INTRODUCTION
In a rapidly changing world, understanding the factors controlling the spatial distribution of parasites is of great importance for biodiversity conservation (Wimberly et al. Reference Wimberly, Yabsley, Baer, Dugan and Davidson2008). For this purpose, it is necessary to acquire precise knowledge of the biological mechanisms underlying the dynamics of parasite populations (Lafferty, Reference Lafferty2009a). Parasite life-history traits such as fecundity, body size and growth are perfect candidates for such a purpose since they determine the survival, propagule pressure and transmission rate of parasites (Poulin, Reference Poulin1995a).
The probability of a free-living parasite encountering and infecting an appropriate host is likely to be low and stochastic (Combes, Reference Combes2001; Hakalahti et al. Reference Hakalahti, Hakkinen and Valtonen2004). Hence, for most parasite species, increased fecundity is likely to be a selected strategy for improving host encounter rate (Poulin, Reference Poulin1995b). An increased fecundity is likely to improve host encounter by producing larger propagules with a higher rate of survival (Hakalahti et al. Reference Hakalahti, Hakkinen and Valtonen2004; Herreras et al. Reference Herreras, Montero, Marcogliese, Raga and Balbuena2007). Because the number and size of propagules compete directly for a limited energy budget, a phenotypic trade-off is expected between them (Smith and Fretwell, Reference Smith and Fretwell1974; Roff, Reference Roff1992; Stearns, Reference Stearns1992). Even if rarely demonstrated in parasitic organisms (but see Rossin et al. Reference Rossin, Poulin, Timi and Malizia2005; Timi et al. Reference Timi, Lanfranchi and Poulin2005; Herreras et al. Reference Herreras, Montero, Marcogliese, Raga and Balbuena2007), it is assumed that within-population variability in female body size determines the strength and the shape of the trade-off between propagule size and number (Roff, Reference Roff1992). In natural populations of free-living organisms, environmental conditions can also influence this trade-off (Blanckenhorn and Heyland, Reference Blanckenhorn and Heyland2004; Jessup and Bohannan, Reference Jessup and Bohannan2008). For instance, Schluter et al. (Reference Schluter, Price and Rowe1991) showed that beneficial environments, and specifically the positive effects of nutrition on fitness traits, might mask trade-offs expected under some selective regimes. Analysis of the trade-off between egg number and egg size therefore requires knowledge of environmental conditions in which organisms are living, and also on the relationships between those conditions and other life-history traits (Jessup and Bohannan, Reference Jessup and Bohannan2008). In parasites, these relationships remain largely unexplored, mainly because of the difficulty in collecting information.
An additional difficulty arises from the fact that parasites are constrained in a nested scheme in which components are highly inter-dependent (Keitt and Urban, Reference Keitt and Urban2005; McMahon and Diez, Reference McMahon and Diez2007). Specifically, parasites are nested within their hosts that are themselves nested within the surrounding environment (Morand, Reference Morand1996; Seppala et al. Reference Seppala, Liljeroos, Karvonen and Jokela2008). Accordingly, life-history strategies adopted by parasites are likely to be under the combined pressure of many interacting forces such as host density, host condition or abiotic factors (Morand, Reference Morand1996; Eisen and Schall, Reference Eisen and Schall2000; Hakalahti et al. Reference Hakalahti, Hakkinen and Valtonen2004; Seppala et al. Reference Seppala, Liljeroos, Karvonen and Jokela2008). Modelling hierarchically structured data in natural settings has become increasingly efficient through the development of specific statistical models (Keitt and Urban, Reference Keitt and Urban2005; Diez and Pulliam, Reference Diez and Pulliam2007; McMahon and Diez, Reference McMahon and Diez2007). These models should prove powerful for disentangling relationships between physical/biological factors and variation in life-history traits of parasites in natural settings (Byers et al. Reference Byers, Blakeslee, Linder, Cooper and Maguire2008).
Tracheliastes polycolpus von Nordman 1832 (Copepoda: Lernaeopodidae) is an ectoparasite of wild fish populations distributed throughout the northern Paleartic region. Tracheliastes polycolpus completes its holoxenous life cycle in cyprinid fishes. The common host in Western Europe is the dace Leuciscus leuciscus Linnaeus (Loot et al. Reference Loot, Poulet, Reyjol, Blanchet and Lek2004). Only sexually mature females are parasitic and they are anchored to host fins and feed on epithelial cells and mucus of the host, characteristically damaging fins (Loot et al. Reference Loot, Poulet, Reyjol, Blanchet and Lek2004). During their parasitic phase, females develop 2 egg sacs that each carry several hundred eggs. Eggs hatch throughout the year (Fryer, Reference Fryer1982) as free-living copepodids, which then develop into 4 successive chalimus stages. Within egg sacs, eggs are at the same stage of maturation indicating that all eggs hatch simultaneously. In females, the last stage is morphologically modified for attachment to the host (Kabata and Cousens, Reference Kabata and Cousens1973). Adult males are free-swimming and dwarfed. They usually die very soon after mating, and never become parasitic (Kabata, Reference Kabata1986).
The goals of this study were 2-fold. First, we aimed to evaluate how the variability observed in the wild for 4 life-history traits of T. polycolpus (body size, egg presence, egg number and egg size) segregates across multiple levels of measurement (site level, host level, fin level and parasite level). To assess the relative roles of abiotic and biotic variables in explaining life-history variation we sampled T. polycolpus individuals along a river gradient and used a hierarchical modelling framework which explicitly integrates possible associations across the above-cited levels (McMahon and Diez, Reference McMahon and Diez2007). Second, we studied the phenotypic trade-off between egg number and egg volume of T. polycolpus within and between these sampling sites. We analysed co-variation in clutch investment and allocation using female body size as a control in order to gain insight into the factors determining the presence or absence of this trade-off.
MATERIALS AND METHODS
Sampling design
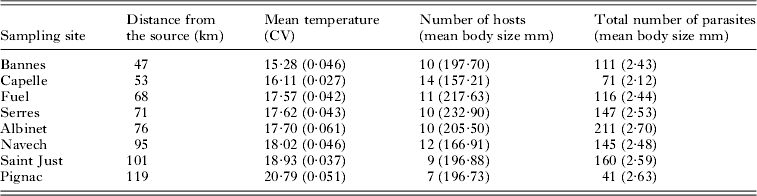
A hierarchical design was used to capture a range of environmental conditions across an altitudinal gradient of approximately 70 km along the Viaur river continuum in southwestern France. Eight study sites were sampled along the river gradient, each site was between 1000 and 2000 m2 in size (see Table 1). Sampling was performed in early summer within the same week (16–20 June 2005). Each site was separated by at least a 3–18 km river stretch to cover all environmental characteristics met by L. leuciscus and T. polycolpus. We hereafter use the term ‘site level’ to refer to inference at the level of the study site. Between 7 and 14 Leuciscus leuciscus were sampled by electrofishing (Deka 6000 DC, Deka, Marsberg, Germany) within each site (see Table 1 for more details). The term ‘host level’ hereafter refers to inference at the level of the individual host. We use the term ‘microhabitat level’ to refer to inference at the level of the host fins and the term ‘parasite level’ to refer to inference at the level of the individual parasite.
Table 1. Distance from the source, mean temperature, number of hosts and parasite samples for each sampling site
Parasite life-history trait measurements
Overall, 1002 individual parasites were collected on 83 fish from 8 sampling sites (see Table 1 for more details). All parasites for a given host were sampled. Each parasite was measured for 4 life-history traits: body size, presence/absence of eggs, egg size, and number of eggs. Tracheliastes polycolpus were individually placed in 1·5 ml Eppendorf® tubes containing river water to keep them alive during transport to the laboratory. At the laboratory, a picture of each parasite was taken with a digital camera. The camera (Nikon Coolpix 4500®) was fixed on a binocular microscope, and a metric ruler was placed to provide a baseline scale. The same focal length was kept in order to avoid any picture distortion. We used the ImageTool software (free download at http://ddsdx.uthscsa.edu/dig/itdesc.html) to measure body size (measured as the trunk length) of all parasites. We noted whether females carried egg sacs. When egg sacs were present, the egg diameter (as a surrogate of the size of the egg) was measured for a random sample of 10 eggs per female. We identified 2 distinct developmental stages of eggs according to their colouration (4 black dots were visible on one stage but not on the other one) and size. The first egg stage was significantly smaller (mean±s.d.; 0·159 mm±0·015) than the second stage (0·169 mm±0·019) (ANOVA, d.f.=1, 447; F=37·91; P<0·001). To avoid statistical biases in egg size analysis we only considered females with egg sacs containing eggs of the second stage (that we considered as mature eggs). After the picture, egg sacs were removed from each parasite and individually stored in Gilson's solution for 2 months to digest the sac tissues and separate eggs. Then, the number of eggs in 1 egg sac was determined at 10× magnification.
Biotic and abiotic measurements
Site level
Abiotic variables considered at the site level were the mean annual water temperature (°C), the mean water velocity (cm.s−1) and a measure of water quality. Tracheliastes polycolpus is anchored to the fins of its host, and it can directly be affected by flow rate (energy could be allocated for facing the current velocity rather than for developing gonads) and by the temperature of the surrounding environment (Boxshall, Reference Boxshall and Rohde2005). Mean annual water temperature at each site was extracted from a continuous data recorder (Tynitag®). Temperature was recorded (to the nearest 0·1°C) every 6 h during the year preceding the parasite sampling. During the sampling period, water velocity was recorded at 25 haphazardly selected points within each site using a wading rod and an electronic flow meter (to the nearest 0·05 m.s−1). An average value was calculated and used to characterize flow rate in each site. We finally calculated the diatom index that is an integrative indicator of water quality and environmental conditions (water chemistry and water oxygenation) of a given site (Cox, Reference Cox, Whitton, Rott and Friedrich1991; Taylor et al. Reference Taylor, Prygiel, Vosloo, de la Rey and van Rensburg2007). This index is based on the presence/absence of certain diatom species in samples, some species being indicators of good water quality, others not. Epilithic diatom assemblages were sampled at each site by brushing 5 cobbles located in riffle or running sections. Each sample was cleaned in boiling hydrogen peroxide and chloridric acid. After removing all traces of chloridric acid, the diatom frustules were dried onto cover-slips and mounted onto glass slides in Naphrax® (Northern Biological Supplies, Ipswich, UK). At least 400 frustules per sample were identified to species level and counted under a photonic microscope at ×1000 magnification (see Grenouillet et al. Reference Grenouillet, Brosse, Tudesque, Lek, Baraille and Loot2008 for more details). The diatom index was calculated with the OMNIDIA 4.2 software (Lecointe et al. Reference Lecointe, Coste and Prygiel1993). The diatom index varies from 1 to 20, higher values indicating better water quality. The only biotic variable considered at the site level was the host density. The dynamics of parasite populations is known to be partly influenced by the density of hosts in a given habitat (Grenfell and Harwood, Reference Grenfell and Harwood1997; Arneberg et al. Reference Arneberg, Skorping, Grenfell and Read1998). We therefore evaluated dace density in each site by electrofishing (see above) using the 2-pass depletion method classically used for evaluating fish density in streams (Bohlin et al. Reference Bohlin, Hamrin, Heggberget, Rasmussen and Saltveit1989). Within each site, we delimited a stream reach (from 300 to 780 m2) that was as representative as possible of the entire site, and we captured dace in this reach. Dace were stored in aerated tanks between the 2 passes. Dace density was estimated using the method of Carle and Strub (Reference Carle and Strub1978).
Host level
Studies have shown that parasite life-history traits can be affected by characteristics of the host such as its age, size, diet, or immune status (Tsai et al. Reference Tsai, Li and Dai2001; Krasnov et al. Reference Krasnov, Khokhlova and Shenbrot2002; Barber, Reference Barber2005). Thus, after being captured, dace were anaesthetized (eugenol solution), measured (total body length, ±1 mm) and weighed (±1 g) before being released in their respective sites. The overall condition of each host was estimated using the Fulton's condition factor (K=weight per length3) (Nash et al. Reference Nash, Valencia and Geffen2006). Host body condition has been shown to increase the host immune function and thus the host ability to resist or tolerate parasites (Krist et al. Reference Krist, Jokela, Wiehn and Lively2004; Blanchet et al. Reference Blanchet, Méjean, Bourque, Lek, Thomas, Marcogliese, Dodson and Loot2009). Both the host body length (which is correlated to age in fish) and the condition factor were used as potential predictors of parasite life-history traits at the host level.
Microhabitat level
Two variables related to this level were considered to potentially influence life-history traits of T. polycolpus: the number of conspecifics present on each fin and the identity of the fin where the parasite was sampled (i.e. pelvic, pectoral, dorsal, anal or caudal fin). Changes in fecundity may be explained by the number of conspecifics sharing a host, which could influence the parasite's per capita average fecundity (Anderson, Reference Anderson and Cox1993). Intraspecific competition can be strong in parasites (Crompton, Reference Crompton, Toft, Aeschlimann and Bolis1991; Patrick, Reference Patrick1991; Hawlena et al. Reference Hawlena, Abramsky, Krasnov and Saltz2007), and we thus suppose that the number of conspecifics per fin could negatively influence the quantity of nutrients available for parasite growth. In addition, a recent study (Timi et al. Reference Timi, Lanfranchi and Poulin2010) demonstrated that the attachment site selected by a parasitic copepod (Neobrachiella spinicephala) on its fish host affected both parasite body mass and egg production. Patterns of microhabitat preferences have been highlighted in T. polycolpus, with individuals being anchored preferentially (particularly when at low density) on pectoral, anal and pelvic fins (Loot et al. Reference Loot, Poulet, Reyjol, Blanchet and Lek2004). According to Timi et al. (Reference Timi, Lanfranchi and Poulin2010), we would predict higher fitness in individuals on those fins.
Parasite level
Variability in parasite female body size has been reported as a source of variation in reproductive investment for many parasites (e.g. Rossin et al. Reference Rossin, Poulin, Timi and Malizia2005; Herreras et al. Reference Herreras, Montero, Marcogliese, Raga and Balbuena2007). Parasite body size was thus used as a possible predictor of the presence/absence of eggs, the number of eggs and the size of the eggs at this level.
Statistical analysis
Relative contribution of abiotic and biotic factors on life-history traits
Our sampling design produced either 3 (for the body size of the parasite) or 4 (for the presence/absence of eggs, the number of eggs and the size of the eggs) levels of nesting with respect to the response variable. Therefore, in order to consider the influence of key biotic and abiotic variables on life-history traits of T. polycolpus, and to account for the statistical error structure imposed by the nested data, we implemented hierarchical (or multilevel) generalized linear models based on maximum likelihood to estimate parameters (HGLMs McMahon and Diez, Reference McMahon and Diez2007).
The models developed here were very simple in their formulations and were fitted using the ‘lme4’ package in R (R Development Core Team, 2002). For each model we included predictor variables as fixed-factors. All predictors were initially centred around zero (using the function ‘scale()’ in R) to standardize all predictors and to facilitate the interpretation of slope coefficient estimates. The only exception was ‘fin identity’ as it was treated as a categorical predictor. At the microhabitat level, we included the interaction term between number of parasites and fin identity as we hypothesized that the relationship between parasite number and parasite life-history traits could vary according to fin identity (for instance, pelvic fins are thinner than the caudal fin and could be easier to degrade). Random factors correspond to each of the 3 or 4 levels considered, with the factor of the lower level being nested within the immediate factor of the upper level (e.g. host microhabitat level nested within host level). In summary, a nested structure corresponding to the nested sample design was used for fitting the random part of the model and to account for the statistical error structure imposed by this design (see Pinheiro and Bates, Reference Pinheiro and Bates2002). A 3-level model assuming a Gaussian error-term distribution was built for testing the variables that best predict the parasite body size. A 4-level model assuming a Binomial error-term was built for the presence/absence of eggs. Four-level models assuming a Gaussian error-term were built for the number of eggs and the size of the eggs. Each model was fitted by maximizing the restricted log-likelihood (REML method). The 95% confidence interval for the estimate of each slope coefficient was estimated using Markov chain Monte Carlo (MCMC) sampling methods (5000 iterations). An estimate was considered as significant when its 95% interval does not include zero. The significance of the categorical predictor was assessed using log-ratio tests.
Unconditional models were initially fitted to determine the basic variance partitioning of each of the response variables among the 4 levels of association (Diez and Pulliam, Reference Diez and Pulliam2007). In this kind of model, only the random part of the full model is considered and is used to calculate interclass correlation coefficients. Note that in these unconditional models, the random part contained 4 levels (parasite scale, microhabitat scale, host scale, site scale) for each response variable.
Trade-off between egg number and egg volume
We performed HGLM to test (i) for a trade-off between egg number and egg size in T. polycolpus and (ii) if the strength of this trade-off varied according to the surrounding environment. Female body size is known to affect this trade off (Herreras et al. Reference Herreras, Montero, Marcogliese, Raga and Balbuena2007). Hence in this analysis, we used the residuals of linear models between egg size and parasite body size and between egg number and parasite body size as corrected measures of egg size and egg number respectively. In this HGLM, egg number (residuals) was the dependent variable and we included sampling sites' identity, host body size and egg size (residuals) as fixed predictors. A negative relationship between egg number and egg size would indicate a trade-off between these traits. To test for a potential effect of the surrounding environment on the strength of this trade-off, we tested 2 interaction terms since the surrounding environment of the parasite has 2 components: its host and the characteristics of the sampling location. A significant interaction between egg size and host body size would indicate that the relationship between egg number and egg size varied according to the body size of the host. If a large host body size provides more energy for parasites, the strength of the trade-off should be weaker on larger hosts. The second interaction concerned egg size and sample site identity; a significant interaction would indicate that the relationship between egg number and egg size varied according to the sampling location. Random effects were host identity nested within sampling sites.
RESULTS
Variance partitioning
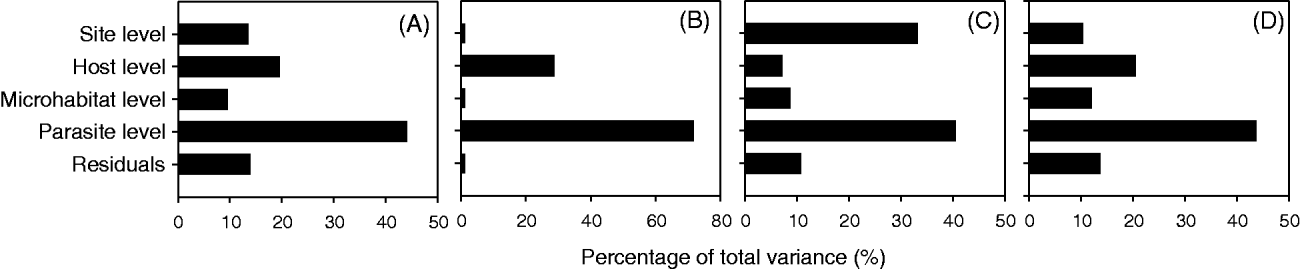
Variance partitioning using unconditional models revealed that for the 4 life-history traits, more than 40% of the variation was distributed among parasites (Fig. 1). For the parasite body size, the number of eggs per parasite and the average size of eggs, greater than 20% of the variation was partitioned among hosts (Fig. 1A, C and D). Greater than 30% of the variation observed for the presence of eggs was distributed among sites (Fig. 1B).

Fig. 1. Pattern of variance decomposition for 4 life-history traits of the ectoparasite Tracheliastes polycolpus, (A) parasite body size, (B) presence of eggs, (C) average size of the eggs per parasite and (D) number of eggs per parasite. The total observed variance for each trait was decomposed among 4 hierarchical levels of sampling, namely within site, within host, within microhabitat (i.e. the fins on which the parasite was sampled) and within parasite. For each trait, variance was decomposed using hierarchical linear models. ‘Residuals’ is the percentage of variance that was not explained by the 4 spatial levels under consideration.
Relative contribution of abiotic and biotic factors on life-history traits
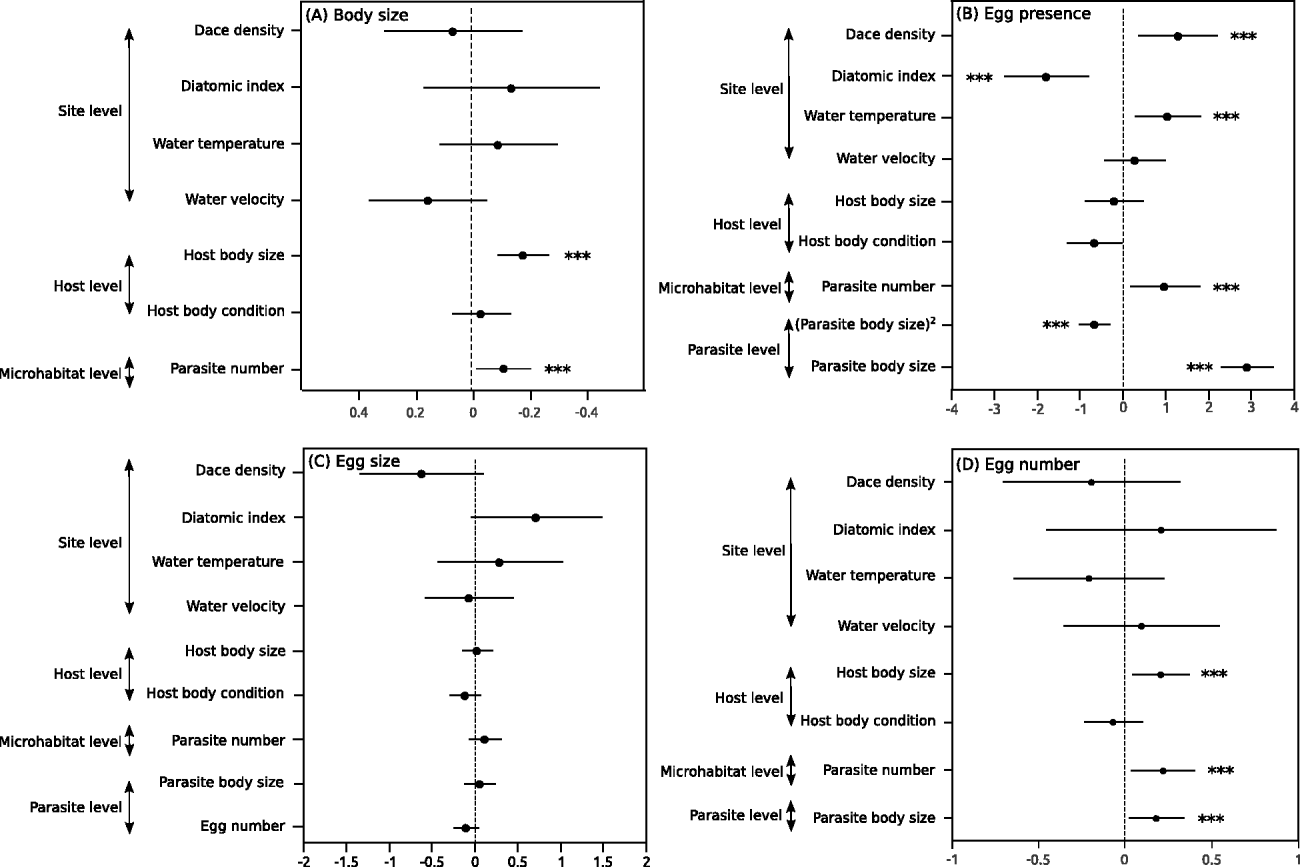
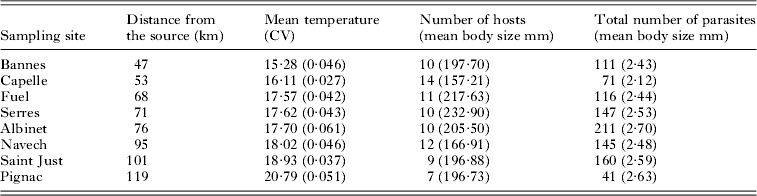
The 4 traits exhibited different relationships with the biotic and abiotic variables in the full hierarchical models. For all traits except egg size (Fig. 2C) at least 1 of the variables selected had a significant effect on the response variable.
Fig. 2. Scale-explicit coefficient estimates. Solid lines represent 95% confidence interval effects of variables at 3–4 levels of the model (site level, host level, microhabitat level and parasite level). Intervals not overlapping the zero line are significantly different from zero. Dots represent point estimate of parameter value. Predictors of (A) parasite body size, (B) egg presence, (C) egg size, and (D) egg number. The asterisks (***) indicate significant results (P<0·05).
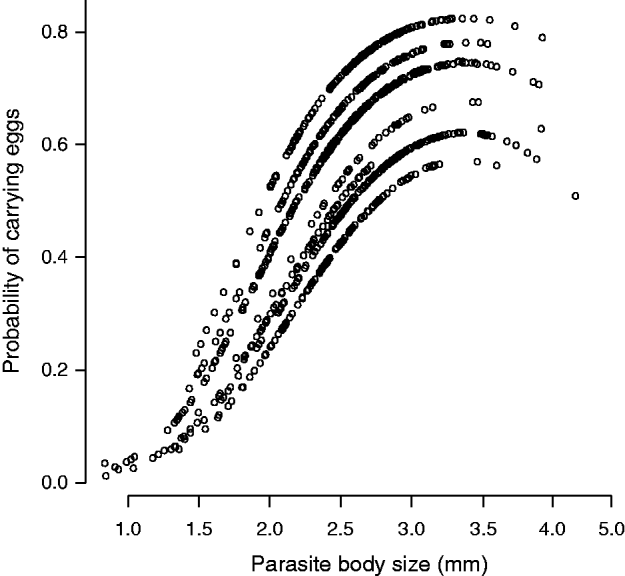
Both the parasite body size and the number of eggs showed similar patterns, with both traits being positively affected by the host body size and the number of parasites on each fin (Fig. 2A, D). The number of eggs was also significantly greater in larger parasites (Fig. 2D). The probability of carrying eggs was affected by several variables operating at multiple levels. First, the presence of eggs was affected by the size of the parasite, with the probability of carrying eggs being highest for parasites of intermediate body size (i.e. ∼3·5 mm) (Fig. 2B, Fig. 3). This pattern was consistent for all sampled sites (Fig. 3). This indicates that the probability of carrying eggs increased until a maximum body size was reached, probably the size at which the parasite lays eggs. Second, the parasite number positively affected the probability of carrying eggs (Fig. 2B). Finally, the probability of carrying eggs increased as the host density and the water temperature within a site increased and decreased as the diatom index (i.e. the level of water quality) decreased (Fig. 2B).

Fig. 3. Influence of the body size of the ectoparasite Tracheliastes polycolpus on the probability of carrying eggs. The probabilities of carrying eggs are those predicted from a linear-mixed model with binomial error term (see the main text). Parasites with intermediate body size had the higher probability of carrying eggs. The relationship is presented for each sampling site independently.
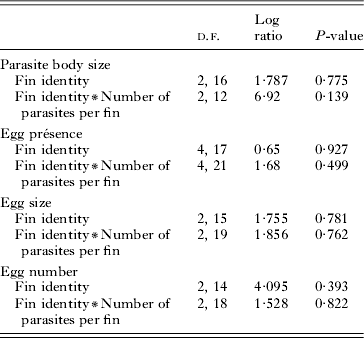
There was no significant ‘fin identity’ effect for any of the 4 traits, and similarly no interaction term between fin identity and parasite number was significant (Table 2). This indicates that the relationships between parasite number, parasite body size, egg presence and egg number did not differ among fins.
Table 2. Results of general linear mixed models used to evaluate the effects of various predictors on parasite body size, egg presence, egg size and egg number of the parasite Tracheliastes polycolpus

Trade-off between egg number and egg volume
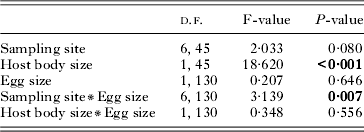
As above, we found a significant positive relationship between host body size and egg number (Table 3, slope coefficients not shown). Although no significant relationship between egg number and size was found, we highlighted a significant interaction between egg size and sampling site identity (Table 3).
Table 3. Results of a General linear model used to evaluate the effects of sampling site, host body size and the egg size on the egg number of the parasite Tracheliastes polycolpus
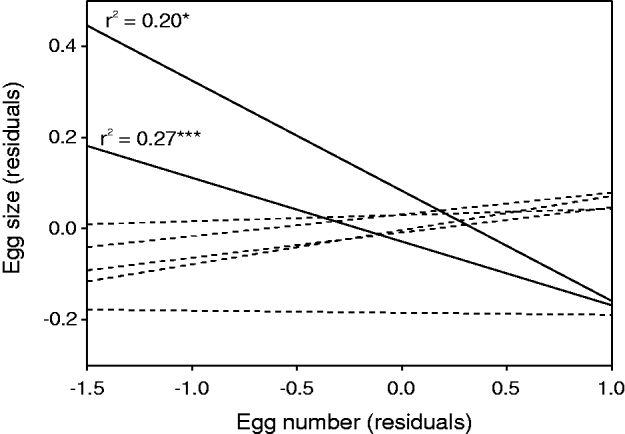
Such an interaction indicates that the relationship between egg number and egg size varied according to sampling location. Particularly, a significant (P<0·05) and negative relationship between egg number and egg size was found for 2 study sites (Fig. 4). We found no evidence for a significant relationship at the other sites. Similarly, we found no significant interaction term between egg size and host body size, indicating that the strength of the trade-off did not vary according to host body size. One sampling site was discarded from this analysis because no females sampled had eggs.

Fig. 4. Results of regression analysis between egg number (residuals) and egg size (residuals) of 7 populations of Tracheliastes polycolpus. Straight solid lines indicate significant trends (* P<0·05; *** P<0·001). Stippled lines indicate non-significant relationship.
DISCUSSION
In this study, we demonstrated that life-history traits (body size and fecundity components) of a fish ectoparasite strikingly varied along a short environmental gradient. For most life-history traits, a large amount of this variation was observed among individual parasites. In addition, we found that each of the 4 life-history traits analysed was unique in its response to the determinants considered. Indeed, some traits demonstrated significant relationships with factors measured at the parasite level (e.g. the presence of eggs), while others showed significant relationships with factors measured at the microhabitat level (parasite body size, presence of eggs and eggs' number), the host level (parasite body size and eggs' number) and/or the environmental level (presence of eggs). Finally, we found evidence for a phenotypic trade-off between the number and the size of eggs, albeit the strength of this trade-off was site dependent.
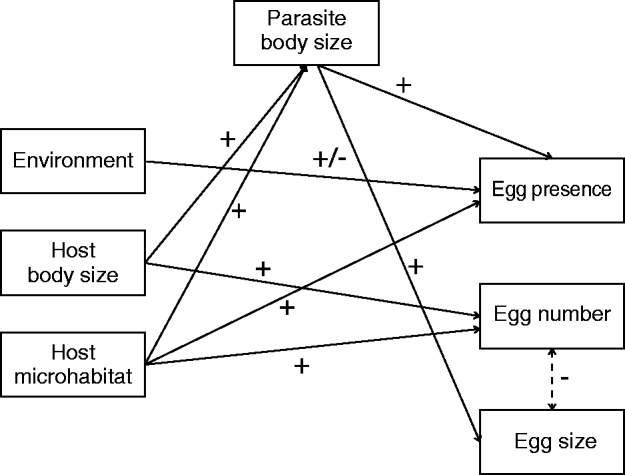
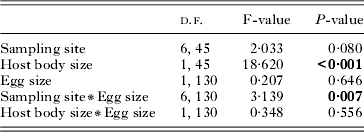
We summarized in Fig. 5 the significant relationships between life-history traits and biotic and abiotic factors considered in this study. This figure highlights that parasite number measured at the microhabitat level (i.e. fins) is a significant predictor for 3 out of the 4 life-history traits we investigated. Particularly, we demonstrate significant positive relationships between fitness-related parameters and local parasite number, with parasites being larger, having a higher probability of laying eggs and producing a larger number of eggs when anchored to fins with high parasite numbers. This result was independent of the fin identity because parasite fitness did not differ among fins, which contrasts with the findings of Timi et al. (Reference Timi, Lanfranchi and Poulin2010). Host body size is highly correlated to fin surface (Drucker and Lauder, Reference Drucker and Lauder2002), so we can assume that the parasite number per fin is a good surrogate of parasite density in our analyses. Hence, although correlation is not causation, we can reasonably argue that higher local parasite numbers, irrespective of host body size and fin identity, favour individual parasite fitness. This result is in opposition to the general expectation that the number of conspecifics sharing a host constrains resource availability (i.e. the carrying capacity hypothesis; Hughes et al. Reference Hughes, Petersen, Ugelvig, Pedersen, Thomsen, Poulsen and Boomsma2004), and hence decreases individual fitness. For instance, parasites have the potential of reaching their maximum body size only when they do not share their host with conspecifics (Poulin, Reference Poulin1998). In addition, it has been shown that high within-host competition negatively affects per capita egg production of many helminth parasite species (Jones et al. Reference Jones, Breeze and Kusel1989). Our study is one of the first to reveal a positive effect of conspecifics on individual fitness traits (but see Rossin et al. Reference Rossin, Poulin, Timi and Malizia2005 for a weak tendency with egg number). This positive density dependence (a kind of Allee effect) may result from within-host additive actions of parasites sharing a fin. Parasites may, for instance, act synergistically to produce tissue-damaging compounds (Whiteman et al. Reference Whiteman, Santiago-Alarcon, Johnson and Parker2004), or suppress the immune system.
Fig. 5. Path diagram revealing the interactions between life-history traits of parasite Tracheliastes polycolpus(parasite body size and reproductive output variables) and host and environmental factors.
At the host level, we found that host body size was positively correlated with parasite body size and egg number. Several studies have demonstrated that the overall quality of host-related to its age, size, diet or immune status-affected the reproductive success, and hence the fitness of parasites (Quinnell, Reference Quinnell1988; Seppala et al. Reference Seppala, Liljeroos, Karvonen and Jokela2008). Tsai et al. (Reference Tsai, Li and Dai2001) showed that the body size of the fish host Varicorhinus bacbatulus positively affected clutch size of the parasitic isopod Ichthyoxenus fushanensis. Similarly, a positive association between host body size and parasite body size has already been documented in other host-parasite systems (Jennings and Calow, Reference Jennings and Calow1975; Poulin, Reference Poulin1995b; Tsai et al. Reference Tsai, Li and Dai2001) and has been proposed as a space and/or food constraint imposed by the host. In our case, we can speculate that large hosts directly provide higher nutritional inputs to parasites, or are weaker in mounting an efficient immune response against parasites (Seppala et al. Reference Seppala, Liljeroos, Karvonen and Jokela2008). However, although the use of standardized coefficient regression is supposed to assess the independent strength of each factor (Murray and Conner, Reference Murray and Conner2009), one cannot totally rule out the possibility of an indirect effect of host body size. Indeed, larger and older hosts display higher T. polycolpus loads (Blanchet et al. Reference Blanchet, Méjean, Bourque, Lek, Thomas, Marcogliese, Dodson and Loot2009); we can hence speculate that because larger and older hosts have a higher parasite load, the number of parasites is higher at the microhabitat level, which increases the individual fitness of parasites. In this context, the positive association between parasite number and fitness would be an indirect consequence of differences among host ‘quality’. Additional studies that make use of causal modelling (Grace, Reference Grace2006) should be performed to decipher between direct or indirect effects of bigger hosts on parasite fitness.
At the environmental level, we found that dace density, water quality and water temperature significantly affected the presence of eggs in T. polycolpus. No other life-history traits were affected by environmental level factors. Concerning the presence of eggs, there was a higher probability of finding a T. polycolpus with eggs in sites with a high density of dace, a low diatom index (i.e. site with a weaker than the mean water quality) and a high water temperature. The positive relationship between water temperature and egg presence might indicate a temporal asynchrony among sites, implying that parasites develop eggs earlier in the season in warmer sites. However, at this step, speculation about the process underlying these relationships between environmental characteristics and life-history straits is still complex and uncertain. These results need to be tested in the laboratory before drawing conclusions about mechanisms underlying these patterns. However, a very important result is that environmental variation did not seem to be the most important component of life-history trait variation in T. polycolpus. Indeed, as stated above, environment-level factors were significantly linked to a single trait while microhabitat level factors affected 3 out of the 4 life-history traits investigated here. In addition, unconditional models reveal that most of the variation in all life-history traits was observed at the parasite level. This latter finding suggests that the environment may have a limited effect on T. polycolpus life-history traits, and that unmeasured factors (e.g. hatching date, relatedness, genetic background …) at the parasite level would be better candidate factors for future studies.
Finally, we report interesting and new patterns concerning the trade-off between egg size and egg number in parasites. Indeed, we found no general trade-off between egg size and egg number when all data from all sites were pooled. However, a closer look at this relationship highlights 2 significant trade-offs for 2 out of the 7 sampling sites. It is noteworthy that the 2 sampling sites showing a significant trade-off (Fuel and Serres) were geographically very close to each other (<3 km). Our result provides one of the few demonstrations that the shape and strength of a trade-off can vary within a parasite's population. Hosts are often viewed as unlimited food sources for parasites, which has been argued to override conflicts between energetically demanding traits such as egg size and the number of eggs (Rossin et al. Reference Rossin, Poulin, Timi and Malizia2005; Timi et al. Reference Timi, Lanfranchi and Poulin2005; Herreras et al. Reference Herreras, Montero, Marcogliese, Raga and Balbuena2007). Accordingly, we found no evidence that the host body size affected the existence and strength of this trade-off. Another hypothesis suggests that, in nematodes, spatial constrictions imposed by uterine size may play an important role in determining the emergence of a trade-off between egg size and the number of eggs at the interspecific level (Herreras et al. Reference Herreras, Montero, Marcogliese, Raga and Balbuena2007). This hypothesis is rather unlikely in our case since we are dealing with intraspecific variation, and because egg sacs are external in T. polycolpus. Therefore, our study provides a potentially new explanation for understanding intraspecific variability in the trade-off between egg size and the number of eggs. Indeed, our result suggests that the strength (in fact the existence) of this trade-off strikingly depends upon the surrounding environment. It is noteworthy that, even if not demonstrated for T. polycolpus, some parasitic Copepods are able to produce repeated clutches in their lifetime (de Meeûs et al. Reference de Meeûs, Raibaut, Renaud, Boxsiiat and Defaye1993), which could mask the trade-off between egg number and size. Hence, if confirmed in the laboratory and after accounting for potential multiple clutches, our finding would indicate that, as for free-living organisms (Dobson and Murie, Reference Dobson and Murie1987; Stearns, Reference Stearns1992), the evolution of life-history trade-offs in parasites might depend upon environmental factors that could affect (either directly or indirectly) the acquisition of resources and/or the differential allocation of resources in life-history traits.
Conclusion
Although each trait had a unique response to biotic and abiotic factors, we found that variation in life-history traits was particularly related to unmeasured parasite-level factors and host-level factors rather than environmental-level factors. Reproductive strategies of parasites affect the opportunities for transmission and determine the spatial and temporal dynamic of pathogens (Wimberly et al. Reference Wimberly, Yabsley, Baer, Dugan and Davidson2008). Thus, understanding the forces that shape the life-history traits of parasites is of interest for anyone interested in epidemiology, control strategies, and evolutionary medicine (Thomas et al. Reference Thomas, Brown, Sukhdeo and Renaud2002). In such a context, our results highlight the importance of considering parasite life-history traits in a statistical framework that explicitly considers multiple biological levels (Vignon and Sasal, Reference Vignon and Sasal2010). Changes in host conditions and parasite characteristics must, for instance, also be considered in order to predict parasite spread after environmental changes (Lafferty, Reference Lafferty2009b).
This paper also showed that variation in the trade-off between egg size and egg number exists in parasites, even within a single population. The existence of this trade-off varied between sites, which shows that the allocation of energy in parasites depends not only on intrinsic factors and host conditions. Hence, under particular conditions, the evolution of life-history traits in parasites might be constrained by competing resource allocations. This result shows that trade-offs can arise even if parasites are supposed to live in resource-rich habitats; other mechanisms than direct resource competition can indeed interfere with the allocation between egg size and egg number (Herreras et al. Reference Herreras, Montero, Marcogliese, Raga and Balbuena2007).
Overall, this paper provides an example where a greater coupling of hierarchical study designs and appropriate analyses allow the determination of the ecological processes that drive the spatial variation of life-history traits of parasites and phenotypic trade-offs. This kind of correlative approach is a first step towards better designed and targeted laboratory experiments.
ACKNOWLEDGMENTS
We are indebted to Nicolas Soubiran, Fabien Leprieur, Muriel Gevrey, Yasmina Younes-Baraillé, Marc Dubois, Gaël Grenouillet, Roselyne Etienne, Alain Blanchet and Marie-Madeleine Furet who helped us in the field. The study was supported by the Adour-Garonne French Water Agency (Project no. 0601711).