INTRODUCTION
Class Rudimicrosporea Sprague 1977, a monotypic taxon with a single family Metchnikovellidae, has always been treated as an ancestral group within the phylum Microsporidia based mainly on ‘primitive’ ultrastructural features of metchnikovellidean spores, such as short straight polar filaments (manubriums) without a coiled region, absence of polaroplasts and poorly developed spore walls (Sprague, Reference Sprague, Bulla and Cheng1977; Larsson and Køie, Reference Larsson and Køie2006). Another distinctive and presumably plesiomorphic trait of metchnikovellids is production of spore sacs (‘cysts’) (Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013) with thick walls corresponding to a reinforced plasma membrane of the sporont mother cell. Individual sporonts segregate by internal budding within the spore sac, where they eventually develop into spores.
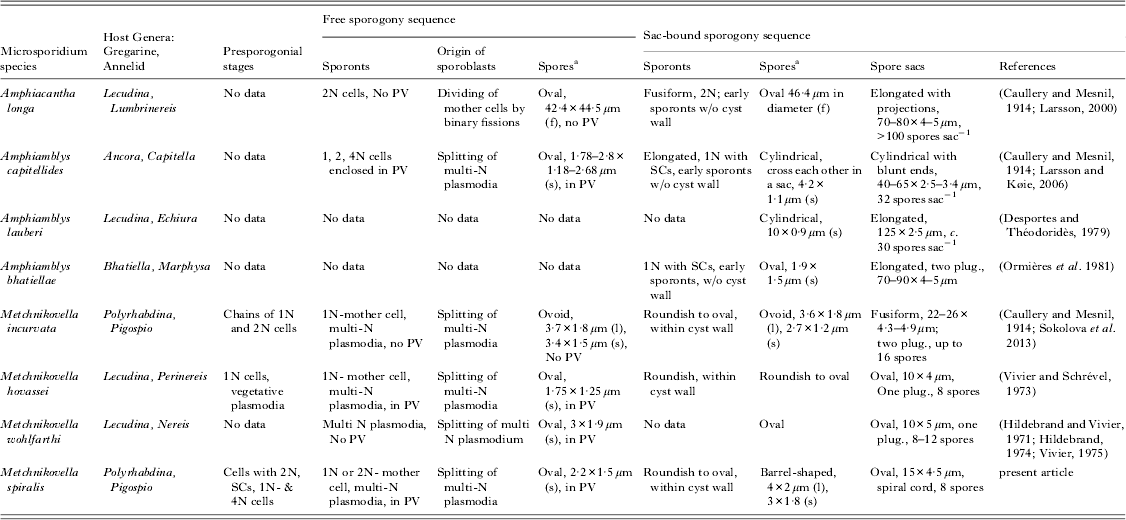
Metchnikovellids have a long history of discovery: all three genera – Metchnikovella, Amphiamblys and Amphiacantha, have been known since the first decades of the 20th century (Caullery and Mesnil, Reference Caullery and Mesnil1914, Reference Caullery and Mesnil1919). Large (10–65 μm long) spore sacs (‘cysts’) produced by all metchnikovellids were easily recognized by early microscopists and allowed differentiation of the genera: elongated sacs with filamentous projections are characteristic for Amphiacantha, long and cylindrical for Amphiamblys and oval or slightly bent for Metchnikovella (Vivier, Reference Vivier1975). Presently, 25 metchnikovellid species are known, seven of which have been investigated by electron microscopy (TEM) (Vivier and Schrével, Reference Vivier and Schrével1973; Hildebrand, Reference Hildebrand1974; Desportes and Théodoridès, Reference Desportes and Théodoridès1979; Ormières et al. Reference Ormières, Loubès and Maurand1981; Larsson, Reference Larsson2000; Larsson and Køie, Reference Larsson and Køie2006; Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013). TEM revealed further differences among the three genera, mostly in the ultrastructure of spores (Larsson and Køie, Reference Larsson and Køie2006).
Since 1984 a metchnikovellid with unusual morphology of spore sacs has been regularly observed in gregarines Polyrhabdina sp. from intestines of polychaetes Pygospio elegans inhabiting the silt littoral zone in vicinity of the Biological Station of St. Petersburg State University at the White Sea (Rotari, Reference Rotari1988; Rotari and Paskerova, Reference Rotari and Paskerova2007). Oval spore sacs of this microsporidium, were enclosed in an additional envelope composed of characteristic spiral structures embracing the sac and visible in light microscopy as striations of the sac surface. Such organization of spore sacs has never been recorded for any representative of metchnikovellids, and it was concluded that the microsporidium belonged to an as-yet undescribed genus (Rotari, Reference Rotari1988). In 2007 the White Sea metchnikovellid with spiral cysts was mentioned under the name Vivieria spiralis in the Proceedings of the V European Congress of Protistology (Rotari and Paskerova, Reference Rotari and Paskerova2007). Because the Article 9·10 of the amended International Code of Zoological Nomenclature (ICZN) says that ‘abstracts … of presentations’ … ‘does not constitute published work’ and thus cannot contain legitimate descriptions of new taxa (International Commission on Zoological Nomenclature, 2012), and given that microsporidia names are governed by ICZN (Redhead et al. Reference Redhead, Kirk, Keeling and Weiss2009), the combination Vivieria spiralis Rotari and Paskerova (Reference Rotari and Paskerova2007) is not valid. Comparison of ultrastructural characters among metchnikovellids presented in this paper argues for placement of the new microsporidium in the genus Metchnikovella, rather than creation of a new genus. Here we describe the organism, reported originally as Vivieria spiralis (and representing an invalid taxon), as a new species of the genus Metchnikovella basing on light and electron microscopy observations of the White Sea metchnikovellid with spiral cysts sampled in the years 1984–2011. We also extend the diagnosis of the genus Metchnikovella Caullery and Mesnil (Reference Caullery and Mesnil1897) by including electron microscopy data.
MATERIALS AND METHODS
Polychaetes P. elegans Claparède, 1863 were collected in July–August in 1984–1987 and 2009–2011 at the silt littoral zone of the Levin reach (66°17·878′N, 33°27·774′E), Chupa Inlet, Kandalaksha Gulf of the White Sea. Polychaetes were dissected under a stereomicroscope using laboratory needles. Gregarines, provisionally identified as Polyrhabdina sp. (Apicomplexa: Lecudiniidae), were found inside the gut, either attached to the gut epithelium, or freely in the gut lumen. Infected gregarines were identified under stereomicroscope by presence of conspicuous cysts or characteristic oval inclusions inside the cytoplasm. These gregarines were isolated on microscopic slides, observed and photographed. During the years 1984–1987 live gregarines, as well as fixed in Bouin solution and stained with Boehmer haematoxylin, were studied using phase contrast Biolam microscope (Lomo, Russia), equipped with a film camera. In 2009–2011 observations were done using a field PZO Biolam microscope equipped with DIC optics and fitted with a Canon EOS 300 digital camera.
All gregarines from the polychaete in which at least one gregarine was infected, were fixed for TEM. Small pieces of polychaete intestine with the attached gregarines, or free gregarines released from the gut lumen, were fixed in 2·5% glutaraldehyde in 0·2 m cacodylate buffer containing 0·05% MgCl2 (pH 7·4, final osmolarity 700 mOsm) for 1–4 h, washed in filtered seawater and post fixed in 2% osmium tetroxide in the same buffer for 1 h at 4 °C. Fixed gregarines were dehydrated in ascending ethanol series, transferred to acetone and embedded in Epon-Araldite in flat moulds. Embeddings were examined in bright field under 5× objective of the inverted OLYMPUS IX50 microscope. Gregarines containing cysts and/or suspicious inclusions were sectioned with ultratome MT XL (RMC products) and EM UC 6 (Leica). Thick (0·5–1 μm) sections were stained with methylene blue, examined and photographed under Zeiss Axioplan microscope equipped with a Microfire digital camera. Thin (70–80 nm) sections were stained with uranyl acetate and Reynolds’ lead citrate and examined using a Jeol JEM 1011 microscope equipped with a Hamamatsu ORCA-HR digital camera or Leo 910 microscope equipped with a film camera (Zeiss). In total, four infected gregarines were sectioned and examined by TEM.
RESULTS
Occurrence and light microscopy
In the surveys performed during the years 1984–1987 nearly all examined P. elegans polychaetes were infected with the gregarines of the genus Polyrhabdina Mingazzini, 1891. Among them only 5·6% of worms (6 of 106) harboured gregarines infected with a metchnikovellid, showing oval spore sacs decorated by spiral structures from outside (‘spiral sacs’) (Fig. 1a–d) (Rotari, Reference Rotari1988). In 2010 the survey was performed at the same locality, and gregarines of the genus Polyrhabdina sp. were recorded in gut lumens of less than a half of examined polychaetes (145 of 323). Eleven of 145 gregarine-infected polychaetes (7·6%) contained at least one gregarine with visible spore sacs. Overall, 20 of 509 (3·9%) gregarines examined in 2010 harboured microsporidian infection. However, spiral spore sacs were found only in five gregarines, while the rest of the gregarines contained elongated boomerang-shaped spore sacs of Metchnikovella incurvata (Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013). In one of 11 polychaetes, gregarines containing spiral oval sacs and gregarines with boomerang-shaped sacs of M. incurvata occurred simultaneously. However, mixed infection of the same gregarine with both species of microsporidia was never observed. Inside a gregarine, spiral sacs were enclosed in parasitophorous vacuoles (PV) (Fig. 1a, b); outlines of some vacuoles were in contact with the gregarine pellicle (Fig. 1b, c, c′). Spore sacs isolated from the host displayed spiral structures on the surface (Fig. 1d). Striations corresponding to these structures were hardly noticeable in spore sacs located inside live gregarines. Live cysts measured 11·6±0·24 μm (n = 19, range 10·0–13·5)×4·7±0·10 (n = 22, range 3·5–5·3); fixed – 9·8±0·27 μm (n = 8, range 9·0–11·3)×3·3±0·1 (n = 8, range 2·7–3·6). They contained eight barrel-shaped spores approximately 4 μm long and 2 μm wide (3·9±0·10 μm (n = 10, range 3·3–4·4)×2·4±0·06 μm (n = 7, range 2·3–2·8). The number of spore sacs per gregarine visible in one focal plane varied from one to 20. Infected gregarines looked slightly broader and shorter than uninfected gregarines and maintained the ability to glide. No stages other than spore sacs with spores could be distinguished in live gregarines.

Fig. 1. Metchnikovella spiralis: (a–e), light microscopy; (f, g), low power electron microscopy. (a, b) Gregarines with spore sacs. Spacious parasitophorous vacuoles (PV) surround each spore sac (black arrows). About eight refractive spores are visible inside each spore sac. Some PVs contact gregarine pellicle (white arrows). (a) Staining with Boehmer haematoxylin, bright field. (b) Infected gregarines pressed with the coverslip, DIC. (c) A spore sac with eight spores attached to the gregarine pellicle, staining with Boehmer haematoxylin, bright field. (c′) Graphical interpretation of (c). Black arrow indicates the boundary of parasitophorous vacuole, small arrows – external envelope made of spiral structures, arrowheads – the cyst wall. (d) Spore sacs isolated from the host cell. Note striations on the sac surface corresponding to external spiral carcass. Staining with Boehmer haematoxylin, bright field. (e) Thick section through the epoxy resin-embedded gregarine stained with methylene blue, bright field. Spore sacs are encircled by transversally sectioned spiral structures (arrows) and submerged into spacious vacuoles. Asterisks mark parasitophorous vacuoles with spore sacs. Similar sized vacuoles are packed with free spores (VFS). GP, gregarine pellicle; N, gregarine nucleus; SP, multinucleate sporogonial plasmodium; VFS, parasitophorous vacuoles with free spores. Scale bar = 10 μm. (f, g) Thin sections through two infected gregarines displaying various life cycle stages of the parasite. GP, gregarine pellicle; FS, free spores in parasitophorous vacuoles; SP, sporogonial plasmodium; VSS, parasitophorous vacuoles with spore sacs surrounded by spiral structures (arrowhead). Presporogonial stages are indicated by white asterisks. (f), Scale bar, 6 μm; (g), 10 μm.
On thick sections of epoxy resin-embedded gregarines, spore sacs were detected inside vacuoles and were encircled by structures obviously corresponding to spirals visible on the surface of isolated spore sacs. Similar sized vacuoles packed with free spores (2·5±0·03 μm (n = 18, range 2·2–2·8)×1·5±0·07 μm (n = 8, range 1·2–1·7), were seen in gregarine cytoplasm (Fig. 1e).
Electron microscopy
Thin sections of gregarines contained parasites at different stages of the life cycle. Vacuoles with free spores or sporoblasts, alongside with spore sacs and earlier vegetative stages were most often seen within the host cells (Figs 1f, g and 3h).
Presporogonial stages
The following cell types corresponding to presporogonial stages were identified on sections through infected gregarines. (i) Roundish cells with large dikarya, enclosed in a single membrane. Zones of contact between two nuclei displayed characteristic ‘widening’ in the central part, where the membranes of the nuclear envelopes were loosely aligned (Fig. 2a). Uncommon for typical diplokarya of microsporidia, ribosomes ornamented the nuclear membranes of both nuclei along the zone of their contact (insert 2a′), except for the central region. The nucleoplasm contained structures resembling synaptonemal complexes. Those were composed of two parallel bar structures about 200 nm long with electron-dense substance in-between (insert 2a″). This finding probably indicates the occurrence of meiosis. (ii) Cells with a large nucleus, numerous intranuclear electron-dense inclusions, and well-developed ER. These cells were surrounded by an additional membrane (Fig. 2b). (iii) Tetranucleate elongated plasmodia with two sets of coupled nuclei (dikarya) (Fig. 2c), which divided in (iv) individual cells with two isolated nuclei (Fig. 2d). The latter were probably sporont mother cells (or their precursors), which presumably transformed into (v) multinucleate sporogonial plasmodia after multiple nuclear divisions (Fig. 2e, f). All stages except roundish dikaryotic cells were enclosed in an additional envelope, tightly delineating the plasma membrane from the outside (Fig. 2b, b′, d, d′).

Fig. 2. Metchnikovella spiralis: electron microscopy of presporogonial stages. (a) A dikaryotic cell enclosed in a single membrane (short black arrows). Nuclear membranes are loosely arranged in the central part of the zone of contact between two adjacent nuclei forming a characteristic ‘opening’ (asterisk). Structures resembling synaptonemal complexes can be distinguished in the nucleoplasm (white arrows). N, dikaryon counterparts. Bar, 500 nm. (a′) Zone of the contact between two nuclei at higher magnification. Nuclear membranes of the contacting nuclei are not merged; each of the membranes is ornamented with ribosomes. Scale bar, 250 nm. (a″) Synaptonemal-like complexes at higher magnification, white arrow. Scale bar, 250 nm. (b) A section through a stage with a large nucleus (N) containing electron-dense inclusions. Two-layered membrane delineates the cell (arrows). ER, endoplasmic reticulum. Scale bar, 500 nm. (b′) Additional fine membrane outlining the plasmalemma at higher magnification. Scale bar 125 nm. (c) Four-nucleate cell with coupled nuclei (N). Scale bar, 2 μm. (d) A stage with two isolated nuclei (N), presumably a sporont mother cell or its precursor. Scale bar, 1 μm. (d′) The structure of the periphery of this stage at higher magnification: plasma membrane (arrow) is covered by a thicker external envelope. Scale bar, 250 nm. (e) Multinucleate sporogonial plasmodium. N, nucleus. Scale bar, 2 μm. (f) Multinucleate plasmodium splitting into sporonts (Spt). Scale bar, 2 μm. (g) The surface of the irregular-shaped large cell with patches of tubular-shaped cisternae 60–100 nm in diameter (arrows). N, nucleus. Scale bar, 500 nm. (g′) Periphery of the cell with cross-sectioned cisternae at higher magnification. (g″) Section through a stack of longitudinally sectioned cisternae. (g′, g″), Scale bar, 250 nm.
In addition to the above-mentioned stages, irregularly shaped large cells with homogeneous cytoplasm and occasional spacious vacuoles were spotted rarely in our sections (Fig. 2g). The surface of these cells was covered with patches of material composed of stacks of tubular-shaped cisternae, 60–100 nm in diameter (Fig. 2g, g′, g″).
Free sporogony (FS)
The multinucleate plasmodium underwent plasmotomy (Fig. 2f). We did not observe sporoblasts, probably because they rapidly transformed into spores, which resided in spacious PVs (Figs 1g and 3a, h). The surface of PVs occasionally displayed numerous convexities produced by bulging spores. Thus on sections through the vacuole periphery each of these protrusions was often seen as an individual spore encircled by the PV membrane (Fig. 1f). Spores were typically metchnikovellidean, roundish to oval, slightly angular at the top of the polar cap. They measured on section 1·3–1·9 μm in height (along the manubrium) and 2·0–3·2 μm in width (the longest dimension), on average 1·6±0·4×2·5±0·12 (N = 13). The spore fine structure was very much like the one described for Metchnikovella spp. (Vivier and Schrével, Reference Vivier and Schrével1973; Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013). The spore envelope was 26–36 nm thick and displayed three layers: 5–7 nm thick plasma membrane, electron-lucent endospore 12–17 nm thick and 9–12 nm-thick exospore ornamented with electron-dense elements. The latter most often appeared as globules of 14–22 nm in diameter (Fig. 3a, b), but on a few sections at least some of them were seen as thin tubules or strands of granular material expanding into a PV lumen (Fig. 3d, e). PVs containing mature spores were filled with short threads of granular material originating from exospore surfaces (Fig. 3d). Cisternae with electron-dense content underlined the surface of spores (Fig. 3f) probably contributing to secretion of the exospore material. The manubrium displayed a multilayered structure (Fig. 3a, c). The manubrium was cylindrical, maximally 800 nm long with thinner apical and distal regions (c. 170 nm) and a thick central ‘bulbal’ part (up to 230 nm) (Fig. 3a–c). The polar cap closely adjoined the apical end of the manubrium (Fig. 3b). The polar cap was of typical mushroom shape with slightly curved edges and uniform thickness of 60–80 nm (Fig. 3a, b). Manubrial cisternae derived from the distal end of the manubrium and were associated with a cluster of vesicles or short tubules (Fig. 3c–e). The nucleus was horseshoe-shaped (Fig. 3a, f). Several layers of rough endoplasmic reticulum wrapped the nucleus (Fig. 3a, f). Round double membrane profiles were occasionally seen in sections through PV lumens (Fig. 3g). The diameter of the profiles was approximately equal to one of the manubrium (Fig. 3f, g). We hypothesize that those were sections through inverted manubriums, through which sporoplasms had liberated from PV.

Fig. 3. Metchnikovella spiralis: ultrastructure of free spores. (a) A section through a spore showing major features of a metchnikovellidean spore: specific proportions of the spores with the short axis traversing the polar cap and manubrium (spore height) perpendicular to the long axis (spore width), mushroom-shaped polar cap (PC), straight manubrium (M) with its wide ‘bulbal’ part (asterisk), manubrial cisterna (MC), and numerous coated vesicles (V) associated with MC. A horseshoe-shaped nucleus (N); 3-layered thin envelope (arrows) incrusted with globular structures; ER, endoplasmic reticulum; VFS, parasitophorous vacuole with free spores. Scale bar, 500 nm. (b) The section through the spore demonstrating the shape of the polar cap and a loose association of the polar cap and manubrium (white arrow), unusual for typical microsporidia. A narrow bridge of cytoplasm separates the polar cap from manubrium. A black arrow indicates globular structures on the surface of the three-layered spore wall. Scale bar, 500 nm. (c) A section through a polar cap (PC) and manubrium with its three parts: thinner apical (black arrowhead) and distal (white arrowhead) regions, and a thicker central ‘bulbal’ part (asterisks). A manubrial cisterna (white arrow) derives from the distal part of the manubrium. Granular material filling the parasitophorous vacuole (VFS), originates on the spore surface (black arrows). V, vesicles. Scale bar, 500 nm. (d) Distal part of the manubrium (M) splitting in two manubrial cisternae (MC). Some vesicles (V) are budding off the cisterna (white arrow). Black arrows indicate fine threads and granules of exospore material filling the parasitophorous vacuole. Scale bar, 500 nm. (e) A section through the bulbal (asterisk) and distal parts of manubrium (M) with two branching off manubrial cisternae (MC). Arrow points to a strand of granular material deriving from the exospore. Scale bar, 500 nm. (f) A section along the long axis and through the central region of the spore displaying transversally sectioned manubrium (M), coated vesicles (V) and cisternae with electron-dense content (arrows), which probably contribute to secretion of exospore material. ER, endoplasmic reticulum; N, nucleus. Scale bar, 500 nm. (g) Round profiles, presumably inverted manubria (arrows), in the peripheral areas of sporophorous vesicle (VFS). Scale bar, 500 nm. (h) A parasitophorous vacuole with free spores (VFS), and the one with a spore sac (VSS), in which a sporont mother cell is undergoing internal budding. VFS is filled with fine granular secretion. Sections through the spiral structures are indicated by arrows. GP, gregarine pellicle. Scale bar, 500 nm.
Sac-bound sporogony sequence (BS)
The earliest observed stage of BS was sporont mother cells with one multi-lobed nucleus, layers of ER adjacent to the nucleus, and a few peripheral inconspicuous bodies or vacuoles, probably indicating the start of vacuolation (Fig. 4a). The vacuoles increased in volume and number (Fig. 4b, c), and at more advanced stages occupied a good part of the cell volume, however, the multi-lobed nucleus and endoplasmic reticulum were still noticeable (Fig. 4b). Some vacuoles contained whorls of membrane material and round bodies which looked like miniature cells with electron-dense nuclei (Figs 3h and 4b, c). The nature of these vacuoles is unclear: their size was too small and the number too big, to be newly segregated sporoblasts. Sporoblasts (Fig. 4d) and spores (Fig. 4e) were similar in ultrastructure. Sporoblasts displayed undulating edges with projections spreading within the cyst lumen (Fig. 4d). Mature BS spores exhibited internal structure similar to free spores. They measured 0·9–1·2×1·7–2·5 μm on thin sections, displayed a 3-layered envelope 25–30 nm thick, manubrium, and horseshoe-shaped nucleus. Other ultrastructural details of BS spores were obscure due to poor preservation (Fig. 4e).

Fig. 4. Metchnikovella spiralis: sac-bound sporogony. (a) A cyst enclosing sporont mother cell with a multilobed nucleus (N) surrounded by layers of endoplasmic reticulum (ER). Vacuoles located in vicinity of the plasma membrane are indicated by arrows. Arrowheads point to the membrane of the parasitophorous vacuole. The lumen of this vacuole is filled with sheath of fine filamentous material (asterisks) and cords (SS) correspondent to spiral structures visible in light microscope (Fig. 1d). Scale bar, 500 nm. (b, c) A sporont mother cell undergoing internal budding (‘vacuolation′). Vacuoles are getting larger and increase in number. Multilobed nucleus (N) and endoplasmic reticulum (ER) are still noticeable. The vacuoles contain whorls of membrane material (white asterisks) and round bodies that look like miniature cells with nuclei (white arrows). The spore sac envelope consists of the internal uniform electron-dense wall (W), and external thin uneven layer of less electron density (small black arrows). Sheath of fine filamentous material (black asterisks) and spiral structures (SS) are extended from this surface. Connection of SS with the surface layer is indicated by the black arrow. Some SS underline the membrane of the parasitophorous vacuole (arrowheads). Scale bar, 500 nm. (d) Sporoblasts (Spb) inside the spore sac exhibit undulating edges with projections spreading within the cyst lumen. Fine filamentous material of the sheath (asterisks) concentrates around spiral cords (SS). Scale bar, 500 nm. (d′) Each cord is composed of bundles of spirally arranged filaments. Scale bar, 500 nm. (e) Cysts with mature spores (S) were poorly preserved; a few spore organelles were yet distinguishable. Arrows indicate three-layered spore envelopes, arrowhead indictes cross-sectioned manubrium. N, nucleus, V, coated vesicles. Scale bar, 500 nm. (f) A schematic drawing of a cyst inside a parasitophorous vacuole membrane (arrows). Electron-dense cyst wall is indicated by arrowheads. External envelope built of spiral structures (seen as striations) is associated with filamentous sheath (asterisks).
All stages of bound sporogony occurred within cysts. On thin sections cysts were seen within electron-lucent PVs limited from the host cytoplasm by the interfacial membrane. Lumens of PVs were filled with ribbon-like structures (‘cords’) of moderate electron density, composed of bundles of spirally arranged filaments (Fig. 4d, insert 4d′). These structures apparently corresponded to spiral cords visible at the light microscopy level. Closer inspection of a cyst revealed a two-layered structure of its envelope; an internal uniform electron-dense wall 50–100 nm thick, and an external thin and uneven layer of less electron density (Fig. 4a–d). At the earlier stages of bound sporogony the connection of the cyst surface layer with ribbon-like structures could be traced (Fig. 4c). Thin threads originating from the surface layer formed a sheath of fine filamentous material intermingling in anastomosing networks and filling PVs (Fig. 4b, d). Around the cords this material appeared to be more concentrated, giving the impression that the cords themselves originated from its clotting (Fig. 4d). The cords often underlined the PV limiting membrane (Fig. 4a, b). Figure 4f summarizes our light and electron microscopy observations on the cyst morphology.
DISCUSSION
Cytology and life cycle
Presporogonial stages were rare in the examined gregarines, and our observations only allow us to hypothesize the sequence of the life cycle events (Fig. 5). The most intriguing is the stage with coupled nuclei (dikaryon). A dikaryon looks much like a diplokaryon of typical microsporidia, except for the presence of ribosomes on the adjacent membranes in the zone of contact. The ‘opening’, formed by loosely aligned nuclear membranes in the centre of this zone, resembles the ‘breach’ observed in diplokarya of microsporidia undergoing meiosis before octosporogony, for example in Kneallhazia solenopsae (Sokolova and Fuxa, Reference Sokolova and Fuxa2008) and Agmasoma penaei (YS, unpublished data). The synaptonemal complexes (SCs) and electron-dense intranuclear particles characteristic of the meiotic nuclei in many microsporidia (corresponding probably to chromosome bivalents in diakinesis) suggest occurrence of meiosis in the studied metchnikovellid. It may be tentatively suggested that counterparts of a dikaryon (Fig. 2a) merge (Fig. 2b), undergo sexual process and meiosis and produce 4-nucleate cells (Fig. 2c) after two rounds of division. Among typical microsporidia, meiosis marked by the presence of SCs is most often connected with the transition from merogony to sporogony and shifting between diplokaryotic and monokaryotic stages. In metchnikovellids SCs were found at the onset of bound sporogony in Amphiamblys bhatiellae (Ormières et al. Reference Ormières, Loubès and Maurand1981), Amphiamblys laubieri (Desportes and Théodoridès, Reference Desportes and Théodoridès1979) and Amphiacantha longa (Larsson, Reference Larsson2000). The stages with paired (=coupled) nuclei were described at the early stage of FS in Amphiamblys capitellides (Rotari, Reference Rotari1988; Larsson and Køie, Reference Larsson and Køie2006), and in all life cycle stages including free and sac-bound spores of Amphiacanta longa (Larsson, Reference Larsson2000). In the present study we demonstrated stages with dikaria in a Metchnikovella-like species. Thus, the representatives of all metchnikovellid genera might have retained coupled nuclei and meiosis, suggesting that sexuality is common among Metchnikovellidae, and was probably inherited from a sexual microsporidian-fungal ancestor (Lee et al. Reference Lee, Corradi, Byrnes Iii, Torres-Martinez, Dietrich, Keeling and Heitman2008; Capella-Gutiérrez et al. Reference Capella-Gutiérrez, Marcet-Houben and Gabaldón2012). It seems likely that metchnikovellidean dikarya are homologous to diplokarya of typical microsporidia though association of nuclei in metchnikovellids seems less tight than in typical microsporidia. For example, nuclear pores in A. capitellides (fig. 8 in Larsson and Køie, Reference Larsson and Køie2006), and ribosomes in A. longa (fig. 15 in Larsson, Reference Larsson2000), and herein were observed in the zone of contact. In typical microsporidia the zone of contact of nuclear counterparts is smooth with the external (adjacent) membranes of the nuclear envelopes being tightly associated.

Fig. 5. Hypothetical scheme of Metchnikovella spiralis life cycle. This scheme reflects our current working hypothesis. The stages numbered by the framed figures were in fact seen. Solid arrows mark the transition between stages supported by observations; those marked by dashed arrows were assumed. Green (dark-grey in print version) letters and arrows belong to the free sporogony sequence (FS), yellow (light grey) – to the sac-bound sporogony sequence (BS). We hypothesize that a polychaete ingests metchnikovellidean cysts (spore sacs) with food. In the gut lumen the cyst wall dissolves, affected probably by gut enzymes, and BS spores are released into the lumen inhabited by gregarines. We do not know how a sporoplasm appears inside the gregarine. Injection of sporoplasms via polar tubes seems unlikely. A short and wide manubrium can hardly pierce the thick gregarine pellicle mechanically. Theoretically, manubria can adhere to the pellicle, form tunnels for sporoplasms and induce pellicle remodelling and phagocytosis, though the latter was never observed in eugregarines. Maybe spores predominantly release sporoplasms at the special areas, where the gregarine coat is thin and/or consists of only plasma membrane, like micropores; in Polyrhabdina sp. numerous micropores are located on the sides of epicytic folds. The gregarine might be infected at the sporozoite stage as well. Inside the gregarine cytoplasm, a sporoplasm initiates FS, i.e. it transforms into a meront (1, FS), grows in size (2, FS), generates additional envelope outside the plasma membrane and undergoes nuclear divisions (3, FS) to produce finally multinucleate sporogonial plasmodium (4, FS), which splits into sporoblasts (5, FS). Mature FS spores reside within a parasitophorous vacuole (6, FS). FS spores release sporoplasms through everted manubria, and several rounds of FS take place to allow dissemination of the infection within the gregarine. We presume that meiosis (asterisk) rather takes place in the BS sequence at the onset of cyst-bound sporogony, like in some other metchnikovellids. Gametogamy (2, BS), dikaryotic stage (3, BS), caryogamy (4, BS) and meiosis (asterisk), may precede splitting the cycle in two sequences, as well. In BS a sporogonial plasmodium divides by internal budding within the dense-walled spore sac (5, BS). A spore sac with mature spores exits the gregarine through the ruptures of the pellicle upon host death, or via exocytosis-like event, during which parasitophorous vacuole membrane merges with the pellicle releasing the cyst (6, BS).
Our results do not allow determination unequivocally whether the certain presporogonial stages represent BS or FS, and one may only guess at which of the following stages the life cycle splits in two sequences: the dikaryotic cell, 4-nucleate plasmodium or product of its division. Elongated plasmodia with coupled nuclei surrounded by a double membrane, splitting in two binucleate cells, might be a part of either BS or FS sequence. We hypothesize that the stages depicted on Figs 2c–f belonged to FS. Dikaryotic stage (Fig. 2a), a stage with a large single nucleus (Fig. 2b), and large cells with tubular structures on the surface (Fig. 2g) might indicate transition to sac-bound sporogony for the following reasons: (i) in metchnikovellids SCs were recorded exclusively in BS sporonts (Desportes and Théodoridès, Reference Desportes and Théodoridès1979; Ormières et al. Reference Ormières, Loubès and Maurand1981; Larsson, Reference Larsson2000); (ii) the stages at Fig. 2a, b exhibit similar structure of nuclei and cytoplasm suggesting they belong to the same sequence; (ii) the stage at Fig. 2g exhibits patches of tubules on the surface structurally similar to scindosomes of A. capitellides (Larsson and Køie, Reference Larsson and Køie2006) and A. laubieri (Desportes and Théodoridès, Reference Desportes and Théodoridès1979), the organelles associated with cyst wall formation. The extracellular localization of the tubular structures in our case suggests that they are different from intracellular scindosomes, but likely also participate in building the cyst wall.
With the exception of the dikaryotic stage presumably at the onset of BS, all stages of the novel microsporidium were delineated with an additional interfacial membrane. Free spores and spore sacs resided in PV, similarly to Metchnikovella hovassei (Vivier and Schrével, Reference Vivier and Schrével1973), but not to M. incurvata (Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013), supporting the idea that the presence or absence of an interfacial envelope cannot be used as a genus characteristic (Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013).
It is noteworthy that connection of the membrane of cysts-containing PVs with the gregarine pellicle (Fig. 1b, c) have never been observed in any metchnikovellid before. It is tempting to hypothesize that the observed phenomenon reflects a special adaptation allowing spore sacs to exit the host cell without rupturing the gregarine pellicle (Rotari, Reference Rotari1988). More evidence, including documentation of a spore sac leaving the intact gregarine, is required to exclude the accidental positioning of some sacs in the vicinity of the pellicle.
The observation of round double membrane profiles on sections through PVs (Fig. 3g), together with visualization of sporoplasms and emptied spores of M. incurvata (fig. 4h in Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013), arouse conjectures concerning possible mechanisms of the sporoplasm exit from a metchnikovellid spore and similarity of this process to the one of typical microsporidia. If the observed profiles were the sections through inverted manubria, it would be the first evidence supporting the idea that metchnikovellids deliver sporoplasms to the host cell cytoplasm in a similar way as typical microsporidia, via the everted polar tube. That would mean that metchnikovellids, presumably a basal group of the phylum Microsporidia, already possessed this unique infection mechanism, a distinctive feature of the phylum Microsporidia. However, the ancestral extrusion apparatus of metchnikovellids is obviously poorly developed and allows spreading the infection only within one cell (autoinvasion). Thus producing ‘environmental cysts’ via sac-bound sporogony remains mandatory to support dissemination of the infection throughout the host population. Sac-bound sporogony (BS) is likely a plesiomorphic trait (Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013), and evolution of microsporidia resulted in elimination of BS, and perfecting the mechanism of extrusion by developing the following structures: (i) a rigid spore wall and (ii) posterior vacuole to build up intrasporal pressure for a powerful discharge; (iii) an advanced anchoring device to sustain the discharge; (iv) a long polar filament to increase the ‘radius of the attack’ to target neighbour cells and organisms; (v) a polaroplast to provide a pool of membranes to envelope the emerged sporoplasms. Metchnikovellids lack all these features. Another group of ‘primitive’ microsporidia, the members of the family Chytridiopsidae, may represent the intermediate evolutionary stage: they are the only group of microsporidia, besides metchnikovellids, possessing sac-bound sporogony alongside functional, though not completely formed extrusion apparatus, which lacks polaroplast and some other features (summarized in Larsson, Reference Larsson1993; Larsson, Reference Larsson2000). It is indeed unfortunate that genomic and proteomic approaches that could answer the key questions about evolution, origin and physiology of microsporidia are not yet applicable to these two groups. So far even phylogenetic relationships between Metchnikovellidae and Chytridiopsidae remain unresolved.
Taxonomy
The three described genera of the order Metchnikovellida – Metchnikovella, Amphiacantha and Amphiamblys – can be differentiated from each other by the morphology of spore sacs (‘cysts’) and ultrastructure of spores. Because the investigated White Sea metchnikovellid displayed a unique feature of cyst morphology – an additional envelope shaped as a spiral carcass enclosing the cyst – upon first discovery this trait was recognized as a genus-level apomorphy, and the species with ‘spiral cysts’ was provisionally assigned to a new genus Vivieria (Rotari, Reference Rotari1988).
However, the results of the present study and meticulous analyses of available data on morphology and intracellular development, alongside comparison to other metchnikovellids, could not justify a new genus for this microsporidium, but suggests placing it within the genus Metchnikovella Caullery and Mesnil, Reference Caullery and Mesnil1897. First of all, the novel microsporidium fits within the original diagnosis of the genus Metchnikovella (see taxonomic summary below). Second, a relatively small number of spores (eight) within the sack, is not uncommon for Metchnikovella sp., yet all known Amphiamblys and Amphiacatha species have larger numbers of spores per cyst (Vivier, Reference Vivier1975). Third, the studied species produces oval sac-bound spores similar in size and shape to free spores, like other Metchnikovella spp. (Hildebrand and Vivier, Reference Hildebrand and Vivier1971; Vivier and Schrével, Reference Vivier and Schrével1973; Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013). For comparison, A. capitellides and A. lauberi have cylindrical sac-bound but oval free spores (Desportes and Théodoridès, Reference Desportes and Théodoridès1979; Larsson and Køie, Reference Larsson and Køie2006). Amphiacantha longa produces round spores in both sequences but free spores half the size of the sac-bound spores (Larsson and Køie, Reference Larsson and Køie2006). For A. bhatiellae, however, ovoid sac-bound spores have been reported but information on free spores is unavailable (Ormières et al. Reference Ormières, Loubès and Maurand1981) (Table 1). A horseshoe-shaped nucleus is another character that the new White Sea metchnikovellid shares with Metchnikovella spp. (Vivier and Schrével, Reference Vivier and Schrével1973; Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013). In addition, ornamentation of the exospore of free spores with globules or fine threads is typical for all three species of Metchnikovella spp. studied ultrastructurally, but not for A. longa or Amphiamblys spp. (Hildebrand and Vivier, Reference Hildebrand and Vivier1971; Vivier and Schrével, Reference Vivier and Schrével1973; Ormières et al. Reference Ormières, Loubès and Maurand1981; Larsson, Reference Larsson2000; Larsson and Køie, Reference Larsson and Køie2006; Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013). And finally, cyst wall modifications such as polar thickenings and extensions are widespread among Metchnikovella spp. (Vivier, Reference Vivier1975). For example, spore sacs of the type species, Metchnikovella spionis, bear long extensions of the cyst surface layer. Caullery and Mesnil, who first described the genus, wrote: ‘The type species M. spionis stays apart due to the special structure of the cyst ends, elongated and not containing spores. Other species, like Metchnikovella legeri, possess massive thickenings at poles …’ (Caullery and Mesnil, Reference Caullery and Mesnil1914). In fact, not only Methchnikovella legeri (Caullery and Mesnil, Reference Caullery and Mesnil1914), but M. hovassei (Vivier and Schrével, Reference Vivier and Schrével1973), M. wohlfarthi (Hildebrand and Vivier, Reference Hildebrand and Vivier1971), M. incurvata (Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013) and some other species (Vivier, Reference Vivier1975) have ‘thickenings’ (called also ‘plugging structures’) on one or both poles of spore sacs. Larsson and Køie, (Reference Larsson and Køie2006) pointed to a thick ‘fibrous’ or ‘reticulate’ surface layer of the cyst wall, a distinctive ultrastructural feature of the genus Metchnikovella. This layer is well developed in M. hovassei (figs 33 and 35 in Vivier and Schrével, Reference Vivier and Schrével1973). Differentiation of the cyst wall in thicker external and thinner internal layers was also observed in M. incurvata (fig. 6a in Sokolova et al. Reference Sokolova, Paskerova, Rotari, Nassonova and Smirnov2013). The species described here, probably, demonstrates an advanced state of the character: the fibres of the external layer form a spiral cord around the spore sac (Fig. 4f). We presume that the fibrous external layer of the cyst wall typical to Metchnikovella spp. is prone to various modifications which over evolutionary history developed into adaptations to increase buoyant properties of cysts, like polar thickenings, cyst wall extensions, spiral carcasses, and possibly other yet undescribed appendages.
Table 1. Diagnostic characters of Metchnikovellids examined by electron microscopy

a Free and sac-bound spores are uninucleate in all examined metchnikovellids, except Amphiacantha.
Abbreviations: f, fixed; l, live; N, nucleus, nuclei, nucleate; PV, parasitophorous vacuole; plug., plugging structure, a thickening at the spore sac edges; s, sectioned; SCs, synaptonemal complexes.
Interestingly, since description of the family Metchnikovellidae and its three genera (Caullery and Mesnil, Reference Caullery and Mesnil1897, Reference Caullery and Mesnil1914) no new genera have been added to the synopsis of the family. There were two unsuccessful attempts to erect a new metchnikovellid genus after Caullery and Mesnil. The genus Caullerietta, for metchnikovellids with one polar thickening, was proposed by Dogiel (Reference Dogiel1922) and the genus Desportesia, for A. laubieri Desportes and Théodoridès (Reference Desportes and Théodoridès1979), by Issi (Reference Issi, Beyer and Issi1986). Further study on diversity of cyst morphology among Metchnikovella spp., and particularly ultrastructural analysis of M. hovassei (Vivier and Schrével, Reference Vivier and Schrével1973) proved Caullerietta to be a junior synonym of Metchnikovella. Desportesia has also been abridged to a junior synonym after Larsson and Køie, (Reference Larsson and Køie2006) discovered ‘Desportesia-like’ spores in the life cycle of A. capitellides, the type species of the genus, and retained A. laubieri and A. bhatiellae back in the genus Amphiamblys (Larsson and Køie, Reference Larsson and Køie2006). So far the original genera diagnoses proposed by Caullery and Mesnil (Reference Caullery and Mesnil1897, Reference Caullery and Mesnil1914) accommodate all findings in spite of (or due to?) the extreme conciseness of these diagnoses. Maybe further studies involving molecular taxonomic/genomic approaches will prove the statement of Sprague and co-authors that the genus Metchnikovella with its 25 species with greatly derived cyst morphology ‘is almost certainly a heterogenous assemblage’ (Sprague et al. Reference Sprague, Becnel and Hazard1992), and justify splitting this genus in several genera.
Taxonomic summary
Metchnikovella Caullery and Mesnil, Reference Caullery and Mesnil 1897 , emend. C. and M., 1914
Original diagnosis
‘We preserve the genus Metchnikovella for all species, in which the length of the cyst does not exceed its width more than ten times. Cyst are cylindrical or fusiform, has round ends. Their general form is quite variable’ (Caullery and Mesnil, Reference Caullery and Mesnil1914, p. 529).
Extended description of the genus based on the data accumulated since 1914, including electron microscopy observations (Table 1)
Presporogonial stages may include dikaryotic and monokaryotic cells, individual or arranged in chains. Life cycle with two types of sporogony, free sporogony (FS) and sac-bound sporogony (SBS). Free spores result from plasmotomy of polynucleate sporogonial plasmodium. Sac-bound sporogony occurs via internal budding of sporont mother cell inside thick-walled cyst. Number of spores per sac varies from 8 to more than 32. Spore sac size and shape are as described in original diagnosis. The reticulate external layer of the spore sac wall produces derivates like polar thickenings, extensions, spiral structures, or remains uniform. Spores of both sequences are structurally similar, roundish to oval measuring 1–7 μm in largest diameter. Spores are of metchnikovellidean type, i.e. with the apical-distal axis, perpendicular to the long axis of the spore, with one horseshoe-shaped nucleus. Extrusion apparatus consists of polar cap (homologous to anchoring disc of ‘higher microsporida’) and short polar filament (PF) traversing the short axis of the spore. PF comprises a straight manubrial part and posterior bulbous region. Membrane cisternae derive from the posterior end of the manubrium and are associated with tubular-vesicular structures. Spores lack polaroplast and posterior vacuole. Spore envelopes are composed of plasma membrane-like exospore and thin endospore.
Type species: Metchnikovella spionis, Caullery and Mesnil, Reference Caullery and Mesnil1897
Metchnikovella spiralis sp. n.
Diagnosis
Presporogonial stages may include (i) enclosed in a single membrane cells with dikarya and synaptonemal complexes in the nucleoplasm; (ii) individual or connected in pairs elongated binucleated cells; and (iii) individual monokaryotic cells. Free sporogony (FS) by plasmatomy of multinucleate sporogonial plasmodium results in numerous (>10) sporoblasts transforming into spores. Except for dikaryotic cells, all presporogonial stages and sporogonial plasmodia are surrounded by interfacial envelope tightly delineating the plasma membrane.
In sac-bound sporogony (BS) sporont mother cell with multilobed nucleus produces sporoblasts by internal budding (‘vacuolation’) within spore sac limited by 50–100 nm thick wall, outer layer of which is modified in spiral carcass.
Free spores
Inside live gregarines free spores can hardly be seen. On sections spores roundish to oval, slightly angular at the top of the polar cap, measure 2·0–3·2×1·3–1·9 μm. Spore envelope about 26–36 nm thick with three layers: 5–7 nm thick plasma membrane, intermediate electron-lucent layer 12–17 nm thick, and 9–12 nm thick exospore ornamented with electron-dense globules or strands of granular material, expanded into parasitophorous vacuole (PV) lumen. Manubrium cylindrical, c. 800 nm long with thinner (c. 170 nm) apical and distal regions and thick central ‘bulbal’ part (c. 230 nm). The polar cap of mushroom shape with slightly curved edges and uniform thickness of 60–80 nm. Nucleus in the shape of a horseshoe. Free spores reside in PVs of irregular shape (>10 per PV).
Spore sacs and sac-bound spores
Live spore sacs measure 11·6±0·24 μm (n = 19, range 10·0–13·5)×4·7±0·10 (n = 22, range 3·5–5·3); fixed −9·8±0·27 μm (n = 8, range 9·0–11·3)×3·3±0·1 (n = 8, range 2·7–3·6). Spore sacs contain eight barrel-shaped spores measured 3·9±0·10 μm (n = 10, range 3·3–4·4)×2·4±0·06 μm (n = 7, range 2·3–2·8) (live). Each spore sac is enclosed within a spiral carcass composed of a cord embracing the sac; resides in PV. Number of spore sacs per gregarine varies from one to >20. Free sporogony occurs concurrently (not consecutively) with sac-bound sporogony (SBS).
Differential diagnosis
The species differentiates from congeners and other metchnikovellids by the unique structure of the spore sac enclosed in two envelopes: light-reflecting thick wall with no thickenings at the poles, and an external carcass composed of dense cords spirally coiled around the spore sack, visible in the light microscope as a regularly arranged striations on the surfaces of spore sacs. On ultrathin sections the external envelope can be seen as tangentially or cross-sectioned filaments, regularly arranged in the lumen of PV, between the cyst wall and PV membrane.
Host and locality
Gregarines Polyrhabdina sp. from a polychaete P. elegans, Levin reach, Chupa Inlet, Kandalaksha Gulf of the White Sea, Russia (66°17·878′N, 33°27·774′E).
Type material
Slides with infected DPX- and glycerol-mounted gregarines, are deposited with the collection of slides of the Department of Invertebrate Zoology, St. Petersburg State University. Slides with semi-thin sections, grids with thin sections, and images of thin sections, labelled 622a, 622e, 622f, 623a and 734a, are in collections of YS and GP. Images of live gregarines are in the image database of the Department of Invertebrate Zoology, St Petersburg State University under the following numbers: Img0502–Img0634.
Etymology
Alludes to the structure of spore sacs reinforced with characteristic spiral carcass.
ACKNOWLEDGEMENTS
The work was supported with grants from St Petersburg State University. The present study utilized equipment of core facility centres of SPSU ‘Culturing of microorganisms’ and ‘Development of molecular and cell technologies’. We acknowledge SVM Microscopy Center at the Louisiana State University in Baton Rouge, LA for excellent conditions for electron microscopy studies. Many thanks to William Todd (Louisiana State University) for critical reading of the manuscript, and to Nicholas George (Louisiana State University) for assistance in electron microscopy. We are grateful to the personnel of the Marine Biological Station of the St Petersburg State University at the White Sea for providing facilities for our accommodation and sampling.