas increased might have been due to volatilization or a decrease in temperature pushing the equilibrium towards NH+4(aq), the toxicity of which has never been described for microbes. NH3(aq) generally has an inhibiting effect on development and viability of A. suum eggs (Nilsson, Reference Nilsson1982; Katakam et al. Reference Katakam, Roepstorff, Popovic, Kyvsgaard, Thamsborg and Dalsgaard2013) and the time needed to inactivate eggs depends on the level of NH3(aq). Based on in vitro experiments in closed containers, Pecson et al. (Reference Pecson, Barrios, Jiménez and Nelson2007) reported a T99 of 87 days at a 58 mm NH3(aq) and 25 days at 294 mm at 20 °C and at a pH 12 in sewage sludge. Nordin et al. (Reference Nordin, Nyberg and Vinnerås2009) reported a T99 of 74 days at a concentration of 58 mm and only 13 days at 220 mm at 24 °C in faeces. The observed much lower mean NH3(aq) levels in the present study might be due to evaporation as higher temperatures of bedding material favour conversion of NH3(aq) to NH3(gas) (Philippe et al. Reference Philippe, Cabaraux and Nicks2011). However, it is not known if higher NH3(aq) levels may have been present and affected the eggs at some point in time in the microenvironment in the current study. Though the present study revealed a negative correlation between viability and temperature and NH3(aq), the exact contribution of each parameter to the inactivation of A. suum eggs cannot be ascertained from the present study as the age of the eggs and duration of their exposure to a specific level of each parameter was not known.
In order to have an inhibitory effect on the development and viability of A. suum eggs, pH should be above 11·5 (Eriksen et al. Reference Eriksen, Andreasen and Ilsøe1996; Gantzer et al. Reference Gantzer, Gaspard, Galvez, Huyard, Dumouthier and Schwartzbrod2001). Though the observed pH was slightly alkaline, the significant effect of pH on development and viability of eggs in the present study might have been indirect as pH affects the equilibrium between NH4+ and NH3 (Philippe et al. Reference Philippe, Cabaraux and Nicks2011).
Moisture in itself plays an important role in the development and viability of A. suum eggs and continuous exposure of eggs to low moisture content may lead to desiccation. Seamster (Reference Seamster1950), who exposed eggs to relative humidity (RH) levels of 80, 85, 95 and 100% at 31·1 °C, reported that eggs that were kept at 100% RH developed whereas those kept at the lower RH were desiccated. In the present study, moisture may have been a limiting factor for development and viability in resting areas as the average observed moisture content of the litter was only 36%. Sanguinetti et al. (Reference Sanguinetti, Tortul, Garcia, Ferrer, Montangero and Strauss2005), who measured viability as described in the present study, observed a reduction in viability of eggs when the humidity dropped below 40%.
A previous study by Holmgren and Nilsson (Reference Holmgren and Nilsson1998) reported that deep litter systems pose a higher risk for helminth infections compared with shallow litter systems, but the present results revealed that most of the eggs were inactivated (or development ceased) within the deep litter, indicating that this may not be as risky as previously presumed. The study thus demonstrated that the deeper parts of the bedding material in the pens create unfavourable conditions for the development of eggs to infectivity although a significant proportion remains viable. Transmission is, however, unavoidable because eggs become infective in the upper layers of the cleaner parts of the pen. Application of used bedding material (spent litter) to crops without proper treatment may potentially contaminate the fields with A. suum eggs. A thorough composting of the bedding material before application to fields that may at some point be used for animals is therefore imperative.
ACKNOWLEDGEMENTS
The help of Lise-Lotte Christiansen, Jakob Felsager, Orla Nielsen, Sundar Thapa, Uffe Christian Braae, Lars Stoumann Jensen, Lene Vigh and Ea Larsen is gratefully acknowledged.
FINANCIAL SUPPORT
PhD fellowship of Kiran Kumar Katakam was jointly financed by the Faculty of Health and Medical Sciences, University of Copenhagen and the PhD Research School, RECETO. The project (Parasites in Organic Livestock: innovative solutions to new challenges) is part of the Organic RDD programme, which is coordinated by the International Centre for Research in Organic Food Systems, ICROFS and is funded by the Danish AgriFish Agency, Ministry of Food, Agriculture and Fisheries.