INTRODUCTION
Canine demodicosis is a common but exigent non-contagious parasitic dermatosis caused by over-proliferating host-specific follicular Demodex mites (Ravera et al. Reference Ravera, Altet, Francino, Sánchez, Roldán, Villanueva, Bardagí and Ferrer2013; Singh and Dimri, Reference Singh and Dimri2014). In canine demodicosis, cutaneous inflammation is associated with excessive proliferation of mites and clinical recovery of the diseased dogs often entails parasiticidal treatment and reduction in the mites population (Forton, Reference Forton2012; Miller et al. Reference Miller, Griffin, Campbell, Miller, Griffin and Campbell2013). Management of canine demodicosis remains one of the main challenges in veterinary dermatology. Albeit, newer parasiticides such as members of isoxazoline class of compounds have recently opened novel window in therapeutic management of canine demodicosis (Beugnet et al. Reference Beugnet, Halos, Larsen and de Vos2016; Six et al. Reference Six, Becskei, Mazaleski, Fourie, Mahabir, Myers and Slootmans2016), hitherto many aspects of the disease have remained unidentified and thus prevention and management of canine demodicosis has still remained a big challenge. Exact pathogenesis of generalized canine demodicosis is obscure, however, an aberration in immune status is thought to be most significant (De Bosschere et al. Reference De Bosschere, Casaer, Neukermans, Baert, Ceulemans, Tavernier and Roels2007; Singh et al. Reference Singh, Dimri, Sharma, Sharma and Saxena2010). Host immune response and mechanisms associated with Demodex mites population control requires extrapolation to unravel the host–parasite interface in demodicosis (Ferrer et al. Reference Ferrer, Ravera and Silbermayr2014). Host's immune system appears to detect and tolerate the presence of these mites, and also has an inhibitory effect on mites proliferation and keeps the mites number low without inducing an inflammatory response (Akilov and Mumcuoglu, Reference Akilov and Mumcuoglu2004; Forton, Reference Forton2012). Obstinately, it is quite possible that Demodex canis mites regulate the host immune response and induce immunosuppressive pathways to evade the host's defence system and perpetuate in the microenvironment (Akilov and Mumcuoglu, Reference Akilov and Mumcuoglu2004; Singh et al. Reference Singh, Dimri, Sharma, Sharma and Saxena2010; Singh et al. Reference Singh, Dimri, Sharma, Swarup, Sharma, Pandey and Kumari2011). But, the immunological mechanisms associated with inhibition of D. canis mites over-proliferation by the host and/or immunosuppressive mechanisms utilized by the mites for their sustained perpetuation and induction of demodicosis in dogs are baffling so far.
Innate immunity is the major component of the defensive strategy of the organism against pathogens invasion, injury and trauma. Immediate activation of the innate immune system by infection is influenced by the ability to recognize non-self motifs. It has recently put forth that the parasympathetic system can be involved in control of immunity and inflammation (Tracey, Reference Tracey2007; Rosas-Ballina et al. Reference Rosas-Ballina, Olofsson, Ochani, Valdés-Ferrer, Levine, Reardon, Tusche, Pavlov, Andersson, Chavan, Mak and Tracey2011). Neural circuits play a role in regulating immune function and inflammation by modulating the cytokines production through cholinergic pathway (Pavlov and Tracey, Reference Pavlov and Tracey2004; Rosas-Ballina et al. Reference Rosas-Ballina, Olofsson, Ochani, Valdés-Ferrer, Levine, Reardon, Tusche, Pavlov, Andersson, Chavan, Mak and Tracey2011). Acetylcholine (ACh) plays an important role in attenuating the release of pro-inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), interleukin-6 (IL-6) and interleukin-18 (IL-18), without affecting the production of interleukin-10 (IL-10), an immunosuppressive cytokine (Pavlov and Tracey, Reference Pavlov and Tracey2004, Reference Pavlov and Tracey2006). Scientific studies have demonstrated the potential association of anti-inflammatory and/or immunosuppressive cytokines including transforming growth factor beta (TGF-β) and IL-10 with immunosuppressive pathogenesis of canine demodicosis (Tani et al. Reference Tani, Morimoto, Hayashi, Inokuma, Ohnishi, Hayashiya, Nomura, Une, Nakaichi and Taura2002; Felix et al. Reference Felix, Guiot, Stein, Felix, Silva and Nobre2013; Yarim et al. Reference Yarim, Yagci and Ciftci2013). Involvement of T lymphocytes mediated immunogenic response in dogs with demodicosis, suggesting the cellular reaction has previously been documented (Fukata et al. Reference Fukata, Fuoki, Yoshikawa, Kambayashi, Kito and Kitagawa2005). Possibility of increased apoptosis or immunological exhaustion of CD4+ T cells along with downregulation of CD4+ T cells activity has been hypothesized by Singh et al. (Reference Singh, Dimri, Sharma, Sharma and Saxena2010) in canine demodicosis. Moreover, hastened peripheral leukocytes apoptosis in dogs with demodicosis and its potential association with the progression of demodicosis has been previously demonstrated by us (Singh et al. Reference Singh, Dimri, Sharma, Swarup, Sharma, Pandey and Kumari2011). But, the précised mechanism of immune regulation in canine demodicosis is still obscure. Therefore, understanding of the immuno-pathology of canine demodicosis is warranted for opening the novel and newer therapeutic windows for management of clinical manifestations in canines. Exploration of vagal pathway in association with cytokines regulation in clinical cases of demodicosis will be of significance in further understanding the immuno-pathogenesis and immune evasion mechanisms of Demodex mites in dogs. Apparently, no study has been undertaken to unravel the nexus between the neural immunosuppressive pathway and over-proliferation of D. canis mites in dogs, therefore, the present study was undertaken to evaluate the association of cholinergic pathways with the immunosuppressive/anti-inflammatory cytokines regulation in dogs with localized and generalized demodicosis.
MATERIALS AND METHODS
All the diseased dogs included in the study were client owned and were presented to the Teaching Veterinary Clinical Complex (Kothari Hospital) of the University for clinical and dermatological examination. Skin scrapings, hair plucks and skin lesions impression smears were obtained. The dogs positive for D. canis and with skin lesions distributed over more than 50% of the body surface and involvement of two or more feet were classified as cases with generalized demodicosis (GD). Dogs with small alopecic, erythematous, scaly and hyperpigmented skin lesions over the face and forelegs covering less than 50% of the total body area were classified as cases with localized demodicosis (LD) (Gortel, Reference Gortel2006). Smears obtained from the affected skin lesions were diagnosed concurrent pyoderma when the skin cytology revealed presence of neutrophils and intracellular cocci. All dogs enrolled in the study were between 12 and 36 months of age and of various breeds. All demodicosed dogs included in the study had the history of clinical disease before attaining 18-months-age and were considered to have juvenile onset of demodicosis. The dogs were found to be free from endo-parasites infestations and any other concurrent disease. The dogs found negative for haemoprotozoa on thin blood smear examination were also free from other ecto-parasites infestations, except for D. canis mites. On clinical examination, all the physiological parameters like body temperature, respiratory-rate and heart-rate of the participated dogs were found within the normal reference range. Haemato-biochemical profile estimations of these dogs revealed no remarkable alterations in the liver and renal function marker parameters. But conspicuous alterations in haemogram and leukogram were observed (data not shown). The dogs had received no miticidal treatment during the last 30 days when presented for clinical examination. A total of 30 dogs with demodicosis were allocated into three groups. Group 2 included nine dogs with LD, Group 3 included 14 with GD but no pyoderma and Group 4 included seven dogs with GD and concurrent pyoderma. Another eight healthy age-matched dogs were used as controls (Group 1). With the informed consent of animal owners, approximately 3 mL of blood samples were obtained from each dog in clot activators containing tubes before start of any therapy and used for harvesting serum. The tubes containing blood samples were kept in a slanted position for 15–20 min at normal room temperature, followed by subjected to centrifuge at 1500 rpm for 5 min. Supernatant serum was aspirated using micropipette, which was transferred into cryovials and stored at −20 °C until used for estimation of different immunological and biochemical parameters. Serum cholinesterase activity was estimated by an optimized version of Ellman method by using cholinesterase activity assay kit (MAK-119, Sigma-Aldrich, USA) following the procedure as described by the manufacturer. Cholinesterase activity was expressed as unit L−1 (One unit of cholinesterase is the amount of enzyme that catalyzes the production of 1·0 µ m of thiocholine per minute at room temperature at pH 7.5). IL-10, TNF-α and interferon-gamma (IFN-γ) levels in serum were estimated using canine specific ELISA kits (RAB0524-IL-10; RAB0526-TNF-α; RAB0523-IFN-γ, Sigma-Aldrich, USA) following the procedure as described by the manufacturer. Levels of IL-10 and IFN-γ were expressed as ng mL−1, where TNF-α level was expressed as pg mL−1. The intra-assay and inter-assay reproducibility coefficients of variation of the assayed cytokines were <10 and <12%, respectively. Statistical differences between the data of different groups were determined using one-way analysis of variance followed by the Tukey's test with general linear models in SPSS 16. The level of statistical significance for all the comparisons made was established at P < 0·05.
RESULTS
Circulatory cholinesterase activity and estimated circulatory cytokines levels in serum of diseased and healthy dogs are presented in Table 1. Figure 1 illustrates the schematic presentation of the possible association between cholinergic pathway and cytokine regulation in canine demodicosis. Compared with the control group (Group 1), dogs with GD (Group 3 and 4) as well as the dogs with LD (Group 2) were found to have significantly (P < 0·01) elevated cholinesterase activity. Further, compared with dogs with LD (Group 2), significantly higher cholinesterase activities were observed in dogs with GD (P < 0·01) and in dogs with GD along with concurrent pyoderma (P < 0·05). Significantly lower TNF-α levels were found in dogs with LD (P < 0·05) and in the dogs with GD (P < 0·01) compared with those in dogs of control group. On the contrary, TNF-α level was found be significantly (P < 0·01) higher in dogs with GD along with concurrent pyoderma (Group 4) in comparison with the dogs of Group 2 and 3 with LD and GD, respectively. Dogs with GD (Group 3 and 4) were found to have significantly (P < 0·01) elevated IL-10 levels in comparison with those in control (Group I). Furthermore, the dogs with GD along with concurrent pyoderma (Group 4) were found to have even significantly (P < 0·01) higher circulatory IL-10 level than the dogs with LD and GD. But, alteration in IL-10 level in dogs with LD was not significant when compared with the control (Group I). Compared with the effects on other, no remarkable alterations in IFN-γ levels were observed in dogs of any of the examined groups.
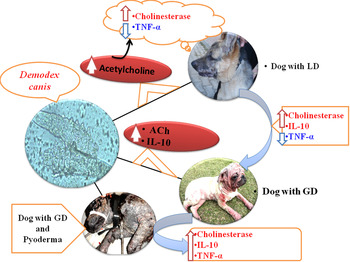
Fig. 1. Regulation of circulatory cytokines and cholinesterase activities of dogs with demodicosis.
Table 1. Circulatory cholinesterase activity and cytokines levels in healthy and demodicosed dogs

Data presented are Mean ± s.e.m.
aSignificant (P < 0·01) compared with Group 1.
bSignificant (P < 0·05) compared with Group 1.
ASignificant (P < 0·01) compared with Group 2.
BSignificant (P < 0·05) compared with Group 2.
CSignificant (P < 0·01) compared with Group 3.
DISCUSSION
Concentration of ACh at cholinergic synapses is regulated by cholinesterases, namely acetylcholinesterase, which is expressed in nerve cells and erythrocytes, and butyrylcholinesterase, which is found in plasma. Cholinesterase activity indicates about the release of ACh, therefore, cholinesterase activity is considered as an indirect biomarker of ACh status (Rosas-Ballina and Tracey, Reference Rosas-Ballina and Tracey2009; Tracey, Reference Tracey2009; D'Elia Zanella et al. Reference D'Elia Zanella, Chies, Spadella, Therezo, Rossignoli Pde, Frei and Martins2014). ACh plays an important role in neuronal stimulation and is also important as a mediator in inflammatory process (Tracey, Reference Tracey2009). ACh stimulus suppresses the production of inflammatory cytokines in early stages of inflammatory diseases to maintain the homeostasis. Cholinergic anti-inflammatory pathway through ACh acts to inhibit the production of TNF-α, interleukin-1β and macrophage migration inhibitory factor, is well documented (Pavlov and Tracey, Reference Pavlov and Tracey2006; Das, Reference Das2007). Significantly increased cholinesterase activity in dogs with LD and GD in the present study evidently suggests an overproduction of ACh in dogs with demodicosis. Further, cholinesterase activity in dogs with GD was even remarkably higher than in dogs with LD. These observations suggest that cholinergic pathways are activated during progression of canine demodicosis and concurrent secondary bacterial infections. Cholinergic activation could be the dog's reactive physiological response to inflammatory process due to Demodex mites proliferation and bacterial infection. Therefore, host's response indirectly seems in favour of the maintenance of D. canis mites and progression of the disease condition. Our study demonstrates for the first time the association of cholinergic activation with demodicosis in dogs. Apparently, no scientific reports demonstrating the association between cholinergic immunosuppressive pathway and mites-induced dermatological conditions in animals and human patients are available. However, our observations in the present study are in agreement with the previous scientific reports demonstrating the role of the cholinergic anti-inflammatory pathways in maintenance and progression of other parasitic diseases in dogs and experimental animals (Da Silva et al. Reference Da Silva, Pimentel, Fiorenza, França, Tonin, Jaques, Leal, Da Silva, Morsch, Sschetinger, Lopes and Monteiro2011; Wolkmer et al. Reference Wolkmer, Paim, Da Silva, Gai, Carvalho, Da Souza, Da Rosa, Da Silva, Pereira, Lopes, Nogueira, Rubin, Monteiro and Mazzanti2013; D'Elia Zanella et al. Reference D'Elia Zanella, Chies, Spadella, Therezo, Rossignoli Pde, Frei and Martins2014; Tonin et al. Reference Tonin, Calado, Bottari, Dalenogare, Thomé, Duarte, Duarte, Morsch, Schetinger, Alves, Tinucci-Costa and Da Silva2016). T helper (Th) cells play a central role in activation of immune system against infectious agents through secretion of lymphokines or cytokines. Demodex mites target the Th cells and alter their activation, possibly for their own survival and perpetuity (Akilov and Mumcuoglu, Reference Akilov and Mumcuoglu2004; Singh et al. Reference Singh, Dimri, Sharma, Sharma and Saxena2010). Demodex mites may evade the host immune responses by inducing irrelevant immune responses through enhancement of Th2 cytokines production and regulatory T-cell activation, which result in increased production of IL-10 and TGF-β (Tani et al. Reference Tani, Morimoto, Hayashi, Inokuma, Ohnishi, Hayashiya, Nomura, Une, Nakaichi and Taura2002; Felix et al. Reference Felix, Guiot, Stein, Felix, Silva and Nobre2013), both of which may suppress the early protective Th1 responses and hence could favour the survival of parasite and its incessant proliferation. Role of ACh in attenuating the release of pro-inflammatory cytokines such as TNF-α without affecting the production of IL-10, an immunosuppressive cytokine has been documented (Pavlov and Tracey, Reference Pavlov and Tracey2006). Our observation of significant decrease in TNF-α level in dogs with demodicosis (dogs with LD as well as with GD) further corroborate well with the potential role of cholinergic pathway in anti-inflammatory/immunosuppressive response in demodicosed dogs. Albeit, dogs with GD along with concurrent pyoderma were found to have an elevated level of TNF-α and cholinesterase activity as compared with the dogs of control group and demodicosed dogs without pyoderma. Elevated circulatory TNF-α level could be the inflammatory response against concurrent bacterial infections in dogs with GD along with pyoderma. Moreover, dogs with GD along with or without pyoderma were found to have remarkably elevated IL-10 levels in comparison with controls. But, remarkable alteration in IL-10 contents between the dogs with LD and controls was not observed. This clearly indicates the potential association of IL-10 overproduction in progression of the disease condition from localized to generalized form. Our observations are in line with the findings of previous scientific studies where in the potential association of immunosuppressive cytokines with canine demodicosis has been demonstrated (Tani et al. Reference Tani, Morimoto, Hayashi, Inokuma, Ohnishi, Hayashiya, Nomura, Une, Nakaichi and Taura2002; Felix et al. Reference Felix, Guiot, Stein, Felix, Silva and Nobre2013; Yarim et al. Reference Yarim, Yagci and Ciftci2013). Therefore, it is quite possible that D. canis mites might be utilizing overproduction of IL-10 in adjunct to the vagal cholinergic anti-inflammatory host response to impair the Th1 immune response or to enhance the Th2 anti-inflammatory response in diseased dogs possibly for their own survival and incessant proliferation.
In conclusion, findings of the present study evidently demonstrate the involvement of cholinergic anti-inflammatory pathways and markedly elevated circulatory IL-10 content during generalized demodicosis process.
ACKNOWLEDGEMENT
The authors are highly thankful to the Head, Department of Pharmacology and Toxicology for providing the necessary facilities established under the ICAR Niche Area of Excellence Programme (Grant No. 10(10)/2012-EPD dated 23.03.2012) and also to the Hon'ble Vice-Chancellor of the University for providing the necessary facilities and funds.
FINANCIAL SUPPORT
Partial financial assistance from ICAR under ICAR outreach programme on Ethnoveterinary Medicine (Grant No. 1-72/(EVM-Outreach Programme)/2009/Med dated 05.02.2010) is also thankfully acknowledged.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL
The study was in accordance with the ethical standards of the Institutional Animal Ethics Committee of U.P. Pandit Deen Dayal Upadhyaya Pashu-Chikitsa Vigyan Vishwavidyalaya Evam Go Anusandhan Sansthan (DUVASU) Mathura, India (Approval No. 77/IAEC/2015; Dated 02.02.2016).





