Introduction
Pathogen pressure, inadequate nutrition and the interaction between these stressors can drastically impair pollinator health (Goulson et al., Reference Goulson, Nicholls, Botías and Rotheray2015). The relationship between nutrition and bee disease dynamics is complex, as diet quality and diversity can support immunocompetence and reduce infections in some instances, while increasing disease burdens in other cases (Koch et al., Reference Koch, Brown and Stevenson2017; Dolezal and Toth, Reference Dolezal and Toth2018). A bee's diet is made up of nectar and pollen, both of which have primary metabolites such as sugars, amino acids and lipids, as well as secondary compounds, such as flavonoids, terpenoids and alkaloids, with the potential to decrease (Richardson et al., Reference Richardson, Adler, Leonard, Andicoechea, Regan, Anthony, Manson and Irwin2015) or increase (Palmer-Young and Thursfield, Reference Palmer-Young and Thursfield2017) infections. In a world dominated by agricultural landscapes, with low floral diversity and frequent movement of commercial bee colonies that can potentially introduce pathogens into wild solitary bee populations (Kremen et al., Reference Kremen, Williams and Thorp2002; Otterstatter and Thomson, Reference Otterstatter and Thomson2008; Furst et al., Reference Furst, McMahon, Osborne, Paxton and Brown2014; Alger et al., Reference Alger, Burnham, Boncristiani and Brody2019), understanding the interplay between nutrition and pathogen burdens is important for protecting pollinator health.
Immune defence against pathogens is energetically costly, yet the resulting physiological trade-offs between immunity and other fitness components can be compensated by changes in diet (Moret and Schmid-Hempel, Reference Moret and Schmid-Hempel2000). When Bombus terrestris bees are starved of pollen, infection with Crithidia bombi, a trypanosomatid gut pathogen, markedly increases host mortality compared to bees with access to pollen (Brown et al., Reference Brown, Loosli and Schmid-Hempel2000). However, when both B. terrestris and B. impatiens have access to pollen, they can present higher C. bombi loads than pollen-starved counterparts (Logan et al., Reference Logan, Ruiz-González and Brown2005; Conroy et al., Reference Conroy, Palmer-Young, Irwin and Adler2016). Similarly, access to pollen can increase honey bee survival, while simultaneously increasing pathogen loads of the microsporidians Nosema apis (Rinderer and Dell Elliott, Reference Rinderer and Dell Elliott1977) and Nosema ceranae (Zheng et al., Reference Zheng, Lin, Huang, Sohr, Wu and Chen2014; Jack et al., Reference Jack, Uppala, Lucas and Sagili2016). As such, disentangling the effects of nutritional stress and pathogen infection on bee survival is important for understanding pollinator health, especially for solitary bees, which contribute substantially to pollination services worldwide yet have been historically understudied (Danforth et al., Reference Danforth, Minckley, Neff and Fawcett2019).
Honey bees and bumble bees have been the predominant model systems for addressing questions regarding bee health, especially for nutritional and epidemiological evaluations (Schmid-Hempel, Reference Schmid-Hempel1998). Recent advances in molecular surveillance have revealed widespread pathogen prevalence across solitary bee taxa from most bee families (Andrenidae, Apidae, Colletidae, Halictidae and Megachilidae). This includes pathogens known to infect honey bees and bumble bees (Apidae), including Apicystis bombi, Ascosphaera spp., C. bombi, C. mellificae, N. ceranae and numerous viruses (Singh et al., Reference Singh, Levitt, Rajotte, Holmes, Ostiguy, vanEngelsdorp, Lipkin, dePamphilis, Toth and Cox-Foster2010; Evison et al., Reference Evison, Roberts, Laurenson, Pietravalle, Hui, Biesmeijer, Smith, Budge and Hughes2012; Ravoet et al., Reference Ravoet, De Smet, Meeus, Smagghe, Wenseleers and de Graaf2014; Schoonvaere et al., Reference Schoonvaere, Smagghe, Francis and de Graaf2018; Figueroa et al., Reference Figueroa, Grab, Ng, Myers, Graystock, McFrederick and McArt2020). However, except for single-stranded RNA viruses which allow for strand-specific PCR assays to detect viral replication, we currently cannot distinguish between transient passage through the bee gut and active infections, nor do we know if there are negative consequences for the host based solely on molecular screenings (Bramke et al., Reference Bramke, Müller, McMahon and Rolff2019). Some existing studies that have experimentally evaluated the impacts on solitary bee health have shown increased mortality associated with infections [e.g. Megachile rotundata larvae infected with the fungus Ascosphera aggregata (James, Reference James2005) and Osmia bicornis infected with the neogregarine A. bombi and the microsporidian N. ceranae (Tian et al., Reference Tian, Piot, Meeus and Smagghe2018; Bramke et al., Reference Bramke, Müller, McMahon and Rolff2019)], further highlighting the need to address the host range of bee pathogens and negative consequences for these understudied bee species.
Pathogens are spread between bee species via shared use of floral resources (Graystock et al., Reference Graystock, Goulson and Hughes2015). Despite ample possible routes of indirect transmission via flowers at the community level (Figueroa et al., Reference Figueroa, Grab, Ng, Myers, Graystock, McFrederick and McArt2020), the host range of many bee pathogens remains currently unknown. Infection with the trypanosomatid C. bombi can affect bumble bee foraging behaviour, cognitive function (Gegear et al., Reference Gegear, Otterstatter and Thomson2005, Reference Gegear, Otterstatter and Thomson2006) and reproduction (Goulson et al., Reference Goulson, O'Connor and Park2018). While C. bombi is known to infect multiple bumble bee species (Colla et al., Reference Colla, Otterstatter, Gegear and Thomson2006; Cordes et al., Reference Cordes, Huang, Strange, Cameron, Griswold, Lozier and Solter2012; Ruiz-González et al., Reference Ruiz-González, Bryden, Moret, Reber-Funk, Schmid-Hempel and Brown2012), honey bees are not a known host (Ruiz-González and Brown, Reference Ruiz-González and Brown2006; Graystock et al., Reference Graystock, Goulson and Hughes2015), even though both groups belong to the same family. It is largely unknown whether solitary bee species, which frequently test positive for C. bombi via PCR-based screenings (Figueroa et al., Reference Figueroa, Grab, Ng, Myers, Graystock, McFrederick and McArt2020; Graystock et al., Reference Graystock, Ng, Parks, Tripodi, Muñiz, Fersch, Myers, McFrederick and McArt2020), are actually infected by this pathogen (Ravoet et al., Reference Ravoet, De Smet, Meeus, Smagghe, Wenseleers and de Graaf2014). The solitary bees Osmia lignaria and M. rotundata are cavity-nesting species that provide important pollination services for fruits and vegetables in North America and Europe (Velthuis and van Doorn, Reference Velthuis and van Doorn2006; Pitts-Singer and Cane, Reference Pitts-Singer, Cane, Berenbaum, Carde and Robinson2011; Brittain et al., Reference Brittain, Williams, Kremen and Klein2013) and can serve as model organisms for experimentally evaluating epidemiological questions due to their commercial availability. Recent experimental work has shown that C. bombi collected from B. impatiens can infect these species (Ngor et al., Reference Ngor, Palmer-Young, Nevarez, Russell, Leger, Giacomini, Pinilla-Gallego, Irwin and McFrederick2020), further highlighting the need to understand the biotic and abiotic factors, such as diet, that influence disease dynamics in species beyond honey bees and bumble bees.
To fill this critical knowledge gap, we conducted a study to understand the influence of nutritional stress on the susceptibility of solitary bees to pathogens. Specifically, we asked: (1) how frequently after exposure to C. bombi do the solitary bee species O. lignaria and M. rotundata become infected, (2) does pollen access influence the likelihood of infection and/or subsequent load of C. bombi in O. lignaria and M. rotundata, and (3) does pollen access and/or C. bombi exposure influence O. lignaria and M. rotundata survival?
Materials and methods
Study system
For our experiments, we used the trypanosomatid pathogen C. bombi (Kinetoplastea, Trypanosomatida) and two solitary bee species: M. rotundata (Hymenoptera, Megachilidae; Crown Bees, Woodinville, WA, USA) and O. lignaria (Hymenoptera, Megachilidae; Watts bees, Bothell, WA, USA). The bees were obtained as cocoons and allowed to eclose in individually marked housing units: inverted 59 mL salsa cups (Fabi-Kal® Greenware, Lancaster, PA, USA) lined with filter paper (Whatman®, Marlborough, PA, USA) that provided access to 30% sucrose solution through a small opening at the tip of a 1.5 mL microcentrifuge tube (VWR™, Radnor, PA, USA) (Fig. S1). The sex of these individuals can be easily determined visually upon eclosion. As such, only females were maintained for the experiment with O. lignaria. Females represented <10% of the eclosed M. rotundata, and we therefore conducted the experiments exclusively with males for this species in order to have sufficient sample sizes. As such, we do not make any formal species or sex comparisons in our analyses. The C. bombi we used in our trials was collected from wild Bombus impatiens (Hymenoptera, Apidae) workers from Massachusetts, USA (GPS: 42822’17.5300N, 72835’13.5200W). The strain was sustained in laboratory bumble bee colonies (Biobest, Leamington, Ontario, Canada). The two solitary bee species are commonly used in commercial agriculture in the USA, though O. lignaria is native to North America while M. rotundata is European (Velthuis and van Doorn, Reference Velthuis and van Doorn2006).
Experimental design
To investigate C. bombi replication and its effect on bee survival, we conducted a 2 × 2 factorial experiment for each bee species, contrasting nutritional stress (presence/absence of pollen) and infection status (inoculated/sham-inoculated with C. bombi). We prepared C. bombi inoculum fresh for each inoculation day by dissecting the gut of infected B. impatiens bees from the laboratory source colony. We homogenized the bee guts in distilled water and quantified C. bombi cells using a haemocytometer. We diluted the mixture to 1200 C. bombi cells μL−1, which we then combined 1:1 with 30% sucrose solution for an inoculum of 600 cells μL−1, a standard inoculum concentration for infecting bumble bees with C. bombi (Richardson et al., Reference Richardson, Adler, Leonard, Andicoechea, Regan, Anthony, Manson and Irwin2015; Figueroa et al., Reference Figueroa, Blinder, Grincavitch, Jelinek, Mann, Merva, Metz, Zhao, Irwin and McArt2019). We used 30% sucrose without C. bombi as a control (sham) inoculum. The sucrose solution was coloured with blue food colouring (McCormick & Company, Baltimore, MD, USA) to facilitate confirmation of consumption and availability.
For each trial, half of the bees were inoculated with C. bombi and half received the sham inoculation. Each bee species was inoculated on separate dates: O. lignaria on 27 June 2019 (n = 97), 22 July 2019 (n = 48) and 23 July 2019 (n = 52), while M. rotundata was inoculated on 7 July 2019 (n = 100). The unit of replication for this study was individual bees. The lower sample size for M. rotundata was due to lower availability of bees. The bees had eclosed 1–2 days before being inoculated in their individual housing units. Each treatment was evenly represented on each inoculation date. Standard C. bombi inoculations in bumble bees are conducted by eliciting proboscis extension from the sugar in the inoculum and directly feeding the bees 10 μL of inoculum (Richardson et al., Reference Richardson, Adler, Leonard, Andicoechea, Regan, Anthony, Manson and Irwin2015; Figueroa et al., Reference Figueroa, Blinder, Grincavitch, Jelinek, Mann, Merva, Metz, Zhao, Irwin and McArt2019). However, in a pilot study, we found that O. lignaria and M. rotundata bees were uncooperative and would not consume the inoculum droplets using the standardized bumble bee protocol. When we subdued the bees using CO2, we were able to place the inoculum droplet directly on their extended proboscis, finding that this method was effective at infecting the solitary bees.
To administer the inoculum for this experiment, we exposed individual bees to CO2 gas for 45 s, during which time most bees extended their proboscis. We then placed 5 μL of inoculum on their proboscis and on the pollen, if present, or on the side of the feeding tube for pollen-free treatments, to maximize exposure to the pathogen (10 μL on the first day of trial), O. lignaria received an additional 5 μL dose on the pollen/feeding tube for two consecutive days for a total of 20 μL (12 000 C. bombi cells) administered compared to the 10 μL (6000 C. bombi cells) for M. rotundata. The additional doses were administered to increase the likelihood of exposure to the pathogen in the larger of the two species (O. lignaria). Trays containing the bees were separated by treatment to avoid cross-contamination, but were all maintained on the same laboratory bench for the duration of the assay.
Half of the bees in both the inoculated and sham-inoculated treatments were provided ~36 mg balls of pollen, made from a mixture of honey bee collected poly-floral pollen (Bee Pollen Granules, CC Pollen High Desert, Phoenix AZ, USA), and all bees were provided 30% sucrose solution. The sucrose solution was replaced every 3 days and pollen balls were given to the pollen treatment bees every other day. We verified that the pollen had little to no pesticides by screening a pollen sample for 267 pesticides using liquid chromatography/mass spectrometry (Urbanowicz et al., Reference Urbanowicz, Baert, Bluher, Böröczky, Ramos and McArt2019). We detected only one pesticide, the acaricide coumaphos, at a level below the limit of quantification (<0.525 ng g−1), and thus concluded that pesticide exposure would not be a primary driver of any pattern found with pollen access. The pollen was not a source of C. bombi as can be verified by the absence of C. bombi in our sham-inoculated bees fed pollen (Fig. 1). While O. lignaria larvae tend to develop more quickly and larger body sizes on pollen collected by members of its own species, they will nonetheless develop on honey bee-collected pollen (Levin and Haydak, Reference Levin and Haydak1957). Whether this translates into differences in pollen feeding by adults (both female O. lignaria and male M. rotundata) has not been tested. Though we did not quantify pollen consumption, we observed frass with pollen residues for some of the bees in the pollen-access treatments, indicating consumption. The bees were maintained in laboratory conditions at an average temperature of approximately 20°C in constant dark.
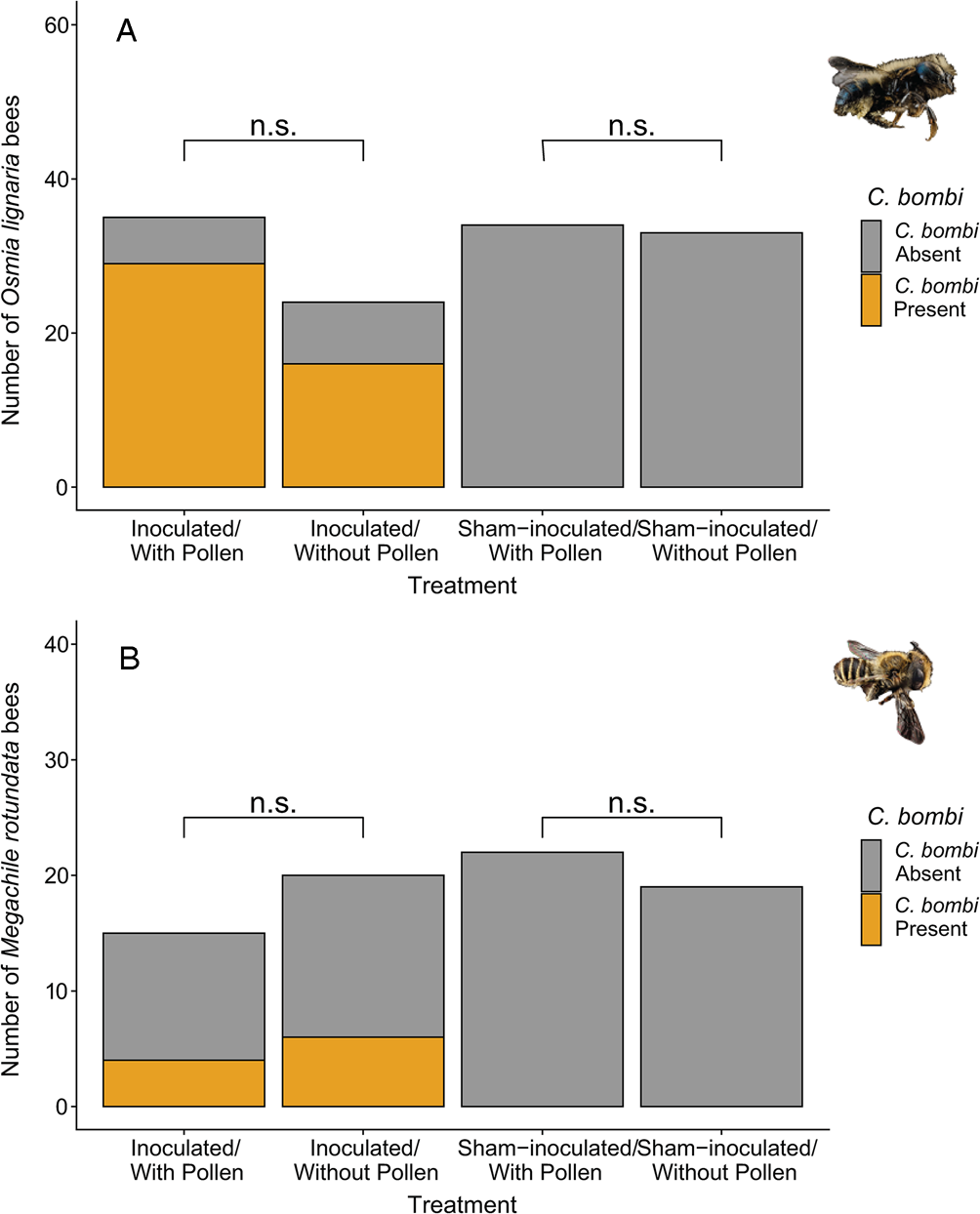
Fig. 1. Effect of pollen access on motile C. bombi presence in solitary bees that survived the length of the trial. Pollen access did not affect the likelihood of C. bombi presence in (A) Osmia lignaria females or (B) Megachile rotundata males. Differences in sample size are a product of mortality. (n.s.) indicates P > 0.05.
We checked bee survival daily for the duration of the trial (terminated after 11 days for M. rotundata, and 8, 6 and 5 days for the three O. lignaria trials, respectively), and recorded daily mortality for each bee. Trial lengths varied due to differences in mortality; while M. rotundata had low overall mortality (18% died by the end of the 11-day trial), mortality for O. lignaria was overall higher and varied greatly (ranged from 14 to 54% depending on the trial). We dissected any bee that had died within 24 h to screen for C. bombi (checked daily), as well as all bees that survived until the end of the trial. Given that our pathogen counts are based on motile cells, we expected the most accurate counts from bees that were alive. As such, we shortened the trial times for O. lignaria in order to have enough live bees to accurately quantify infection because observing live C. bombi in dead bees, while possible, is likely less accurate. For example, only 8% of recorded C. bombi-positive O. lignaria bees had died before the end of the trial and had much lower corresponding pathogen loads (mean of 71 active C. bombi cells μL−1 compared to 171 cells μL−1 in bees that survived the length of the trial). We cannot determine whether the lower likelihood of detection and lower counts were products of insufficient time for the pathogen to replicate or whether the pathogen had died within the host before dissection and could not be visualized. As such, infection analyses were conducted only on bees that survived until the end of the trial. Nonetheless, the shorter trial times for O. lignaria are within time frames relevant for C. bombi replication in bumble bees (Schmid-Hempel and Schmid-Hempel, Reference Schmid-Hempel and Schmid-Hempel1993). We homogenized the dissected bee guts in 200 μL of distilled water, incubated the mixture for 4 h at room temperature and finally quantified motile C. bombi using a haemocytometer (Richardson et al., Reference Richardson, Adler, Leonard, Andicoechea, Regan, Anthony, Manson and Irwin2015; Figueroa et al., Reference Figueroa, Blinder, Grincavitch, Jelinek, Mann, Merva, Metz, Zhao, Irwin and McArt2019).
For the bees that had motile C. bombi, we evaluated whether the values indicated active replication by the pathogen. To do this, we compared estimated whole gut counts to the value in the entirety of the inoculum provided to the bees. The C. bombi μL−1 observed for each bee was multiplied by 200 μL, the volume of water in which the gut was incubated, indicating the total number of C. bombi cells estimated to be in the bee gut. This is a conservative estimate as we are not including the volume of the gut itself. Values above 12 000 for O. lignaria and 6000 for M. rotundata indicate active pathogen replication in the bees as these values correspond to the maximum possible cells consumed in the inoculum. We report these numbers as ‘whole gut C. bombi estimates’ in the results.
At the end of the test periods, we measured the inter-tegular distance (ITD) of all of the bees using an Olympus SZX10 microscope and cellSens Standard software (Olympus Corporation of the Americas, Scientific Solutions Group, Waltham, Massachusetts, USA). The ITD is commonly used as a proxy for bee body size (Greenleaf et al., Reference Greenleaf, Williams, Winfree and Kremen2007), which is a factor related to immunity in bumble bees (Otterstatter and Thomson, Reference Otterstatter and Thomson2006).
Statistical analyses
Data analyses were conducted using R version 3.5.1 (R Development Core Team, 2008) with the lme4, glmmTMB and coxme packages (Bates et al., Reference Bates, Mächler, Bolker and Walker2015; Therneau and Therneau, Reference Therneau and Therneau2015; Brooks et al., Reference Brooks, Kristensen, van Benthem, Magnusson, Berg, Nielsen, Skaug, Machler and Bolker2017). We conducted analyses on M. rotundata and O. lignaria separately.
To determine the role of pollen access on C. bombi infection patterns in the solitary bees, we employed a manual two-step hurdle model due to zero-inflation and overdispersion in the data (Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009). As mentioned above, these analyses were only conducted on inoculated bees that survived until the last day of the trial to increase the accuracy of C. bombi counts (11 days for M. rotundata and 5, 6 or 8 days for the three O. lignaria trials). We first evaluated whether pollen access predicted the presence or absence of C. bombi in the gut of inoculated bees, followed by an evaluation of the C. bombi counts for those that were infected. For O. lignaria, we first constructed a generalized linear mixed model (GLMM) to evaluate whether the solitary bee species harboured C. bombi in their gut as the response (yes/no), predicted by access to pollen (yes/no) and bee size (n = 59). The model included inoculation date as the random effect and a binomial distribution (logit link). To determine significance, we employed a likelihood ratio test and compared the model to one with the same response, distribution and random-effect structure, but which excluded pollen as a predictor. We then developed a GLMM that included C. bombi count in C. bombi-positive bees as the response variable, predicted by access to pollen (presence/absence) and bee size (n = 49). The model included inoculation date as the random effect and fit a truncated negative binomial distribution, which is suitable for count data with overdispersion (Brooks et al., Reference Brooks, Kristensen, van Benthem, Magnusson, Berg, Nielsen, Skaug, Machler and Bolker2017). Significance was similarly determined using a likelihood ratio test. We verified model assumptions using the DHARMa package (Hartig, Reference Hartig2017). The statistical analyses for M. rotundata were likewise evaluated, with the sole difference that the manual hurdle model was conducted using a Generalized Linear Model instead of a GLMM because the species was inoculated in a single day and thus did not require inoculation date as a random effect (n = 35 for binomial response and n = 10 for C. bombi count as a response).
To evaluate whether exposure to C. bombi, access to pollen and their interactions influenced solitary bee survival, we conducted survival analyses using Cox proportional hazards models (n = 179 and n = 93 for O. lignaria and M. rotundata, respectively). For O. lignaria, the survival analysis evaluated bee survival (death/days elapsed) as the response, C. bombi inoculation and access to pollen as explanatory variables, and inoculation date as the random effect. To determine the significance of the treatments (pollen and inoculation), we conducted a likelihood ratio test comparing the full model with a model that included the same random-effect structure but excluded either explanatory variable or included an additive relationship instead of an interaction. The analyses for M. rotundata were conducted using the coxph function (no random effect since all bees were inoculated on the same day) while the coxme function was used for O. lignaria (inoculation date as a random effect) (Therneau and Therneau, Reference Therneau and Therneau2015).
Results
We found that both O. lignaria females and M. rotundata males became infected with C. bombi sourced from the common eastern bumble bee (B. impatiens). Specifically, 76% of inoculated O. lignaria and 29% of M. rotundata harboured active C. bombi in their gut 5–11 days post inoculation; we did not detect C. bombi in any sham-inoculated bees (Fig. 1). The median C. bombi load at the end of the trial for inoculated O. lignaria females was 100 moving cells μL−1 of bee gut (range: 0–1600 cells), while for M. rotundata males, the median was 0 cells μL−1 (range: 0–300 cells). The O. lignaria females were inoculated with 12 000 C. bombi cells and final counts averaged 20 000 cells (range: 0–320 000 whole gut cell estimates), indicating active replication of C. bombi in O. lignaria (Fig. 2). While the median C. bombi whole gut count was zero in M. rotundata males, the bees with the highest gut counts in this species (60 000) had more than the administered 6000 C. bombi cells, indicating replication of C. bombi in M. rotundata was also possible (Fig. 2). Specifically, 56% of inoculated O. lignaria females and 29% of M. rotundata males had estimated whole gut C. bombi counts above the inoculation concentration indicating active pathogen replication (Fig. 2).
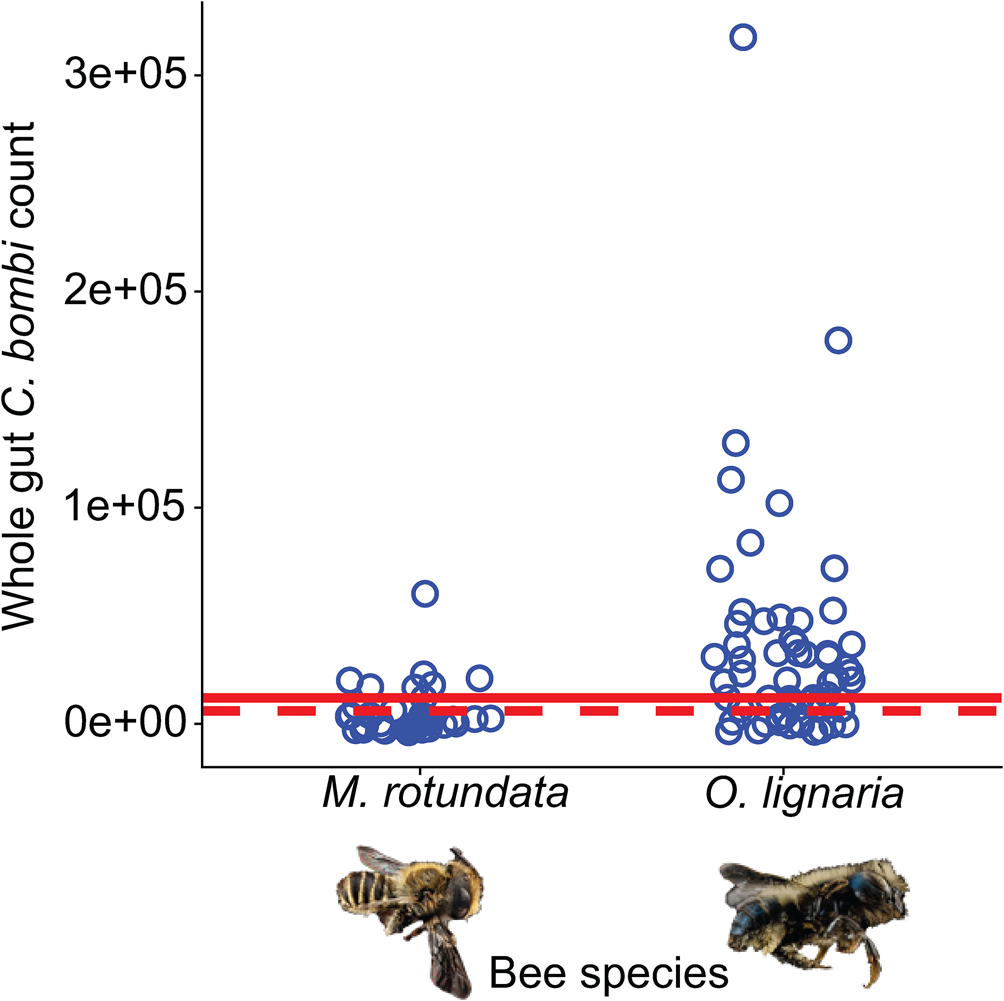
Fig. 2. Estimated whole gut counts of C. bombi in solitary bees 5–11 days post-inoculation. Blue circles indicate estimated whole gut counts in inoculated M. rotundata males and O. lignaria females (see Methods). The dashed red line indicates the total number of C. bombi cells in inoculum provided to M. rotundata males and the solid red line indicates the total number of C. bombi cells in inoculum provided to O. lignaria females. Values above these thresholds indicate active pathogen replication in the host bees.
Pollen access did not influence the likelihood of becoming infected with C. bombi for O. lignaria females or M. rotundata males (χ 21 = 1.86, P = 0.173 and z = 0.05, P = 0.962, respectively; Fig. 1) nor subsequent load for infected bees (χ 21 = 0.05, P = 0.830 and z = 1.78, P = 0.076, respectively for O. lignaria females and M. rotundata males; Fig. 3). Bee size did not explain the likelihood of infection with C. bombi nor subsequent load for the infected bees in O. lignaria females or M. rotundata males (z < 0.84 and P > 0.402 for all).
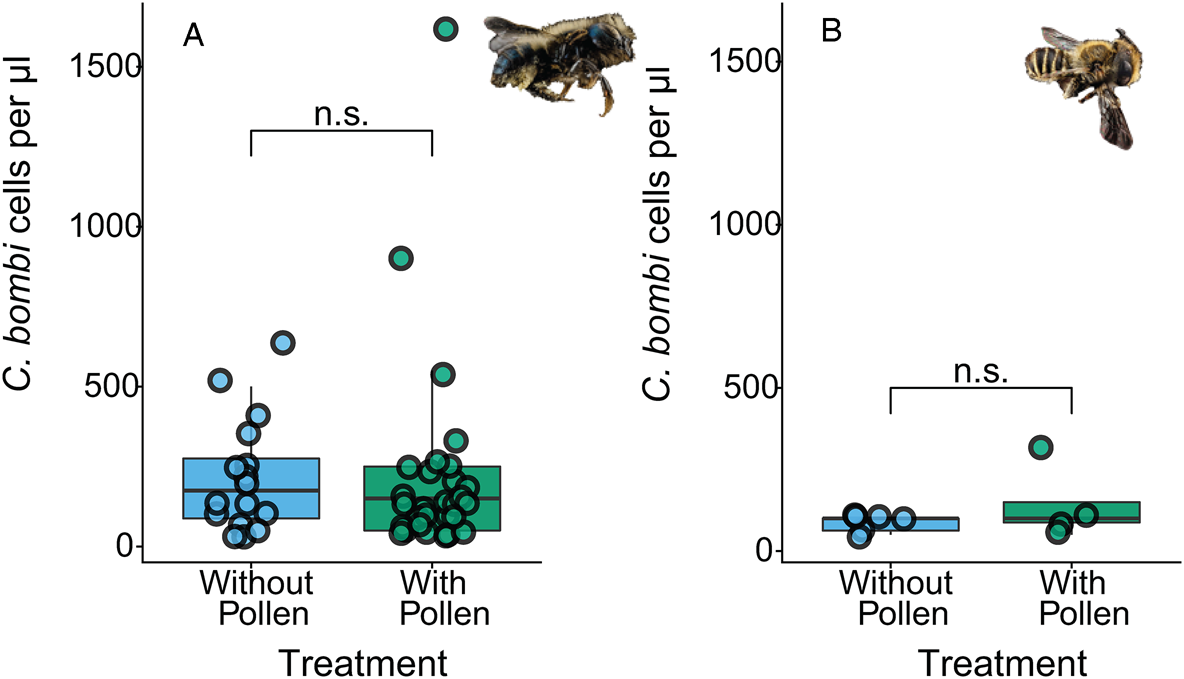
Fig. 3. Effect of pollen access on C. bombi load in solitary bees. Pollen access did not affect C. bombi load (cells μL−1) in (A) Osmia lignaria females or (B) Megachile rotundata males. (n.s.) indicates P > 0.05. This figure represents subsequent load for inoculated bees that presented motile C. bombi.
Access to pollen increased O. lignaria female bee survival rates (χ 21 = 4.13, P = 0.042; Fig. 4A), though not for M. rotundata males (χ 21 = 1.19, P = 0.275; Fig. 4C). Inoculation with C. bombi did not influence survival in O. lignaria females or M. rotundata males (χ 21 = 1.56, P = 0.212 and χ 21 = 2.02, P = 0.155, respectively; Fig. 4B and D), nor was there pollen access by inoculation interaction for survival in O. lignaria females (χ 21 = 2.26, P = 0.133) or M. rotundata males (χ 21 = 1.73, P = 0.189).

Fig. 4. Solitary bee survival across pollen access and C. bombi inoculation treatments. (A) Osmia lignaria females with access to pollen survived longer than those without access to pollen. (B). Inoculation with C. bombi did not influence O. lignaria survivorship. (C) Pollen access did not affect Megachile rotundata male survivorship, (D) nor did inoculation with C. bombi. Trial lengths varied according to general mortality, which was greater in M. rotundata than for O. lignaria (see Methods). Shaded error bands indicate 95% confidence intervals. (*) denotes P < 0.05 while (n.s.) indicates P > 0.05.
Discussion
In this study, we found that the solitary bee species O. lignaria and M. rotundata could become infected with C. bombi collected from bumble bees. We found evidence for C. bombi replication in 56% of inoculated O. lignaria females and 29% of inoculated M. rotundata males. Access to pollen did not affect C. bombi infection in O. lignaria females or M. rotundata males. However, pollen-fed O. lignaria females survived longer than their pollen-starved counterparts. Inoculation with C. bombi did not affect survival in either solitary bee species during the 5–11-day laboratory assay. Overall, our study illustrates that solitary bees can be hosts for a pathogen known to infect bumble bees and that diet can play a key role in the health of these important but historically understudied solitary bee taxa.
Most bee species within a community can be exposed to numerous pathogens when foraging at flowers, including C. bombi (Figueroa et al., Reference Figueroa, Grab, Ng, Myers, Graystock, McFrederick and McArt2020; Graystock et al., Reference Graystock, Ng, Parks, Tripodi, Muñiz, Fersch, Myers, McFrederick and McArt2020). Our results support a growing body of literature indicating the need to assess the host range of bee pathogens, including assessments of replication and impacts on survival (Bramke et al., Reference Bramke, Müller, McMahon and Rolff2019; Müller et al., Reference Müller, McMahon and Rolff2019). The presence of C. bombi in bee feces has been used to identify active infections in bumble bees (Imhoof and Schmid-Hempel, Reference Imhoof and Schmid-Hempel1999), where it can be detected in as quickly as 5 days post-inoculation (Logan et al., Reference Logan, Ruiz-González and Brown2005). We detected active C. bombi in solitary bee guts at the end of the trials, 5–11 days post-inoculation, for a total of 76% of inoculated O. lignaria females and 29% of inoculated M. rotundata males harbouring motile cells. Moreover, 56% of the inoculated O. lignaria females and 29% of the inoculated M. rotundata males had estimated whole gut counts above the concentration of inoculum provided, indicating active pathogen replication. These values are comparable to infection probability in bumble bees [e.g. 57% of inoculated B. terrestris bees presented active infections after a 7-day trial (Näpflin and Schmid-Hempel, Reference Näpflin and Schmid-Hempel2018)]. Furthermore, our estimates for the percentage of bees experiencing C. bombi replication are conservative (i.e. low) since we assume the bee gut did not contribute to the volume of the extraction and that the bees consumed the entirety of the inoculum droplet, further highlighting the extent to which solitary bees can develop active C. bombi infections.
While phylogenetic distance often predicts the ability of hosts to be infected by a given pathogen (Gilbert and Webb, Reference Gilbert and Webb2007; Streicker et al., Reference Streicker, Turmelle, Vonhof, Kuzmin, McCracken and Rupprecht2010), this may not be the case with C. bombi and its known hosts. Specifically, one recent study (Ngor et al., Reference Ngor, Palmer-Young, Nevarez, Russell, Leger, Giacomini, Pinilla-Gallego, Irwin and McFrederick2020) has found that C. bombi does not replicate in honey bees (Apis mellifera), but this pathogen does replicate in bumble bees (Bombus spp.). Both honey bees and bumble bees are in the Apidae family, while the two evaluated solitary bees evaluated in the present study are in the Megachilidae family. The factors that enable honey bees to avoid chronic C. bombi infections, despite being able to transmit the pathogen via flowers (Ruiz-González and Brown, Reference Ruiz-González and Brown2006; Graystock et al., Reference Graystock, Goulson and Hughes2015) and being more phylogenetically similar to bumble bees than O. lignaria and M. rotundata, remain unknown. Future evaluations of C. bombi infection and health impacts on solitary and social species across additional bee families are clearly warranted.
Host nutrition can increase infection intensity by providing resources to pathogens, but may also support host tolerance of infection. Specifically, increased access to food could increase infections by (1) directly providing nutrients to the pathogen; or (2) improving host quality for the pathogen. Conversely, food availability could suppress pathogens by (3) enabling physiologically costly immune responses; and/or (4) providing antimicrobial compounds (Conroy et al., Reference Conroy, Palmer-Young, Irwin and Adler2016; Palmer-Young and Thursfield, Reference Palmer-Young and Thursfield2017). While we did not find that pollen access directly influenced the likelihood or severity of C. bombi infections (Figs 1 and 3), others have found that pollen-deprivation in bumble bees can reduce C. bombi infections (Logan et al., Reference Logan, Ruiz-González and Brown2005; Conroy et al., Reference Conroy, Palmer-Young, Irwin and Adler2016). We found that pollen access increased O. lignaria female survival, independent of C. bombi infection. While we did not find an effect of pollen on M. rotundata, we cannot determine whether this was due to low mortality during the 11-day trial and whether patterns would differ in longer time scales. Similarly to O. lignaria, access to pollen can increase honey bee survival rates despite greater pathogen loads [e.g. N. apis, N. ceranae and numerous RNA viruses (Rinderer and Dell Elliott, Reference Rinderer and Dell Elliott1977; Zheng et al., Reference Zheng, Lin, Huang, Sohr, Wu and Chen2014; Jack et al., Reference Jack, Uppala, Lucas and Sagili2016; Zhang et al., Reference Zhang, St. Clair, Dolezal, Toth and O'Neal2020)], further illustrating the importance of diet in pollinator health. Access to pollen in provisioning female solitary bees has been shown to increase the number of brood cells and proportion of offspring surviving to adulthood [e.g. in O. bicornis and O. californica (Cane, Reference Cane2016; Bukovinszky et al., Reference Bukovinszky, Rikken, Evers, Wäckers, Biesmeijer, Prins and Kleijn2017)], suggesting that differences in physiological demands between sexes could result in differential effects of pollen access. Furthermore, a recent study that experimentally inoculated O. cornuta with C. mellificae, a trypanosomatid known to infect honey bees, found that while both sexes had higher pathogen loads over time, only male survival was significantly reduced by infection (Strobl et al., Reference Strobl, Yañez, Straub, Albrecht and Neumann2019). Future evaluations of the impact of C. bombi on physiology and survival in solitary bees across sexes are well justified, especially in the field, and could incorporate assessments of resource availability and bee immune responses.
Bumble bees infected with C. bombi (Richardson et al., Reference Richardson, Adler, Leonard, Andicoechea, Regan, Anthony, Manson and Irwin2015) and honey bees infected with N. ceranae (Mayack and Naug, Reference Mayack and Naug2009) often consume more pollen, likely to compensate for the energetic cost of mounting an immune response (Moret and Schmid-Hempel, Reference Moret and Schmid-Hempel2000). Interestingly, the role of pollen on C. bombi growth varies depending on whether the assessments are conducted in vitro or in vivo; for example, sunflower pollen in vitro increases pathogen growth (Palmer-Young and Thursfield, Reference Palmer-Young and Thursfield2017), while in vivo this pollen type has a strong inhibitory effect on the pathogen when consumed by bumble bees (Giacomini et al., Reference Giacomini, Leslie, Tarpy, Palmer-Young, Irwin and Adler2018; LoCascio et al., Reference LoCascio, Aguirre, Irwin and Adler2019). When inhibitory, secondary compounds appear to facilitate these anti-C. bombi interactions in bumble bees (Richardson et al., Reference Richardson, Adler, Leonard, Andicoechea, Regan, Anthony, Manson and Irwin2015; Koch et al., Reference Koch, Brown and Stevenson2017). These differences suggest that pollen may interact in important ways within the host, leading to varying effects on C. bombi growth. Future evaluations of the role of pollen quality, quantity and diversity on susceptibility to pathogens mediated by interactions between immune responses, gut physiology and microbiota are important avenues for pollinator research (Alaux et al., Reference Alaux, Ducloz, Crauser and Conte2010; Cotter et al., Reference Cotter, Simpson, Raubenheimer and Wilson2011; Dolezal and Toth, Reference Dolezal and Toth2018), especially focusing on solitary bees and other understudied pollinators.
Life-history and functional traits are important mediators of disease transmission and dynamics within a host. While we did not find a role of pollen in M. rotundata male infection patterns, recent work has shown that adult male solitary bees (Andrena spp.) frequently harbour pollen in their digestive tract (Urban-Mead et al., unpublished results). The importance of males in dispersing pathogens in plant–pollinator networks is not well established, despite male and female solitary bees having comparable infection rates (Müller et al., Reference Müller, McMahon and Rolff2019; Strobl et al., Reference Strobl, Yañez, Straub, Albrecht and Neumann2019; Ngor et al., Reference Ngor, Palmer-Young, Nevarez, Russell, Leger, Giacomini, Pinilla-Gallego, Irwin and McFrederick2020) and marked differences in floral preference (Roswell et al., Reference Roswell, Dushoff and Winfree2019). Moreover, natural rates of pathogen acquisition or deposition by solitary bees while foraging is not known (Figueroa et al., Reference Figueroa, Blinder, Grincavitch, Jelinek, Mann, Merva, Metz, Zhao, Irwin and McArt2019), including the threshold of pathogen cells necessary for solitary bees to develop active infections, all of which could strengthen disease spread models. While we report high rates of C. bombi infection for the two solitary species evaluated, especially for O. lignaria females, we did not find that experimentally exposing the solitary bees to C. bombi influenced host survival in the 5–11-day laboratory assay. Characterizing naturally occurring infection rates as well as the corresponding impacts on mortality and reproduction in the field, especially alongside numerous co-occurring stressors (e.g. inadequate diet, pesticide exposure and co-infections), is an important future direction. Differences in life histories across solitary bees may influence these dynamics. For example, O. lignaria overwinter as adults, similar to bumble bee queens [a life stage highly vulnerable to C. bombi (Brown et al., Reference Brown, Schmid-Hempel and Schmid-Hempel2003)], while M. rotundata overwinter as prepupae (Kemp et al., Reference Kemp, Bosch and Dennis2004). Similarly, given that we know infection with C. bombi alters resource allocation patterns in B. terrestris, whereby infected bees invest more in their fat body and less in reproduction than non-infected bees (Brown et al., Reference Brown, Loosli and Schmid-Hempel2000), assessing differences in resource allocation, foraging behaviour and pollen-provisioning abilities for infected solitary bees compared to uninfected counterparts could advance our understanding of pollinator health in wild bee communities.
Overall, our work supports the importance of improving knowledge concerning solitary bee species in pollinator epidemiology, including their assessment as hosts for what have traditionally been considered social bee pathogens, and considering resource availability when evaluating host–pathogen interactions. Future evaluations of the likelihood of transmission between solitary and social species on flowers would benefit from this knowledge, as transmission probability is already known to vary between bumble bee species (Ruiz-González et al., Reference Ruiz-González, Bryden, Moret, Reber-Funk, Schmid-Hempel and Brown2012). Moreover, the activity period of Osmia spp. and Megachile spp. often greatly overlaps with bumble bees, further highlighting the need to understand interspecies disease transmission networks (Figueroa et al., Reference Figueroa, Grab, Ng, Myers, Graystock, McFrederick and McArt2020). In addition, pathogen transmission between social and solitary bees could occur via spillover by introduced commercial colonies (Colla et al., Reference Colla, Otterstatter, Gegear and Thomson2006; Otterstatter and Thomson, Reference Otterstatter and Thomson2008; Graystock et al., Reference Graystock, Yates, Evison, Darvill, Goulson and Hughes2013), and could occur in various directions (Graystock et al., Reference Graystock, Blane, McFrederick, Goulson and Hughes2016), further highlighting the need to understand host ranges and impacts of disease on pollinator communities. In order to respond to the growing dependence of pollinators for food security (Aizen et al., Reference Aizen, Aguiar, Biesmeijer, Garibaldi, Inouye, Jung, Martins, Medel, Morales, Ngo, Pauw, Paxton, Sáez and Seymour2019), which is in large part contributed by wild bees (Garibaldi et al., Reference Garibaldi, Steffan-Dewenter, Winfree, Aizen, Bommarco, Cunningham, Kremen, Carvalheiro, Harder, Afik, Bartomeus, Benjamin, Boreux, Cariveau, Chacoff, Dudenhoffer, Freitas, Ghazoul, Greenleaf, Hipolito, Holzschuh, Howlett, Isaacs, Javorek, Kennedy, Krewenka, Krishnan, Mandelik, Mayfield, Motzke, Munyuli, Nault, Otieno, Petersen, Pisanty, Potts, Rader, Ricketts, Rundlof, Seymour, Schuepp, Szentgyorgyi, Taki, Tscharntke, Vergara, Viana, Wanger, Westphal, Williams and Klein2013; Winfree et al., Reference Winfree, Reilly, Bartomeus, Cariveau, Williams and Gibbs2018), we must expand our understanding of the role of pathogens, nutrition and other stressors on solitary bee health.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020002218
Acknowledgements
We thank Abby Davis, Julie Davis, Mesly Mata Loya and Alondra Torres Gonzalez for assistance with the experimental set-up and trials, Laura Harrington, Quinn McFrederick, Evan Palmer-Young, Todd Ugine and members of the Cornell Pollinator Reading Group for reading a previous version of the manuscript, and Wee Hao Ng for assistance with statistical analyses. We would also like to thank Jonathan Giacomini, Bryanna Joyce, Simon Pinilla-Gallego and Jenny Van Wyk for assistance in obtaining source bees for making the inoculum used in the trials.
Financial support
This work was supported by the National Science Foundation Graduate Research Fellowship (grant number DGE-1650441), the National Science Foundation Postdoctoral Research Fellowships in Biology Program (grant number NSF-2010615), and the National Institute of General Medical Sciences of the National Institutes of Health (grant number R01GM122062). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation. This material is based upon work supported by the NSF Postdoctoral Research Fellowships in Biology Program under Grant No. 2010615.
Conflict of interest
None.
Ethical standards
Not applicable.








