Introduction
Chagas disease is a tropical neglected infection caused by the protozoan parasite Trypanosoma cruzi (Lara et al., Reference Lara, Andrade-Lima, Magalhães Calvet, Borsoi, Lopes Alberto Duque, Henriques-Pons, Souza Pereira and Veiga2018; Ribeiro et al., Reference Ribeiro2018). About 6–7 million people are infected by T. cruzi worldwide, mainly in Latin American countries, where the disease is a public health problem and at least 25 million people are at risk of infection (WHO, 2018). Trypanosoma cruzi infection is a zoonosis in endemic areas and humans are the main accidental hosts of this parasite (Noireau et al., Reference Noireau, Diosque and Jansen2009). Chagas disease exhibits increasing incidence and prevalence in non-endemic areas, especially North America, Europe, Asia and Africa (Jackson et al., Reference Jackson, Pinto and Pett2014; Angheben et al., Reference Angheben, Boix, Buonfrate, Gobbi, Bisoffi, Pupella, Gandini and Aprili2015). In the absence of the insect vector, the spread of Chagas disease in non-endemic countries often occurs due to the migration of infected people from endemic countries, blood transfusion, transplant of infected organs and from infected mother to fetus (vertical transmission) (Rassi et al., Reference Rassi, Rassi and Marin-Neto2009).
Chagas disease courses with non-specific clinical manifestations in the acute phase, especially fever, vomit, lethargy and anorexia (Pinto et al., Reference Pinto, Valente, Valente, Ferreira and Coura2008; Malik et al., Reference Malik, Singh and Amsterdam2015). About 60–70% of infected people remain asymptomatic during many years after infection (Fresno and Gironès, Reference Fresno and Gironès2018). However, 30% of infected people may present anatomical and functional cardiac and/or digestive abnormalities while the disease becomes chronic (Rassi et al., Reference Rassi, Rassi and Rezende2012). Although the pathogenesis is not completely understood, Chagasic cardiomyopathy is the most frequent and serious manifestation of the symptomatic chronic phase, which is related with high morbidity and mortality rates (Medei et al., Reference Medei, Nascimento, Pedrosa and de Carvalho2008; Biolo et al., Reference Biolo, Ribeiro and Clausell2010). The clinical characteristics of chronic Chagas cardiomyopathy (CCC) are associated with four sets of coexisting manifestations: (i) fatigue, dyspnoea, venous congestion systemic, anasarca and adynamia; (ii) heart fibrosis, sinus node dysfunction, atrial and ventricular arrhythmias; (iii) microcirculation disorders and angina; and (iv) dilatation of cardiac chambers, vascular aneurysm, thromboembolism and heart failure. Together, these manifestations are dangerous and closely correlated with CCC progression, morbidity and mortality rates in T. cruzi-infected patients (Biolo et al., Reference Biolo, Ribeiro and Clausell2010; Simões et al., Reference Simões, Romano, Schamidt, Martins and Marin-neto2018).
The specific treatment of Chagas disease is currently based on benznidazole and nifurtimox, which are nitroheterocyclic drugs more effective in acute infections, with cure rates ranging from 50 to 65% (Messenger et al., Reference Messenger, Miles and Bern2015; Nogueira et al., Reference Nogueira, Felizardo, Caldas, Gonçalves and Novaes2018). However, both drugs induce marked systemic toxicity and low effectivity in chronic infections (cure rates ranging from 0 to 30%), which are related with the occurrence of side-effects and the high rates of treatment discontinuation, ranging from 14.5 to 75% (Pérez-Molina et al., Reference Pérez-Molina, Sojo-Dorado, Norman, Monge-Maillo, Díaz-Menéndez, Albajar-Viñas and López-Vélez2013; Rassi et al., Reference Rassi, Marin-Neto and Rassi2017; Gulin et al., Reference Gulin, Bisio, Rocco, Altcheh, Solana and García-Bournissen2018). Despite these drugs have been used for 40 years, no additional effective treatments were developed in the last few decades (Rassi et al., Reference Rassi, Marin-Neto and Rassi2017; Nogueira et al., Reference Nogueira, Felizardo, Caldas, Gonçalves and Novaes2018). This limitation has been associated with parasite resistance to chemotherapy, which is partially mediated by molecular adaptive mechanisms, such as mutation in the nitroreductase TcNTR gene and upregulation of lipoamide dehydrogenase (Campos et al., Reference Campos, Leon, Taylor and Kelly2014; Santos et al., Reference Santos, Moreira, Baba, Volpe, Ruiz, Romanha and Murta2016). As the mechanisms associated with host cell parasitism and parasite survival are poorly understood, development of new effective treatments is still an important challenge. In this sense, there is a continuous effort to identify key mechanisms involved in T. cruzi infection (Romano et al., Reference Romano, Cueto, Casassa, Vanrell, Gottlieb and Colombo2012; Barrias et al., Reference Barrias, de Carvalho and Couza2013), which could be potentially relevant to discover new molecular targets useful in the rational design of new effective antitrypanosomal drugs.
There is evidence that molecules of the renin-angiotensin system (RAS), especially angiotensins, are involved in the pathogenesis of Chagas disease (Botoni et al., Reference Botoni, Poole-Wilson, Ribeiro, Okonko, Oliveira, Pinto, Teixeira, Teixeira, Reis, Dantas, Ferreira, Tavares and Rocha2007; Teixeira et al., Reference Teixeira, Hecht, Guimaro, Sousa and Nitz2011). Considering that molecules such as angiotensin II (Ang II) and angiotensin 1–7 (Ang 1–7) may alter parasitaemia and mortality (Leon et al., Reference Leon, Wang and Engman2003; de Paula Costa et al., Reference de Paula Costa, Silva, Pedrosa, Pinho, de Lima, Teixeira, Bahia and Talvani2010) in T. cruzi-infected mice, chemotherapy based on RAS-modulating drugs such as enalapril, captopril and losartan have been suggested as potentially useful in the treatment of Chagas disease (Botoni et al., Reference Botoni, Ribeiro, Marinho, Lima, Nunes and Rocha2013; Penitente et al., Reference Penitente, Leite, de Paula Costa, Shrestha, Horta, Natali, Neves and Talvani2015). As the immune response is the main line of defence against T. cruzi, the antiparasitic potential of RAS-modulating drugs appears to be dependent on the effect of RAS molecules on innate and acquired immune cells (de Paula Costa et al., Reference de Paula Costa, Silva, Pedrosa, Pinho, de Lima, Teixeira, Bahia and Talvani2010; Santos et al., Reference Santos, Mnezes, Villani, Magalhães, Scharfstein, Gollob and Dutra2010). This aspect becomes even more interesting and complex considering that immune cells are not only targets of RAS molecules, but also are direct effectors of this system (Hoch et al., Reference Hoch, Guzik, Chen, Deans, Maalouf, Gratze, Weyand and Harrison2009). In this sense, dendritic and natural-killer (NK) cells, macrophages, neutrophils, eosinophils, mast cells and T lymphocytes (CD4+ and CD8+) express several RAS molecules, such as renin, angiotensinogen, angiotensin-converting enzyme (ACE), Ang II and angiotensin receptors (Reilly et al., Reference Reilly, Tewksbury, Schechter and Travis1982; Costerousse et al., Reference Costerousse, Allegrini, Lopez and Alhenc-Gelas1993; Resende and Mill, Reference Resende and Mill2002; Jurewicz et al., Reference Jurewicz, McDermott, Sechler, Tinckam, Takakura, Carpenter, Milford and Abdi2007). Although the immune response to RAS stimulation is not fully understood, by exerts chemoattractant effects and modulates the survival, production of cytokines and reactive species in leucocytes (Okamura et al., Reference Okamura, Rakugi, Ohishi, Yanagitani, Takiuchi, Moriguchi, Fennessy, Higaki and Ogihara1999; Hoch et al., Reference Hoch, Guzik, Chen, Deans, Maalouf, Gratze, Weyand and Harrison2009; Barroso et al., Reference Barroso, Magalhaes, Galvão, Reis, Souza, Sousa, Santos, Campagnole-Santos, Pinho and Teixeira2017; Magalhães et al., Reference Magalhães, Barroso, Reis, Rodrigues-Machado, Gregório, Motta-Santos, Oliveira, Perez, Barcelos, Teixeira, Santos, Pinho and Campagnole-Santos2018), angiotensins activate a set of events whose potential implications on the resistance and/or susceptibility of T. cruzi-infected hosts remains overlooked.
Currently, the available evidence on the relation between T. cruzi infection and RAS is rather fragmented and a comprehensive analysis on the impact of RAS molecules and their modulatory drugs on the evolution of T. cruzi infection has never been developed. In this context, it remains poorly understood if and to what extent RAS-modulating drugs exert direct trypanocidal effects or if potential biological effects are secondary to modulation of the host immune system. Thus, we used a systematic review framework to integrate the preclinical (in vitro and in vivo) and clinical evidence to investigate the relevance of angiotensin-modulating drugs in the treatment of T. cruzi infections. In addition to evaluating the potential antiparasitic and immunomodulatory effects of RAS-modulating drugs, the methodological quality of the studies reviewed and the risk of bias associated with the current evidence were also critically analysed.
Methods
Structured search strategy
The review protocol was based on the PRISMA guideline (Preferred Reporting Items for Systematic Reviews and Meta-analyses) (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009). To retrieve all relevant research records, two search strategies were adopted: (i) a primary search in comprehensive electronic databases, and (ii) a secondary search from the reference list of all relevant studies identified in the primary search (Pereira et al., Reference Pereira, Greco, Moreira, Chagas, Caldas, Gonçalves and Novaes2017). The databases PubMed/Medline, Web of Science and Scopus were used in the primary search. The filters developed for the primary search were structured in two levels: (i) disease model: American trypanosomiasis (Chagas disease) and (ii) biological target/pharmacological strategy: RAS-modulating drugs. The search filter for the PubMed/Medline was based on standardized descriptors obtained from the hierarchical Thesaurus MeSH (Medical Subject Headings, http://www.ncbi.nlm.nih.gov/mesh). In PubMed/Medline, the commands MeSH and TIAB (title and abstract) were combined for the retrieval of indexed papers and those citations in the indexing process (epub ahead of print). The same research descriptors were structured according to the specific search algorithms required in Web of Science (TS = descriptor) and Scopus [TITLE-ABS-KEY(descriptor)] databases (Pereira et al., Reference Pereira, Greco, Moreira, Chagas, Caldas, Gonçalves and Novaes2017).
Specific biological systems (non-human animals vs humans) were intentionally omitted from our search filters to enhance the search sensitivity [designed to find as many relevant papers as possible, often at the cost of much ‘noise’ (much time consumed by screening numerous irrelevant studies)] rather than specificity (designed to find a small set of highly relevant papers, with the risk of omitting numerous relevant papers) (Jenkins, Reference Jenkins2004). No chronological or language limits were applied in the primary search. All original full-text studies published up to December 2018 were included in the systematic review. The search strategy is detailed in the Supplementary file (Table S1).
Screening for primary studies
Publication data (journal, volume, number, page and year), title and abstract of all studies identified in the primary and secondary searches were compared and duplicated registers were removed. The title and abstract of all studies were screened and those that were not related to the subject of investigation were excluded. All potentially relevant studies were recovered in full-text and submitted to an eligibility analysis, in which the adequacy to well-defined inclusion and exclusion criteria was analysed. The inclusion criteria were: Preclinical and clinical original studies evaluating the impact of RAS-modulating drugs on T. cruzi-infection. The exclusion criteria were: (i) papers without full-text available, (ii) secondary studies (i.e. literature reviews, comments, letters to the editor and editorials), (iii) grey literature (studies not peer-reviewed or formally published in indexed journals) and (iv) studies with multiple interventions in which the effect of RAS-modulating drugs cannot be isolated. In the eligibility phase, the researchers independently analysed all studies and disagreements were solved by consensus. The reference list of all relevant studies identified in the primary search was manually screened considering a potential identification of additional studies (Pereira et al., Reference Pereira, Greco, Moreira, Chagas, Caldas, Gonçalves and Novaes2017). The flowchart indicating the process of study selection is presented in Fig. 1.

Fig. 1. Flowchart detailing selection of studies included in systematic review. Based on PRISMA statement ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (http://www.prisma-statement.org).
Studies categorization and data extraction
Data extraction was based on basic methodological requirements that should be reported to the proper interpretation of preclinical and clinical evidence. Therefore, the characteristics of all publication such as authors, country in which the study was developed and year of publication were extracted from preclinical and clinical studies. In preclinical studies, additional data were extracted, such as: (i) characteristics of the animal model: species, lineage, sex and age; (ii) disease model: parasitic strain, inoculum (size and route of administration) and duration of infection; (iii) primary outcomes: parasitaemia, parasitic load, mortality; and (iv) secondary outcomes: histopathological data, immunological and neuroendocrine markers. In clinical studies, specific data were extracted, such as: (i) characteristics of the population: country, age and sex; (ii) characteristics of the disease: diagnostic method, time of infection, stage of disease (acute or chronic), form of disease (cardiac, digestive or undetermined); (iii) primary outcomes: mortality, cardiac function; and (iv) secondary outcomes: neuroendocrine markers.
Analysis of reporting quality and research bias in preclinical and clinical studies
The comprehensiveness of the scientific report in preclinical studies was analysed by using an analytical instrument described by Pereira et al. (Reference Pereira, Greco, Moreira, Chagas, Caldas, Gonçalves and Novaes2017). This tool provides a complete screening of all sections of the paper (abstract to acknowledgements and funding) and was developed from basic requirements recommended by the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Mcgrath and Lilley, Reference McGrath and Lilley2015), additionally considering essential elements that should be reported in studies on human trypanosomiasis. The overall mean adherence and the individual quality criteria were expressed as relative and absolute values (Pereira et al., Reference Pereira, Greco, Moreira, Chagas, Caldas, Gonçalves and Novaes2017).
The risk of bias in preclinical studies was evaluated by the SYRCLE's risk of bias tool for animal studies (Hooijmans et al., Reference Hooijmans, Rovers, de Vries, Leenaars, Ritskes-Hoitinga and Langendam2014). This instrument is based on the Cochrane Risk of Bias tool and is adjusted for specific aspects of bias playing a relevant impact in animal intervention studies. The SYRCLE's tool is structured in ten topics related to multiple sources of bias such as: (i) selection, (ii) performance, (iii) detection, (iv) attrition, (v) reporting and (vi) additional sources of bias not covered by other domains.
The Downs and Black Measuring Quality was used to evaluate the reporting quality and potential risk of bias in clinical studies based on case-series (Downs and Black, Reference Downs and Black1998; Nogueira et al., Reference Nogueira, Felizardo, Caldas, Gonçalves and Novaes2018). The scale is based on 27 questions structured in five categories: (i) reporting quality, (ii) external validity, (iii) bias, (iv) confounding and (v) statistical power. This scale presented high test-retest reliability (r = 0.88) and internal consistency (KR20 formula = 0.89). Due to high ambiguity, statistical power (question 27) was omitted as recommended (Simic et al., Reference Simic, Hinman, Wrigley, Bennell and Hunt2011).
Results
In vitro models
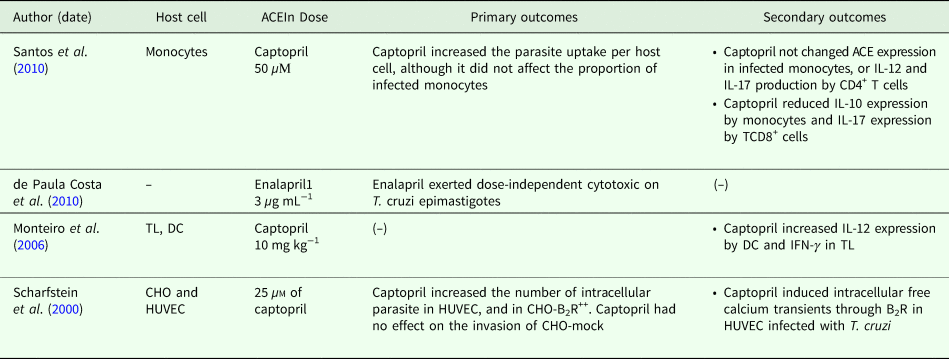
In in vitro assays, heterogeneous cell lineages (i.e. monocytes, dendritic cells (DC), Chinese hamster ovary cells, human primary umbilical vein endothelial cells and T lymphocytes) were used. Although T. cruzi Y strain has been applied in most studies, the size of the parasite inoculum and the time of infection were heterogeneous (3 h to 10 days). Captopril was the drug mainly used, and enalapril was applied in only one study. According to the cell type used, the doses of captopril ranged from 25 to 50 µm, while enalapril was administered between 1 and 3 µm (Table 1).
Table 1. General characteristics of all in vitro models of Trypanosoma cruzi infection exposed to angiotensin-converting enzyme inhibitors (ACEIn)

(–), Data not reported or investigated; CHO, Chinese hamster ovary cells; HUVEC, human primary umbilical vein endothelial cells; TL, T lymphocytes; FCS, fetal calf serum; DC, dendritic cells; BSA, bovine serum albumin.
a Dose administered in mice and TL and DC removed later.
In general, the results obtained from in vitro systems indicated that captopril increased parasite uptake/parasitic load by host cells (Scharfstein et al., Reference Scharfstein, Schmitz, Morandi, Capella, Lima, Morrot, Juliano and Müller-Esterl2000; Santos et al., Reference Santos, Mnezes, Villani, Magalhães, Scharfstein, Gollob and Dutra2010). Only Santos et al. (Reference Santos, Mnezes, Villani, Magalhães, Scharfstein, Gollob and Dutra2010) investigated ACE expression in host cells, which together with IL-12 and IL-17 was unchanged by captopril in infected monocytes and CD4+ T cells, respectively. Conversely, IL-10 and IL-17 expression in CD8+ T cells was reduced by captopril. Only Monteiro et al. (Reference Monteiro, Schmitz, Svensjo, Gazzinelli, Almeida, Todorov, de Arruda, Torrecilhas, Pesquero, Morrot, Bouskela, Bonomo, Lima, Müller-Esterl and Scharfstein2006) indicated increased IL-12 expression in infected DC and IFN-γ by T lymphocytes treated with captopril. Cytotoxic activity of enalapril on T. cruzi epimastigotes was reported in only one study (de Paula Costa et al., Reference de Paula Costa, Silva, Pedrosa, Pinho, de Lima, Teixeira, Bahia and Talvani2010). No in vitro studies included captopril in direct cytotoxic assays on T. cruzi (Table 2).
Table 2. Impact of angiotensin-converting enzyme inhibitors (ACEIn) on in vitro models of Trypanosoma cruzi infection

(–), Data not reported or investigated; TL, T lymphocytes; DC, dendritic cell; ACEIn, angiotensin-converting enzyme inhibitors; CHO-B2R++, Chinese hamster ovary cells with overexpressing B2 type of bradykinin receptor; HUVEC, human primary umbilical vein endothelial cells; CHO-mock, Chinese hamster ovary cells without overexpression of receptors.
In vivo animal models
Only five studies evaluating the impact of RAS-modulating drugs on animal models of T. cruzi infection were identified in the primary and secondary searches. All studies used isogenic animals (i.e. C57BL/6, BALB/c and A/J), especially males (n = 4, 80%) ranging from 4 to 10 weeks. The T. cruzi strains used to induce the infection were quite heterogeneous and the inoculum size varied from 50 to 10 000 parasites per animal, without a direct relation with body mass. The infection time ranged from 25 to 120 days. Only enalapril, captopril and losartan were used, with doses ranging from 15 to 25 mg kg−1 day−1 (Table 3).
Table 3. General characteristics of all preclinical animal models of Trypanosoma cruzi infection treated with angiotensin-converting enzyme inhibitors (ACEIn)

F, female; M, male.
a Number of parasites inoculated in each animal.
Most studies indicated that RAS-modulating drugs were effective in reducing parasitaemia (Chumbinho et al., Reference Chumbinho, Pizzini, Oliveira, Batista and Oliveira2012; Leite et al., Reference Leite, Paula Costa, Lopes, Mota, Vieira and Talvani2017) and mortality (de Paula Costa et al., Reference de Paula Costa, Silva, Pedrosa, Pinho, de Lima, Teixeira, Bahia and Talvani2010; Chumbinho et al., Reference Chumbinho, Pizzini, Oliveira, Batista and Oliveira2012; Penitente et al., Reference Penitente, Leite, de Paula Costa, Shrestha, Horta, Natali, Neves and Talvani2015). Conversely, parasitaemia was unchanged by enalapril (Penitente et al., Reference Penitente, Leite, de Paula Costa, Shrestha, Horta, Natali, Neves and Talvani2015), while high doses of captopril (75 mg L−1) increased the animals’ mortality with no impact on heart parasitism (Leon et al., Reference Leon, Wang and Engman2003). In general, RAS-modulating drugs increased IL-10 plasma levels (Penitente et al., Reference Penitente, Leite, de Paula Costa, Shrestha, Horta, Natali, Neves and Talvani2015; Leite et al., Reference Leite, Paula Costa, Lopes, Mota, Vieira and Talvani2017) and reduced TNF-α, IFN-γ, CCL2 circulating levels and myocarditis severity (de Paula Costa et al., Reference de Paula Costa, Silva, Pedrosa, Pinho, de Lima, Teixeira, Bahia and Talvani2010; Penitente et al., Reference Penitente, Leite, de Paula Costa, Shrestha, Horta, Natali, Neves and Talvani2015; Leite et al., Reference Leite, Paula Costa, Lopes, Mota, Vieira and Talvani2017). Only Chumbinho et al. (Reference Chumbinho, Pizzini, Oliveira, Batista and Oliveira2012) analysed biochemical markers of organ toxicity (i.e. serum creatinine and urea), indicating that losartan increased kidney injury in T. cruzi-infected mice (Table 4).
Table 4. Primary and secondary outcomes in preclinical animal models of Trypanosoma cruzi infection treated with angiotensin-converting enzyme inhibitors (ACEIn)

ACEIn, angiotensin-converting enzyme inhibitors; CK, creatine kinase; CK-MB, creatine kinase isoenzyme MB; DTH, delayed-type hypersensitivity; BUN, blood urea nitrogen; AT1R, angiotensin II receptor type 1.
Clinical studies
Only four clinical studies were identified in our primary and secondary search. No randomized controlled trials were identified. Clinical studies evaluating the effect of RAS-modulating drugs were exclusively based on case series. In all studies identified, patients between 23 and 64 years old of both sexes were investigated. All studies reported the inclusion of infected patients, but only two studies (Roberti et al., Reference Roberti, Martinez, Andrade, Araujo, Brito, Portugal and Horowitz1992; Botoni et al., Reference Botoni, Poole-Wilson, Ribeiro, Okonko, Oliveira, Pinto, Teixeira, Teixeira, Reis, Dantas, Ferreira, Tavares and Rocha2007) reported specific methods for the diagnosis of T. cruzi infection (serology and radioimmunoassay). All studies determined the severity of CCC according to the classification established by the New York Heart Association, ranging from I (no symptoms/no limitation) to IV (symptoms at rest/severe limitations). As in studies with the animal model, captopril, enalapril and losartan were exclusively administered in humans. Doses of 5–150 mg day−1 of these drugs were administered in a protocol of treatment ranging from 4 to 120 days (Table 5).
Table 5. General characteristics of all clinical studies with Chagasic patients treated with angiotensin-converting enzyme inhibitors (ACEIn)

ECHO, echocardiography; ECG, electrocardiography; ChD, Chagas disease; LV, left ventricular; LVDD, LV end-diastolic diameter.
a Classification established according to New York Heart Association.
As indicated in Table 6, no parasitological outcome was investigated in all clinical studies identified. However, all studies reported cardiovascular parameters as primary outcomes. Although therapeutic outcomes have been quite heterogeneous, the treatments exerted no or limited impacts on parameters such as heart rate, systolic and diastolic blood pressure, left ventricular shortening and ejection fraction, atrial and ventricular dimensions and the occurrence of non-sustained ventricular tachycardia. Only three studies analysed plasma markers and only one study reported immunological data. Enalapril and captopril reduced circulating brain natriuretic peptide (BNP) and RANTES circulating levels (Botoni et al., Reference Botoni, Poole-Wilson, Ribeiro, Okonko, Oliveira, Pinto, Teixeira, Teixeira, Reis, Dantas, Ferreira, Tavares and Rocha2007). Captopril also reduced urinary norepinephrine and increased renin plasma levels (Roberti et al., Reference Roberti, Martinez, Andrade, Araujo, Brito, Portugal and Horowitz1992).
Table 6. Primary and secondary outcomes in clinical studies with Chagasic patients treated with angiotensin-converting enzyme inhibitors (ACEIn)

(–), Data not reported or investigated; M, male; F, female; Tei index, myocardial performance index; LAD, left atrial diameter; NSVT, non-sustained ventricular tachycardia; SDBP, systolic and diastolic blood pressure; FS, Framingham score for heart failure; CI, cardiothoracic Index; LVSD, left ventricular systolic dimensions; LVDD, left ventricular end-diastolic dimensions; LVFS, left ventricular fractional shortening; LVEF, left ventricular ejection fraction; RPAD, right pulmonary artery diameter; RBC, red blood cells; WBC, white blood cells.
Reporting quality and risk of bias in preclinical and clinical studies
From the analysis of reporting quality, no preclinical animal studies met all the quality criteria analysed, and about 65% of these criteria were completed. The lower adherence to the criteria of reporting quality (60%) was identified for the study developed by Leite et al. (Reference Leite, Paula Costa, Lopes, Mota, Vieira and Talvani2017) (Fig. 2). The main criteria under-reported were related to animals housing and husbandry, strategy of animal allocation in experimental groups, sample size calculation, baseline data, experimental procedures and adverse events, such as indicated in Table S2.

Fig. 2. Reporting quality in preclinical studies investigating the effect of angiotensin-converting enzyme inhibitors on in vivo models of T. cruzi infection. Based on the reporting quality tool applied to animal models of systemic protozooses (Parasitology 144:1275–1287, 2017).
Based on SYRCLE's tool, the risk of bias in preclinical animal studies was variable among clinical studies, with the most frequent bias elements being: (i) selection bias 1, (ii) performance bias 2 and (iii) detection bias 2. In general, the lowest risk of bias was associated with: (i) reporting bias and (ii) selection bias 2. Considering the specificities of study design, under-reported or incomplete information and the relevance of the information as a criteria of methodological quality; the risk of bias attributed to domains, such as attrition bias, detection bias 1, performance bias 1 and selection bias 3, was unclear (Table S3 and Fig. 3).

Fig. 3. Risk of bias in preclinical studies investigating the effect of angiotensin-converting enzyme inhibitors on in vivo preclinical models of T. cruzi infection. Based on SYRCLE's risk of bias tool for animal studies (BMC Medical Research Methodology 14:43, 2014).
All clinical studies were also evaluated for the risk of bias. No study met all quality criteria, with an average score of 72%. Two studies (Szajnbok et al., Reference Szajnbok, Barretto, Mady, Parga Filho, Gruppi, Alfieri, da Luz and Pileggi1993; Khoury et al., Reference Khoury, Davila, Bellabarba, Donis, Torres, Lemorvan, Hernandez and Bishop1996) reached a below-average score, which was related to a more limited scientific report (Fig. 4). The main limitations attributed to the potential risk of bias were related with a poor description of clinical outcomes, absence of control groups, adjustment of confounding factors, representativeness of the sample investigated, absence of patients and evaluators blinding and strategies of randomization (Table S4).

Fig. 4. Risk of bias in clinical studies investigating the effect of angiotensin-converting enzyme inhibitors on Chagasic patients. Based on Downs and Black Measuring Quality in randomized and non-randomized clinical assays (Journal of Epidemiology and Community Health 52:377–384, 1998).
Discussion
In vitro models
From a comprehensive search, a limited number of in vitro and in vivo studies investigating the effect of RAS-modulating drugs on T. cruzi infection were identified. Although T. cruzi Y strain has been used to induce infection in most in vitro studies, the host cells, culture medium, inoculum size, time of infection and drug dose were quite heterogeneous. As these divergences limit the comparison between these studies, a rational and unbiased systematic approach requires that the relationship between experimental models and biological outcomes be individually analysed.
Even though any nucleated mammal cells can be parasitized by T. cruzi (Brener, Reference Brener1973; Fernandes and Andrews, Reference Fernandes and Andrews2012), the in vitro preclinical modelling should ideally consider if the cell lineages used are relevant for the pathophysiology of Chagas’ disease. Thus, the application of Chinese hamster ovary cells and human primary umbilical vein endothelial cells impairs the construct validity established by Scharfstein et al. (Reference Scharfstein, Schmitz, Morandi, Capella, Lima, Morrot, Juliano and Müller-Esterl2000), which presented a greater distance from explanatory models objectively oriented to the understanding of primary targets or effector mechanisms associated with the development of T. cruzi infections. However, by using monocytes, DC and T lymphocytes as host cells, Santos et al. (Reference Santos, Mnezes, Villani, Magalhães, Scharfstein, Gollob and Dutra2010) and Monteiro et al. (Reference Monteiro, Schmitz, Svensjo, Gazzinelli, Almeida, Todorov, de Arruda, Torrecilhas, Pesquero, Morrot, Bouskela, Bonomo, Lima, Müller-Esterl and Scharfstein2006) presented a well-oriented in vitro model based on the interaction of T. cruzi with effector cells directly involved in the immunological control of parasite survival and replication (Ferraz et al., Reference Ferraz, Gazzinelli, Alves, Urbina and Romanha2009; Da Costa et al., Reference da Costa, Silva, Mendes, Carvalho-Costa, Batista, Lages-Silva, Rodrigues, Oliveira and Ramirez2014; Cardillo et al., Reference Cardillo, de Pinho, Antas and Mengel2015). DC play a central role as antigen-presenting cells in T. cruzi infections, amplifying the antiparasitic immune response from the activation of acquired immune cells, especially lymphocytes (Da Costa et al., Reference da Costa, Silva, Mendes, Carvalho-Costa, Batista, Lages-Silva, Rodrigues, Oliveira and Ramirez2014; Qu et al., Reference Qu, Brinck-Jensen, Zang and Chen2014). While the fast response of DC against T. cruzi is related to a broad repertoire of membrane receptors and costimulatory molecules (i.e. CD40, CD80, MHC-II, PDL1, CCR5 and CCR7), its innate effectivity is directed by an intense production of immunomodulator molecules, especially TNF-α, IFN-γ, IL-12, IL-22, IL-6, IL-10 and CCL2 (Cunha-Neto and Chevillard, Reference Cunha-Neto and Chevillard2014; Cardillo et al., Reference Cardillo, de Pinho, Antas and Mengel2015).
Together with DC, classical monocytes (CD14+CD16− in humans and Ly6C+CD43− in mice) exhibit a marked anti-T. cruzi potential, which is induced by a potent Th1 immunological phenotype (Melo and Machado, Reference Melo and Machado2001; Cardillo et al., Reference Cardillo, de Pinho, Antas and Mengel2015; Cabral-Piccin et al., Reference Cabral-Piccin, Guillermo, Vellozo, Filardy, Pereira-Marques, Rigoni, Pereira-Manfro, Dos Reis and Lopes2016). Upon completion of monocytes maturation in activated macrophages, high chemokine receptor 2 (CCR2) expression, marked migratory and pro-inflammatory potential are detected (Pérez-Mazliah et al., Reference Pérez-Mazliah, Eiro, Álvarez, Lococo, Bertocchi, César, Natale, Alberda, Viotti and Laucella2018). These cells are directly involved in parasitic control, mediating T. cruzi death from its phagocytic activity and production of TNF-α, myeloperoxidase, reactive oxygen (ROS: O2−, OH−, HClO and H2O2,) and nitrogen (RNS: NO and ONOO−) species, whose production is coupled with intense activation of the endosomal–lysosomal system and oxidative burst (Melo and Machado, Reference Melo and Machado2001; Goes et al., Reference Goes, Rocha, Diniz, Aguiar, Machado and Vieira2016; Paiva et al., Reference Paiva, Medei and Bozza2018). Due to its synergistic roles, DC, monocytes and macrophages exert fundamental relevance against T. cruzi, forming an early barrier to infection that orchestrates the assembly and subsequent steps of the adaptive immunity against T. cruzi (Da Costa et al., Reference da Costa, Silva, Mendes, Carvalho-Costa, Batista, Lages-Silva, Rodrigues, Oliveira and Ramirez2014; Poveda et al., Reference Poveda, Fresno, Girones, Martins-Filho, Ramírez, Santi-Rocca, Marin-Neto, Morillo, Rosas and Guhl2014; Cardillo et al., Reference Cardillo, de Pinho, Antas and Mengel2015). In addition to innate immunity cells, CD4+ and CD8+ T lymphocytes reinforce parasitaemia control in acute infections (Ferraz et al., Reference Ferraz, Gazzinelli, Alves, Urbina and Romanha2009), contributing to the polarization of macrophages from Th1 cytokines (i.e. IFN-γ and TNF-α) (Soares et al., Reference Soares, Silva-Mota, Lima, Bellintani, Carvalho and Santos2001; Miranda et al., Reference Miranda, Melo, Almeida, Marinho, Oliveira and Gomes2017). While CD4+ T cells exert immunomodulatory activities, CD8+ T lymphocytes exhibit direct cytotoxic activity that plays a central role against intracellular T. cruzi forms (amastigotes) (Cabral-Piccin et al., Reference Cabral-Piccin, Guillermo, Vellozo, Filardy, Pereira-Marques, Rigoni, Pereira-Manfro, Dos Reis and Lopes2016). The absence of these cells determines inefficient parasite control, more severe cell parasitism and organ damage, which is often associated with infections with rapid progression and high lethality (Martin and Tarleton, Reference Martin and Tarleton2004).
Although the effect of RAS-modulating drugs on T. cruzi infection remains poorly explored, the identification of metabolic pathways linked to RAS in leucocytes (Figs 5 and 6) provides an objective rational basis that supports the use of these drugs as immunomodulatory agents in infectious diseases (Costerousse et al., Reference Costerousse, Allegrini, Lopez and Alhenc-Gelas1993; Jurewicz et al., Reference Jurewicz, McDermott, Sechler, Tinckam, Takakura, Carpenter, Milford and Abdi2007; Hoch et al., Reference Hoch, Guzik, Chen, Deans, Maalouf, Gratze, Weyand and Harrison2009). In this sense, DC, NK cells, CD4+ T and CD8+ T lymphocytes have high ACE activity and express renin, angiotensinogen AT1 and AT2 angiotensin receptors (Costerousse et al., Reference Costerousse, Allegrini, Lopez and Alhenc-Gelas1993; Nataraj et al., Reference Nataraj, Oliverio, Mannon, Mannon, Audoly, Amuchastegui, Ruiz, Smithies and Coffman1999; Jurewicz et al., Reference Jurewicz, McDermott, Sechler, Tinckam, Takakura, Carpenter, Milford and Abdi2007). In CD8+ T cells, ACE also participates in MHC-I processing from endoplasmic reticulum, regulating the specificity of the immune response (Shen et al., Reference Shen, Billet, Lin, Okwan-Duodu, Chen, Lukacher and Bernstein2011). Neutrophils and mast cells exhibit an even more complex endogenous RAS, converting Ang I to Ang II by an ACE-independent route, which is mediated by serine protease cathepsin G in neutrophils and chymase in mast cells (Reilly et al., Reference Reilly, Tewksbury, Schechter and Travis1982; Resende and Mill, Reference Resende and Mill2002). In addition, the presence of intracellular AT1 receptors indicates an intracrine mechanism of Ang II-induced leucocytes activation (Hoch et al., Reference Hoch, Guzik, Chen, Deans, Maalouf, Gratze, Weyand and Harrison2009). As specific responses of RAS activation, Ang II/AT1 stimulation triggers IFN-γ, RANTES and IL-4 secretion by CD4+ T cells (Jurewicz et al., Reference Jurewicz, McDermott, Sechler, Tinckam, Takakura, Carpenter, Milford and Abdi2007), as well as intense IL-1β, IL-6, NF-κB, lipoxygenase (Kranzhofer et al., Reference Kranzhofer, Browatzki, Schmidt and Kübler1999; Okamura et al., Reference Okamura, Rakugi, Ohishi, Yanagitani, Takiuchi, Moriguchi, Fennessy, Higaki and Ogihara1999; Silva et al., Reference Silva, Cunha, Horta, Silva, Gutierrez, Silva and Zamboni2013) and TNF-α and ROS production (Hoch et al., Reference Hoch, Guzik, Chen, Deans, Maalouf, Gratze, Weyand and Harrison2009) in macrophages. Furthermore, Ang II/AT1 stimulation also activates IFN-γ production in DC and NK lymphocytes (Jurewicz et al., Reference Jurewicz, McDermott, Sechler, Tinckam, Takakura, Carpenter, Milford and Abdi2007; Silva et al., Reference Silva, Cunha, Horta, Silva, Gutierrez, Silva and Zamboni2013).

Fig. 5. Synthesis pathways and effects of angiotensin II on immune cells. Ang, angiotensin; Ang I, angiotensin I; Ang II, angiotensin II; ACE, angiotensin-converting enzyme; TNF-α, tumour necrosis factor α, NF-κB, nuclear factor κB; Rn, renin; Lox, lipoxygenase. References to the described routes can be found in file S1.

Fig. 6. Synthesis pathways and effects of angiotensin 1–7 on immune cells. Ang, angiotensin; Ang I, angiotensin I; Ang II, angiotensin II; Ang 1–7, angiotensin 1–7; Ang 1–9, angiotensin 1–9; ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme type 2; NEP, neutral endopeptidase; PEP, prolylcarboxypeptidase; TNF-α, tumour necrosis factor α; MCP-1, monocyte chemoattractant protein 1; NF-κB, nuclear factor κB. References to the described routes can be found in file S1.
Although the effect of Ang 1–7 on leucocytes is poorly understood, the scarce evidence indicates an opposite effect to Ang II stimulation (Fig. 6). Thus, by activating MAS receptor, Ang 1–7 downregulates NF-κB-mediated cell signalling in DC, neutrophils and eosinophils (Silva et al., Reference Silva, Cunha, Horta, Silva, Gutierrez, Silva and Zamboni2013; Barroso et al., Reference Barroso, Magalhaes, Galvão, Reis, Souza, Sousa, Santos, Campagnole-Santos, Pinho and Teixeira2017; Magalhães et al., Reference Magalhães, Barroso, Reis, Rodrigues-Machado, Gregório, Motta-Santos, Oliveira, Perez, Barcelos, Teixeira, Santos, Pinho and Campagnole-Santos2018), a central pathway involved in the activation of genes encoding pro-inflammatory cytokines (Lawrence, Reference Lawrence2009). In addition, Ang 1–7 acts as an anti-inflammatory molecule with potent effect in attenuates TNF-α, IL-6 and MCP-1 expression in macrophages (Guo et al., Reference Guo, Li, Wu, Xie and Cui2008; Thomas et al., Reference Thomas, Pickering, Tsorotes, Koitka, Sheehy, Bernardi, Toffoli, Nguyen-Huu, Head, Fu, Chin-Dusting, Cooper and Tikellis2010; Souza and Costa-Neto, Reference Souza and Costa-Neto2012), also inducing neutrophils and eosinophils apoptosis (Barroso et al., Reference Barroso, Magalhaes, Galvão, Reis, Souza, Sousa, Santos, Campagnole-Santos, Pinho and Teixeira2017; Magalhães et al., Reference Magalhães, Barroso, Reis, Rodrigues-Machado, Gregório, Motta-Santos, Oliveira, Perez, Barcelos, Teixeira, Santos, Pinho and Campagnole-Santos2018).
After establishing that the RAS system can modulate important antiparasitic mechanisms in leucocytes, it is important to consider that the alignment between host cell and parasite strain is also relevant in in vitro assays of T. cruzi infection (Melo and Brener, Reference Melo and Brener1978). Thus, by using a myotropic strain (Dm28c) to infect host cells with epithelial origin, Scharfstein et al. (Reference Scharfstein, Schmitz, Morandi, Capella, Lima, Morrot, Juliano and Müller-Esterl2000) dissociates its experimental model from the natural parasite behaviour (Contreras et al., Reference Contreras, Araujo-Jorge, Bonaldo, Thomaz, Barbosa, Meirelles, Mde and Goldenberg1988). Conversely, Monteiro et al. (Reference Monteiro, Schmitz, Svensjo, Gazzinelli, Almeida, Todorov, de Arruda, Torrecilhas, Pesquero, Morrot, Bouskela, Bonomo, Lima, Müller-Esterl and Scharfstein2006) and Santos et al. (Reference Santos, Mnezes, Villani, Magalhães, Scharfstein, Gollob and Dutra2010) aligned host cell lineage with the reticulotropic/macrophagotropic characteristic of T. cruzi Y strain (Sousa and Alencar, Reference Sousa and Alencar1984; Santiago et al., Reference Santiago, Feng, Bafica, Roffe, Arantes, Cheever, Taylor, Vierira, Aliberti, Gazzinelli and Sher2005), indicating an important element of construct and internal validity of the experimental model used. However, the inoculum size and time of infection were quite heterogeneous in all in vitro models, an important factor that makes these models even more divergent, since parasite load and cellular response to infection are profoundly influenced by the inoculum and time of contact between T. cruzi and host cells (Vazques et al., Reference Vazques, Vazquez, Miguel, Rodrigues, Mendes, Oliveira and Chica2015).
Only captopril and enalapril were tested in vitro as ACEIn, and only de Paula Costa et al. (Reference de Paula Costa, Silva, Pedrosa, Pinho, de Lima, Teixeira, Bahia and Talvani2010) analysed direct cytotoxic effect of enalapril on isolated T. cruzi epimastigotes. Although enalapril exerted a dose-dependent antiparasitic effect, no mechanistic approach was presented in this study. Despite experimental heterogeneity, the main in vitro findings indicated that ACE inhibitors might modulate host–pathogen interactions. In this sense, captopril increased parasite uptake per host cells (Scharfstein et al., Reference Scharfstein, Schmitz, Morandi, Capella, Lima, Morrot, Juliano and Müller-Esterl2000; Santos et al., Reference Santos, Mnezes, Villani, Magalhães, Scharfstein, Gollob and Dutra2010), IL-12 expression by infected DC and IFN-γ by T lymphocytes (Monteiro et al., Reference Monteiro, Schmitz, Svensjo, Gazzinelli, Almeida, Todorov, de Arruda, Torrecilhas, Pesquero, Morrot, Bouskela, Bonomo, Lima, Müller-Esterl and Scharfstein2006). In addition, IL-10 and IL-17 expression in CD8+ T cells was also reduced by this drug (Santos et al., Reference Santos, Mnezes, Villani, Magalhães, Scharfstein, Gollob and Dutra2010), a response potentially independent of ACE expression in host cells (Santos et al., Reference Santos, Mnezes, Villani, Magalhães, Scharfstein, Gollob and Dutra2010). Although no further study on ACEIn and T. cruzi is known, the immunomodulatory properties of captopril were indicated from the suppression of TNF-α and IL-1α synthesis in mononuclear cells challenged with LPS (Schindler et al., Reference Schindler, Dinarello and Koch1995; Peeters et al., Reference Peeters, Netea, Kullberg, Thien and Meer1998). In addition, enalapril and losartan were effective in attenuating the inflammation in mice infected by dengue virus, with reduced IL-1β production by infected peritoneal macrophages (Hernández-Fonseca et al., Reference Hernández-Fonseca, Duran, Valero and Mosquera2015). By enhanced IL-4 and decrease in IFN-γ, TNF-α and IL-17 production, losartan was also beneficial in attenuating chronic viral myocarditis, tissue necrosis and mortality in coxsackievirus B3-infected mice, findings attributed to an improved balance between Th1, Th2 and Th17 phenotypes (Zhang et al., Reference Zhang, Li, Xia, Zhang, Zhong, Wu, Miao and Zhou2013).
In vivo animal models
In preclinical animal models, young isogenic male mice were consistently used to induce T. cruzi infection. Female mice were used in only one study. Although the current evidence does not indicate a relevant effect of sex hormones on T. cruzi infection (Soares et al., Reference Soares, Soares, Moraes, Batista, Kwabara, Sousa, Moreira, Gomes and Araújo2012, Felizardo et al., Reference Felizardo, Marques, Caldas, Gonçalves and Novaes2018), host lineage and age exerts a direct impact on the balance between resistance and susceptibility to T. cruzi infections (Pereira et al., Reference Pereira, Greco, Moreira, Chagas, Caldas, Gonçalves and Novaes2017; Felizardo et al., Reference Felizardo, Marques, Caldas, Gonçalves and Novaes2018). In this sense, young adult animals are highly susceptible to T. cruzi infection than old animals (Felizardo et al., Reference Felizardo, Marques, Caldas, Gonçalves and Novaes2018). In addition, the homogenous genetic background of isogenic animals improves the experimental control, providing a lower immunological variability than outbred animals (Trischmann et al., Reference Trischmann, Tanowitz, Wittner and Bloom1978; Vorraro et al., Reference Vorraro, Cabrera, Ribeiro, Jensen, Franco, Ibãnez and Starobinas2014). Furthermore, most studies used T. cruzi-resistant C57BL/6 mice (de Paula Costa et al., Reference de Paula Costa, Silva, Pedrosa, Pinho, de Lima, Teixeira, Bahia and Talvani2010; Penitente et al., Reference Penitente, Leite, de Paula Costa, Shrestha, Horta, Natali, Neves and Talvani2015; Leite et al., Reference Leite, Paula Costa, Lopes, Mota, Vieira and Talvani2017), while only two cases reported susceptible BALB/c (Chumbinho et al., Reference Chumbinho, Pizzini, Oliveira, Batista and Oliveira2012) or A/J (Leon et al., Reference Leon, Wang and Engman2003) mice. According to the differential parasitic susceptibility, the choice of these lineages (Silva et al., Reference Silva, Cunha, Horta, Silva, Gutierrez, Silva and Zamboni2013) should be essentially aligned with three central characteristics of infection, such as evolution time, parasite virulence/pathogenicity (Marinho et al., Reference Marinho, D'Império Lima, Grisotto and Alvarez1999; Silva et al., Reference Silva, Cunha, Horta, Silva, Gutierrez, Silva and Zamboni2013) and inoculum size (Trischmann et al., Reference Trischmann, Tanowitz, Wittner and Bloom1978).
An appropriated selection of animal lineage and parasite strain is essential to reproduce the different pathological scenarios typically identified in Chagas disease (Oliveira et al., Reference Oliveira, Picka, Nicolete, Calvi and Marcondes-Machado2012; Chatelain and Konar, Reference Chatelain and Konar2015). Thus, longer periods after parasite inoculation are required to induce chronic infections, in which reduced or absent parasitaemia, low-grade inflammation and extensive tissue fibrosis (especially in heart) are pathological outcomes completely divergent to those observed in acute infections (Chatelain and Konar, Reference Chatelain and Konar2015; Lana, Reference Lana, Telleria and Tibayrenc2017). In general, mice lineage, T. cruzi strain and time of infection were aligned in all studies identified in this review. Thus, virulent strains (Y, Brazil and Colombian) (Leon et al., Reference Leon, Wang and Engman2003; de Paula Costa et al., Reference de Paula Costa, Silva, Pedrosa, Pinho, de Lima, Teixeira, Bahia and Talvani2010; Chumbinho et al., Reference Chumbinho, Pizzini, Oliveira, Batista and Oliveira2012) and susceptible mice (BALB/c and A/J) (Leon et al., Reference Leon, Wang and Engman2003; Chumbinho et al., Reference Chumbinho, Pizzini, Oliveira, Batista and Oliveira2012) were used in acute models, while a low virulence strain (VL-10) was selected to induce more prolonged infections (Penitente et al., Reference Penitente, Leite, de Paula Costa, Shrestha, Horta, Natali, Neves and Talvani2015; Leite et al., Reference Leite, Paula Costa, Lopes, Mota, Vieira and Talvani2017). As intense parasitaemia, tissue parasitism, inflammation and damage are the main desirable characteristics in acute models of T. cruzi infection; high inoculum size, highly virulent and pathogenic strains should be a realistic choice for the study of acute outcomes (Lana, Reference Lana, Telleria and Tibayrenc2017; Pereira et al., Reference Pereira, Greco, Moreira, Chagas, Caldas, Gonçalves and Novaes2017). However, more resistant animals (Marinho et al., Reference Marinho, D'Império Lima, Grisotto and Alvarez1999; Silva et al., Reference Silva, Cunha, Horta, Silva, Gutierrez, Silva and Zamboni2013), lower inoculum and less virulent pathogenic strains should be preferred in chronic models, since assures animals survival for a sufficient period for the installation of chronic manifestations (Trischmann et al., Reference Trischmann, Tanowitz, Wittner and Bloom1978; Pereira et al., Reference Pereira, Greco, Moreira, Chagas, Caldas, Gonçalves and Novaes2017).
Unlike in vitro assays, enalapril was preferred in animal studies, which also investigated losartan as an ACE receptor inhibitor. In these studies, suitable doses were used, which have been shown to be effective in modulating the production and/or activity of angiotensin and inducing cardioprotection in murine models of endothelial dysfunction and myocardial infarction (Patten et al., Reference Patten, Aronovitz, Einstein, Lambert, Pandian, Mendelsohn and Konstam2003; Liu et al., Reference Liu, Liu, Wu, Chen and Sun2006). In addition, both RAS-modulating drugs were effective in reducing parasitaemia, tissue parasitism (amastigote nests) and fibrosis, leucocyte infiltration and mortality in T. cruzi-infected animals. In most studies, these effects were partially attributed to the immunomodulatory properties of the drugs tested, which were mainly associated with reduction in TNF-α and IFN-γ levels CCL2/MCP1 and CCL5/RANTES levels (de Paula Costa et al., Reference de Paula Costa, Silva, Pedrosa, Pinho, de Lima, Teixeira, Bahia and Talvani2010; Leite et al., Reference Leite, Paula Costa, Lopes, Mota, Vieira and Talvani2017), as well as the upregulation of IL-10 production (Penitente et al., Reference Penitente, Leite, de Paula Costa, Shrestha, Horta, Natali, Neves and Talvani2015).
As all studies presented a limited immunological approach, it is difficult to determining to what extent the protective effects reported were a result of the immune adaptations induced by captopril, enalapril and losartan. As there is scant evidence on the effect of these drugs in T. cruzi, it is not possible to disregard that the parasitological findings may have been influenced by a direct trypanocidal effect of RAS-modulating drugs, an issue that requires further investigation. However, it is clear that all immunological markers changed by the treatment are directly involved in the pathophysiology of T. cruzi infection (Teixeira et al., Reference Teixeira, Hecht, Guimaro, Sousa and Nitz2011; Cunha-Neto and Chevillard, Reference Cunha-Neto and Chevillard2014; Poveda et al., Reference Poveda, Fresno, Girones, Martins-Filho, Ramírez, Santi-Rocca, Marin-Neto, Morillo, Rosas and Guhl2014). Although Th1 molecules are essential in anti-T. cruzi immunological responses (Marinho et al., Reference Marinho, D'Império Lima, Grisotto and Alvarez1999), a Th1 unbalance has been consistently attributed as the central immunopathological mechanism of tissue damage in Chagas disease (Hunter et al., Reference Hunter, Ellis-Neyes, Slifer, Kanaly, Grünig, Fort, Rennick and Araujo1997; Poveda et al., Reference Poveda, Fresno, Girones, Martins-Filho, Ramírez, Santi-Rocca, Marin-Neto, Morillo, Rosas and Guhl2014). In this sense, downregulation of Th1 effectors (i.e. IFN-γ, TNF-α, CCL2 and CCL5) could be required to reduce the severity of host tissue damage and mortality rates, since high levels of these molecules are associated with intense leucocyte recruitment, heart morphofunctional damage and increased risk of death in chagasic cardiomyopathy (Talvani et al., Reference Talvani, Rocha, Barcelos, Gomes, Ribeiro and Teixeira2004; YAmauchi et al., Reference Yamauchi, Aliberti, Baruffi, Portela, Rossi, Gazzinelli, Mineo and Silva2007; Medeiros et al., Reference Medeiros, Silvério, Marino, Roffê, Vieira, Kroll-Palhares, Carvalho, Silva, Teixeira and Lannes-Vieira2009). Conversely, IL-10 levels exert a strong protective factor against fatal acute myocarditis, reducing mortality in murine models of T. cruzi infection (Reed et al., Reference Reed, Brownell, Russo, Silva, Grabstein and Morrissey1994; Roffê et al., Reference Roffê, Rothfuchs, Santiago, Marino, Ribeiro-Gomes, Eckhaus, Antonelli and Murphy2012).
Clinical studies
Surprisingly, none randomized-controlled clinical trial was identified. Thus, the available evidence on the effect of RAS-modulating drugs in Chagasic patients is entirely based on case series, indicating that further controlled studies are still needed. Unlike in vitro and in vivo studies, these drugs were not used with an antiparasitic purpose, but rather to improve the cardiovascular function in patients with CCC. Although all studies have reported including adult Chagasic patients, specific tests (serology and radioimmunoassay) for the diagnosis of T. cruzi infection were described in only two papers (Roberti et al., Reference Roberti, Martinez, Andrade, Araujo, Brito, Portugal and Horowitz1992; Botoni et al., Reference Botoni, Poole-Wilson, Ribeiro, Okonko, Oliveira, Pinto, Teixeira, Teixeira, Reis, Dantas, Ferreira, Tavares and Rocha2007). However, cardiac function and severity of heart damage were consistently determined in all studies, reinforcing patients’ characterization. The under-reporting of diagnostic tools represents an important methodological limitation, since different diagnostic strategies are required to determine the stage of the disease (Balouz et al., Reference Balouz, Aguero and Buscaglia2017; Rodea et al., Reference Rodea, Cuevas, Ramos and Campos2018). Direct parasitological examination (blood smear or microscopic analysis of tissue fragments) is more indicated in detecting acute infections, which can be confirmed with a high sensitivity (around 99%) by serological diagnostic tools, especially indirect haemagglutination, ELISA and indirect immunofluorescence (Andrade et al., Reference Andrade, Marin Neto, Paola, Vilas-Boas, Oliveira, Bacal, Bocchi, Almeida, Fragata Filho, da Moreira, Xavier, Oliveira Junior and Dias2011; Dias et al., Reference Dias, Ramos, Gontijo, Luquetti, Shikanai-Yasuda, Coura, Torres, Melo, Almeida, Oliveira, Silveira, Rezende, Pinto, Ferreira, Rassi, Fragata, Sousa, Correia, Jansen, Andrade, Britto, Pinto, Rassi, Campos, Abad-Franch, Santos, Chiari, Hasslocher-Moreno, Moreira, Marques, Silva, Marin-Neto, Galvão, Xavier, Valente, Carvalho, Cardoso, Silva, Costa, Vivaldini, Oliveira, Valente, Lima and Alves2016; Nogueira et al., Reference Nogueira, Felizardo, Caldas, Gonçalves and Novaes2018). Since conventional parasitological methods have low sensitivity in cases of sub-patent parasitaemia, serological tests are recommended to detect chronic infections (Dias et al., Reference Dias, Ramos, Gontijo, Luquetti, Shikanai-Yasuda, Coura, Torres, Melo, Almeida, Oliveira, Silveira, Rezende, Pinto, Ferreira, Rassi, Fragata, Sousa, Correia, Jansen, Andrade, Britto, Pinto, Rassi, Campos, Abad-Franch, Santos, Chiari, Hasslocher-Moreno, Moreira, Marques, Silva, Marin-Neto, Galvão, Xavier, Valente, Carvalho, Cardoso, Silva, Costa, Vivaldini, Oliveira, Valente, Lima and Alves2016; Balouz et al., Reference Balouz, Aguero and Buscaglia2017).
Similar to animal studies, only captopril, enalapril and losartan were analysed in clinical investigations. The indication of these drugs was consistent with clinical guidelines for Chagas’ heart disease treatment, which recommend administration of ACE inhibitors in all patients with ventricular dysfunction from NYHA I up to IV (Andrade et al., Reference Andrade, Marin Neto, Paola, Vilas-Boas, Oliveira, Bacal, Bocchi, Almeida, Fragata Filho, da Moreira, Xavier, Oliveira Junior and Dias2011). In general, the doses of each drug tested were coherent with those used in the treatment of systemic arterial hypertension (SAH) in humans (Hodsman et al., Reference Hodsman, Isles, Murray, Usherwood, Webb and Robertson1983; Ikeda et al., Reference Ikeda, Harm, Arcuri, Goldberg and Sweet1997; Akat et al., Reference Akat, Bapat, Murthy, Karande and Burute2010). Dosimetry was also aligned with clinical recommendations, which states that 5–40 mg enalapril (1–2 times day−1), 25–150 mg captopril (2–3 times day−1) and 50–100 mg losartan (once a day) is suitable to control blood pressure (Mion et al., Reference Mion, Gomes, Nobre, Amodeo, Kohlmann, Praxedes and Machado2004). Although the period of treatment has been quite heterogeneous (4–120 days), each study analysed acute or chronic effects from realistic times of treatment. Interestingly, the study that used a larger number of patients also used a longer time of treatment (Botoni et al., Reference Botoni, Poole-Wilson, Ribeiro, Okonko, Oliveira, Pinto, Teixeira, Teixeira, Reis, Dantas, Ferreira, Tavares and Rocha2007), providing a better methodological control for a longer follow-up.
In general, the treatment of Chagasic patients with captopril, enalapril and losartan exerted limited impact on heart rate, blood pressure, left ventricular shortening and ejection fraction, atrial and ventricular dimensions and frequency of ventricular tachycardia. Surprisingly, immunological markers were investigated in only one study (Botoni et al., Reference Botoni, Poole-Wilson, Ribeiro, Okonko, Oliveira, Pinto, Teixeira, Teixeira, Reis, Dantas, Ferreira, Tavares and Rocha2007), which indicated that RAS-modulating drugs were effective in reducing RANTES levels but without effecting MIP-1α. Conversely, severe cardiovascular dysfunction in Chagasic patients was consistently determined in all studies. Inflammatory infiltrate, progressive fibrosis (Cunha-Neto and Chevillard, Reference Cunha-Neto and Chevillard2014) and electromechanical changes (Roman-Campos et al., Reference Roman-Campos, Duarte, Sales, Natali, Ropert, Gazzinelli and Cruz2009; Eickhoff et al., Reference Eickhoff, Lawrence, Sagartz, Bryant, Labovitz, Gala and Hoft2010) are typical manifestations of CCC, contributing to a 55–65% mortality rate of Chagasic patients (Simões et al., Reference Simões, Romano, Schamidt, Martins and Marin-neto2018). As heart morphofunctional damage exhibits a progressive behaviour, control cardiac overload and complacency is a primary therapeutic goal, representing the most challenge task in the clinical management of patients with CCC (Andrade et al., Reference Andrade, Marin Neto, Paola, Vilas-Boas, Oliveira, Bacal, Bocchi, Almeida, Fragata Filho, da Moreira, Xavier, Oliveira Junior and Dias2011; Dias et al., Reference Dias, Ramos, Gontijo, Luquetti, Shikanai-Yasuda, Coura, Torres, Melo, Almeida, Oliveira, Silveira, Rezende, Pinto, Ferreira, Rassi, Fragata, Sousa, Correia, Jansen, Andrade, Britto, Pinto, Rassi, Campos, Abad-Franch, Santos, Chiari, Hasslocher-Moreno, Moreira, Marques, Silva, Marin-Neto, Galvão, Xavier, Valente, Carvalho, Cardoso, Silva, Costa, Vivaldini, Oliveira, Valente, Lima and Alves2016), especially considering that parasitological cure is not a current reality (Biolo et al., Reference Biolo, Ribeiro and Clausell2010; Rassi et al., Reference Rassi, Rassi and Rezende2012; Pereira et al., Reference Pereira, Greco, Moreira, Chagas, Caldas, Gonçalves and Novaes2017).
Although captopril, enalapril and losartan are effective in regulating blood pressure and cardiovascular remodelling in patients with SAH (Ikeda et al., Reference Ikeda, Harm, Arcuri, Goldberg and Sweet1997; Mion et al., Reference Mion, Gomes, Nobre, Amodeo, Kohlmann, Praxedes and Machado2004; Akat et al., Reference Akat, Bapat, Murthy, Karande and Burute2010), the limited effect observed on Chagasic patients remains poorly understood. Although the evidence is still scant, the treatment with RAS-modulating drugs was effective in reducing BNP (Botoni et al., Reference Botoni, Poole-Wilson, Ribeiro, Okonko, Oliveira, Pinto, Teixeira, Teixeira, Reis, Dantas, Ferreira, Tavares and Rocha2007) and norepinephrine levels (Roberti et al., Reference Roberti, Martinez, Andrade, Araujo, Brito, Portugal and Horowitz1992) in Chagasic patients. Furthermore, the treatment had no impact (Khoury et al., Reference Khoury, Davila, Bellabarba, Donis, Torres, Lemorvan, Hernandez and Bishop1996) or increased (Roberti et al., Reference Roberti, Martinez, Andrade, Araujo, Brito, Portugal and Horowitz1992) renin levels. In addition to exerting an important role regulating cardiovascular function, these molecules are relevant markers of cardiac injury in Chagas disease. Thus, high BNP circulating levels is often detected in patients with severe CCC (Talvani et al., Reference Talvani, Rocha, Barcelos, Gomes, Ribeiro and Teixeira2004; Garcia-Alvarez et al., Reference Garcia-Alvarez, Sitges, Pinazo, Regueiro-Cueva, Psada, Poyatos, Ortiz-Pérez, Heras, Azqueta, Gascon and Sanz2010). Additionally, high norepinephrine levels have been associated with a poor prognosis in Chagasic patients, an aspect potentially related with haemodynamic overload and increased risk of heart failure (Kao et al., Reference Kao, Gheorghiade, Hall and Goldstein1989; Roberti et al., Reference Roberti, Martinez, Andrade, Araujo, Brito, Portugal and Horowitz1992).
Reporting quality and risk of bias
Although our systematic review group critically analyses the preclinical and clinical evidence on the effect of RAS-modulating in T. cruzi infections, the interpretation of the results should consider specific limitations of each study design. Surprisingly, a high proportion of essential criteria reported in animal studies were neglected. There is no doubt that under-reported aspects, such as animal housing and husbandry, strategy of animal allocation in experimental groups (i.e. randomization), sample size calculation, baseline data and experimental procedures, are serious limitations to the reproducibility and the reliability of preclinical results, indicating a limited internal and external validity of the individual studies. The analysis of methodological bias corroborated the low reporting quality, pointing a high or unknown risk of bias for most preclinical studies in the majority of categories analysed. In general, the main sources of bias were associated with poor sequence generation (allocation), random outcome assessment, random housing, allocation concealment and experimental blinding. Only selective reporting (reporting bias) did not represent a potential source of bias.
Clinical studies exhibited a better reporting quality than studies with animal models. However, the absence of randomized controlled studies and all methodological limitations observed indicated that the clinical evidence described here should be carefully interpreted. The absence of control groups, a poor description of clinical outcomes, adjustment of confounding factors, small sample size, absence of patients and evaluators blinding and strategies of randomization were the most important inconsistences identified in clinical studies. Considering that the clinical evidence is based on case series and the heterogeneity in patients’ age, cardiac function, drug dose and follow-up period, it is not prudent or advisable to generalize the results to other clinical contexts. However, this review may represent a more comprehensive and up-to-date multilevel analysis on the relationship between RAS-modulating therapy and T. cruzi infection.
Although there is a clear rational basis for the use of RAS-modulating drugs as immunomodulatory agents, the relevance of these drugs as an antiparasitic therapy or as a strategy of cardiovascular support in CCC patients requires more controlled studies with a mechanistic approach. As a simple procedure to improve research quality, essential methodological requirements may be incorporated in preclinical and clinical studies. In this sense, preclinical studies with highest quality can be developed from the use of well-delimited guidelines, including those provided by CAMARADES (Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies – http://www.camarades.info), and SYRCLE (Systematic Review Centre for Laboratory animal Experimentation – http://www.SYRCLE.nl) initiatives. Similarly, clinical research may benefit from the use of SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials; http://www.spirit-statement.org) and CONSORT (Consolidated Standards of Reporting Trials; http://www.consort-statement.org) guidelines, which describes the essential aspects that should be reported when clinical information is publicly disclosed.
Author ORCIDs
Reggiani V. Gonçalves, 0000-0002-5831-3590; Rômulo D. Novaes, 0000-0002-3186-5328
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118201900009X.
Financial support
This work was supported by the Brazilian agencies: Fundação do Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, processes APQ-01895-16 and PPM-00077-18) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, processes 303972/2017-3 and 423594/2018-4). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Conflict of interest
None.
Ethical standards
Not applicable.