Introduction
The vector-borne hemoparasites that parasitize birds are widespread and diverse (Valkiūnas, Reference Valkiūnas2005; Zídková et al., Reference Zídková, Cepicka, Szabová and Svobodová2012). The key taxons involve the intra-erythrocytic hemosporidians (Aconoidasida: Haemosporida): Plasmodium (the agent of avian malaria), Haemoproteus and Leucocytozoon, and the extracellular trypanosomatids of the genus Trypanosoma (Kinetoplastea: Trypanosomatida). To date, 3173 genetic lineages of the three genera of hemosporidians have been identified (MalAvi database, as of December 2018, Bensch et al., Reference Bensch, Hellgren and Pérez-TRIS2009) and at least 80 species of trypanosomes are known (Apanius, Reference Apanius1991). Blood parasites of birds occur worldwide, except for Antarctica, and infect several avian hosts (Kučera, Reference Kučera1982; Sehgal et al., Reference Sehgal, Jones and Smith2001; Valkiūnas, Reference Valkiūnas2005). For instance, in Western Palearctic birds, the most frequently recorded genus is Haemoproteus (51% species sampled), followed by Leucocytozoon (46%), Trypanosoma (36%) and Plasmodium (23%), with the mean prevalence of 11, 10, 4 and 1%, respectively (Arriero and Møller, Reference Arriero and Møller2008). Vectors of bird blood parasites are dipteran hematophagous insects, such as ceratopogonid and hippoboscid flies, simulids and culicine mosquitoes. Birds become infected through a bite (hemosporidians) or digestion (trypanosomes) of an infected arthropod (Molyneux, Reference Molyneux1977; Atkinson et al., Reference Atkinson, Thomas and Hunter2008; Votýpka et al., Reference Votýpka, Szabová, Rádrová, Zídková and Svobodová2012; Svobodová et al., Reference Svobodová, Dolnik, Čepička and Rádrová2017). Blood parasites are important agents of disease in birds and affect their fitness. For example, infection by Plasmodium causes damage of internal organs and destruction of red blood cells, leading to a reduction in metabolism efficiency and acute anaemia (Marzal, Reference Marzal and Okwa2012). As shown by medication experiments, malaria parasites decrease clutch sizes, offspring hatching success, parental provisioning rates and offspring fledging success (Merino et al., Reference Merino, Moreno, Sanz and Arriero2000; Marzal et al., Reference Marzal, de Lope, Navarro and Møller2005; Knowles et al., Reference Knowles, Palinauskas and Sheldon2010). In birds infected by trypanosomes, males arrive later to the breeding grounds in spring [which is known to decrease their breeding success (Verhulst and Nilsson, Reference Verhulst and Nilsson2008)], sire fewer young, and show compromised nest defence against a predator, relative to uninfected males (Rätti et al., Reference Rätti, Dufva and Alatalo1993; Hakkarainen et al., Reference Hakkarainen, Ilmonen, Koivunen and Korpimäki1998; Dyrcz et al., Reference Dyrcz, Wink, Kruszewicz and Leisler2005). Blood parasites are therefore expected to limit bird population growth and viability (Loye and Carroll, Reference Loye and Carroll1995; Holmes, Reference Holmes1996; Marzal, Reference Marzal and Okwa2012). Especially in species of conservation concern, it is important to gain further knowledge of their distribution, diversity and prevalence.
The diversity and prevalence of blood parasites within an avian host species are explained by a range of factors, of which potent ones are landscape features (Sehgal, Reference Sehgal2015; Ferraguti et al., Reference Ferraguti, Martínez-de la Puente, Bensch, Roiz, Ruiz, Viana, Soriguer and Figuerola2018; Pérez-Rodríguez et al., Reference Pérez-Rodríguez, Khimoun, Ollivier, Eraud, Faivre and Garnier2018). Prevalence is related to, for instance, the altitude and distance to water course or water body, as well as habitat degradation (van Riper et al., Reference van Riper, van Riper, Goff and Laird1986; Wood et al., Reference Wood, Cosgrove, Wilkin, Knowles, Day and Sheldon2007; Bonneaud et al., Reference Bonneaud, Sepil, Mila, Buermann, Pollinger, Sehgal, Valkiūnas, Iezhova, Saatchi and Smith2009; Loiseau et al., Reference Loiseau, Iezhova, Valkiūnas, Chasar, Hutchinson, Buermann, Smith and Sehgal2010; Belo et al., Reference Belo, Pinheiro, Reis, Ricklefs and Braga2011; Knowles et al., Reference Knowles, Wood, Alves, Wilkin, Bensch and Sheldon2011; Gonzalez-Quevedo et al., Reference Gonzalez-Quevedo, Davies and Richardson2014; Krama et al., Reference Krama, Krams, Cīrule, Moore, Rantala and Krams2015; Reinoso-Pérez et al., Reference Reinoso-Pérez, Canales-Delgadillo, Chapa-Vargas and Riego-Ruiz2016). Also, habitat fragmentation and heterogeneity are expected to affect parasite infection in birds, through increased edge effects and the introduction of new parasite lineages by species occupying ecotones and areas between fragments (Loye and Carroll, Reference Loye and Carroll1995; Holmes, Reference Holmes1996). However, studies that addressed the relationship between habitat fragmentation/edge and blood parasite infection in birds are scarce, focused solely on forest habitats and only two of them involved intraspecific comparisons (Sebaio et al., Reference Sebaio, Braga, Branquinho, Manica and Marini2010; Laurance et al., Reference Laurance, Jones, Westcott, Mckeown, Harrington and Hilbert2013; Gudex-Cross et al., Reference Gudex-Cross, Barraclough, Brunton and Derraik2015; Pérez-Rodríguez et al., Reference Pérez-Rodríguez, Khimoun, Ollivier, Eraud, Faivre and Garnier2018). For example, in a forest passerine, the Antillean Bullfinch (Loxigilla noctis), prevalence of two hemosporidian lineages is positively related to variables describing habitat fragmentation and amount of edge (Pérez-Rodríguez et al., Reference Pérez-Rodríguez, Khimoun, Ollivier, Eraud, Faivre and Garnier2018). To our knowledge, treeless habitats have not been studied in this respect, while a similar pattern could be expected due to negative edge effects on parasitism in some animal taxa (Ries et al., Reference Ries, Fletcher, Battin and Sisk2004). It appears that in particular habitat specialist birds inhabiting peatlands need to be addressed in this context, as these ecosystems have been globally degrading and shrinking due to habitat loss and fragmentation. Blood parasite prevalence in birds is also explained by individual traits. For example, within species, blood parasite prevalence is female-biased (McCurdy et al., Reference McCurdy, Shutler, Mullie and Forbes1998), higher in older than in younger individuals (Wood et al., Reference Wood, Cosgrove, Wilkin, Knowles, Day and Sheldon2007) and higher in individuals of poorer body condition (Shutler et al., Reference Shutler, Clark, Rutherford and Mullie1999; Dawson and Bortolotti, Reference Dawson and Bortolotti2000; Hatchwell et al., Reference Hatchwell, Wood, Anwar, Chamberlain and Perrins2001; Dyrcz et al., Reference Dyrcz, Wink, Kruszewicz and Leisler2005; Shurulinkov et al., Reference Shurulinkov, Chakarov and Daskalova2012). Negative effects of blood parasite infection on body mass in birds were demonstrated experimentally, through inoculation and medication treatments (Atkinson et al., Reference Atkinson, Forrester and Greiner1988; Merino et al., Reference Merino, Moreno, Sanz and Arriero2000; Valkiūnas et al., Reference Valkiūnas, Z̆ic̆kus, Shapoval and Iezhova2006). To gain a broader understanding of the link between individual features and parasitism, more bird species of diverse ecologies should be studied.
The Aquatic Warbler Acrocephalus paludicola is a globally-threatened passerine bird with the Vulnerable IUCN status. It breeds in temperate regions, in extensive, permanently water-logged fen mires with low vegetation, and winters in sub-Saharan Africa. Following massive habitat loss, the species saw a steep decline. Today, the Aquatic Warbler is fluctuating around approximately 12 200 singing males and breeds in the highly fragmented and scarce remaining fens of East-Central Europe (Aquatic Warbler Conservation Team, 1999; Briedis and Keišs, Reference Briedis and Keišs2016; BirdLife International, 2017; Flade et al., Reference Flade, Malashevich, Krogulec, Poluda, Preiksa, Végvári, Lachmann, Tanneberger and Kubacka2018). Blood parasites have been studied in the Aquatic Warbler during migration and wintering (Neto et al., Reference Neto, Pérez-Rodríguez, Haase, Flade and Bensch2015) but only to a limited extent on the breeding grounds (Dyrcz et al., Reference Dyrcz, Wink, Kruszewicz and Leisler2005). Here, we identified blood parasites in breeding Aquatic Warblers from two core populations using molecular techniques and tested for differences in prevalence and diversity between the populations. We further evaluated whether infection status is related to habitat edge and individual traits. Specifically, we predicted that (1) the amount, proximity and/or complexity of edges is positively related to infection probability, (2) individuals of lower body condition are more likely to be infected than individuals in higher body condition, and (3) females are more likely to be infected than males.
Methods
Study area and sampling
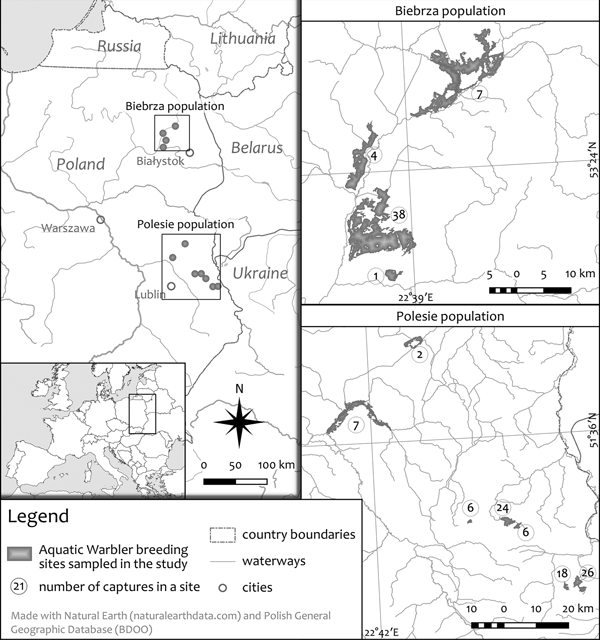
Aquatic Warblers were sampled from two core breeding populations, in the Biebrza Valley (NE Poland; four sites sampled) and the western Polesie region (SE Poland; seven sites sampled, Fig. 1). The distance between the two regions is c. 250 km and they differ by habitat fragmentation, vegetation, patch size, as well as density and numbers of Aquatic Warblers. The Biebrza Valley comprises relatively large, continuous and extensive fen mires, while the Polesie is more fragmented and consists of smaller habitat patches (Fig. 1). The Biebrza Valley holds 2000–2600 singing males, with the density in the sampled areas of about 10 males per 10 ha. The Polesie population is 400–700 singing males and the density is about half that of the Biebrza (Kubacka et al., Reference Kubacka, Oppel, Dyrcz, Lachmann, Barros Da Costa, Kail and Zdunek2014; Polish Society for the Protection of Birds, 2014; Kubacka unpubl.).

Fig. 1. Distribution of the 11 sampling locations including the numbers of Aquatic Warblers. The map was created using QGIS (QGIS Development Team, 2018).
We captured 144 Aquatic Warblers with mist-nets during the breeding season, between May and August of 2014. Locations of captures were saved with a GPS device. We sampled 54 individuals in the Biebrza Valley (17 adult females, 33 adult males and four first-year individuals of unknown sex) and 90 individuals in Polesie (18 adult females, 71 adult males and one first-year individual of unknown sex). Sex was identified by the presence of cloacal protuberance (adult males) or brood-patch (adult females), and age was determined by examination of plumage: partially or completely worn in adults and completely new for first-year birds (Svensson, Reference Svensson1992). Wing length was measured with a ruler to the nearest millimetre, according to the method described by Svensson (Reference Svensson1992). Fat score was assessed on the scale of 0–8 according to Kaiser (Reference Kaiser1993). Body mass was measured with a Pesola spring balance to the nearest 0.1 g. Approximately 5–100 µL of blood was sampled from each individual through brachial venepuncture. The blood samples were immediately transferred to tubes with 1 mL of 96% ethanol and stored at room temperature until laboratory analysis.
Identification and diversity of blood parasites
DNA was isolated using a phenol-chloroform protocol (Sambrook and Russell, Reference Sambrook and Russell2001). With molecular techniques, the blood samples were screened for the presence of blood parasites by amplifying:
1) A 478 bp fragment of the mitochondrial cyt b gene in the case of Leucocytozoon, Haemoproteus and Plasmodium, with the thermal profile according to Hellgren et al. (Reference Hellgren, Waldenström and Bensch2004). The polymerase chain reaction (PCR) was performed in volumes of 25 µL, which included 2 µL of genomic DNA, 0.125 mM of each dNTP, 3 mM MgCl2, 0.6 µM of each primer (first-round primers: HaemNFI and HaemNR3; second-round primers: HaemF and HaemR2 in the case of Plasmodium/Haemoproteus, HaemFL and HaemR2L in the case of Leucocytozoon), and 0.5 units of Taq DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA) with the accompanying PCR buffer at 1 × final concentration.
2) A 326 bp fragment of the small subunit ribosomal RNA (SSU rRNA) gene in the case of Trypanosoma, with the PCR thermal profile according to Sehgal et al. (Reference Sehgal, Jones and Smith2001). Each 25 µL reaction mixture contained 2 µL of genomic DNA, 0.125 mM of each dNTP, 3 mM MgCl2, 1.25 µM of each primer (first-round primers: S-762 and S-763; second-round primers: S-755 and S-823) and 0.5 units of Taq DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA) with the accompanying PCR buffer at 1 × final concentration.
Four μL of PCR products from the second round were run on 2% agarose gels stained with GelRed (Biotium, Hayward, CA, USA) and visualised under ultraviolet light. Each plate contained the positive (DNA from birds with confirmed infection based on blood smear screening) and negative (ddH2O) control. Products of positive samples were purified with FastAP (Fermentas) and sequenced directly with an automated ABI 3130 DNA analyzer (Applied Biosystems), using BigDye terminator v3.1 (Applied Biosystems). The obtained sequences were visually inspected and aligned using the BioEdit software (Hall, Reference Hall1999), which offers a local BLAST (Basic Local Alignment Search Tool) (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990). They were then compared against the MalAvi database (Bensch et al., Reference Bensch, Hellgren and Pérez-TRIS2009) in the case of Leucocytozoon, Haemoproteus and Plasmodium, and GenBank (Benson et al., Reference Benson, Cavanaugh, Clark, Karsch-Mizrachi, Lipman, Ostell and Sayers2013) in the case of Trypanosoma, using nucleotide BLAST and selecting the best hit match. Leucocytozoon, Haemoproteus and Plasmodium were identified at the lineage level, and Trypanosoma was identified at the species/genus level, due to the short length of the sequenced fragment. In the case of double infections (indicated by double peaks in the chromatogram), parasite lineages were assigned visually by comparing DNA sequences with the pool of lineages known to occur in our study (in the pool of other analysed samples). This method was confirmed to be reliable in a previous study, by cloning PCR products and showing that the sets of lineages identified by cloning and by visual comparison of lineages were in complete accordance (Podmokła et al., Reference Podmokła, Dubiec, Drobniak, Arct, Gustafsson and Cichoń2014).
To describe the diversity of the identified blood parasites, two ecological diversity indices were used: the species richness (S; the total number of detected lineages) and the Shannon index (H; combines information on species richness and relative abundance).
Patch and landscape metrics
Each site was a patch of continuous breeding habitat, separated from another site by a stretch of non-breeding habitat (other than fen mire or peat meadow) of at least a few kilometres. Using QGIS software (QGIS Development Team, 2018) and ellipsoidal (GRS 1980) measurement, for each patch and/or capture point, we calculated biologically informative metrics characterizing the proximity, amount and complexity of edge in the breeding habitat (Table 1). These metrics are commonly applied in landscape ecology, also in the context of blood parasitism and vector occurrence (McGarigal et al., Reference McGarigal, Cushman, Neel and Ene2012; Li et al., Reference Li, Roux, Dessay, Girod, Stefani, Nacher, Moiret and Seyler2016; Pérez-Rodríguez et al., Reference Pérez-Rodríguez, Khimoun, Ollivier, Eraud, Faivre and Garnier2018). To ease interpretation of the effects of the metrics on infection status, we provide their correlation matrix, alongside with the total length of edge and the area of a patch (Table S1).
Table 1. Patch and landscape metrics of habitat edge used in the study

Statistical analysis
Data were analysed in the R environment (R Core Team, 2018). We first computed population prevalence, Shannon diversity and species richness, for all the lineages pooled and for each of the identified genera separately. The two diversity indices were calculated in the ‘vegan’ package (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Henry, Stevens, Szoecs and Wagner2019). The prevalences were compared between the two populations with the test for equal proportions (command ‘prop.test’ from the ‘stats’ package) and asymptotic 95% confidence intervals (CIs) were calculated with a custom function. The 95% CIs of the Shannon indices and their differences between the populations were calculated in the ‘boot’ package (Canty and Ripley, Reference Canty and Ripley2017) according to the recommendations of Gardener (Reference Gardener2014). Asymptotic CIs were estimated based on 1000 bootstrap resamples [custom R command ‘H_boot’ from Gardener (Reference Gardener2014)]. The statistical significance of the difference in the diversity index between populations was estimated using a randomisation method based on 2000 bootstrap resamples [custom R command ‘H_bss’ from Gardener (Reference Gardener2014)].
To evaluate the effect of the patch and landscape metrics, and individual variables on infection status, we used generalized linear mixed models with binomial error structure and logit link, fitted with the package ‘lme4’ and the ‘glmer’ command (Bates et al., Reference Bates, Mächler, Bolker and Walker2015). The information theoretic (IT) approach was used for inference and the Akaike information criterion corrected for small sample size (AICc) was applied for model selection (Burnham and Anderson, Reference Burnham and Anderson2002). The IT approach has been increasingly used in ecology, especially in non-experimental studies. In this approach, unlike in null hypothesis testing, an a priori set of biologically plausible candidate models is constructed and each model within the set is evaluated in terms of how well it approximates the relationship between the predictors and the dependent variable relative to the other models, given the data. Then, the models are ranked by AICc and the best-ranking model (or a subset of best models) is selected. Models that are within 2 AICc units of the top model (which has the lowest AICc) are considered to be highly supported (Burnham and Anderson, Reference Burnham and Anderson2004). Including a null model in the candidate set, i.e. a model assuming that the response variable is constant, enables evaluation of ‘absolute’ support for each model. The strength of evidence of each model is provided by computing model likelihood (the relative likelihood of a model in the candidate set, given the data), the Akaike weight (ωAICc) and the evidence ratio. ωAICc is the probability of a model for a given set of candidate models. For a given model, the evidence ratio is the ratio of its probability to the top model probability, which has an evidence ratio equal to one.
We ran two separate analyses, each with four candidate model sets, with the response being infection status (coded as a binary variable: 1 – infected, 0 – uninfected) by (1) any of the blood parasite lineages pooled, (2) Plasmodium, (3) lineage SW2 (the most frequent, Table 3) and (4) Trypanosoma. Each model included the random effect (random intercept) of site, to account for possible non-independence between individuals mist-netted within a given patch (Bolker et al., Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009). Because Leucocytozoon was found only in one individual and no Haemoproteus infections were detected (see Results), we did not run separate models for these genera. The two lineages that are not known to be transmitted in Europe (PAS106 and PAS112, detected in 8 individuals, Table 3) are suggested to have a broad geographical distribution (Šlapeta et al., Reference Šlapeta, Morin-Adeline, Thompson, McDonell, Shiels, Gilchrist, Votýpka and Vogelnest2016), and hence we included all the lineages in both analyses.
In the first analysis, we tested whether the infection status was explained by any of the patch and landscape variables. Each of the four candidate model sets consisted of eight models, with seven models each containing one patch or landscape metric (Table 1) as the fixed variable, and a null model assuming that infection status was constant across individuals. This enabled us to see which of the patch and landscape variables was the best predictor of infection status (by all the parasites pooled, Plasmodium, Trypanosoma and the SW2 lineage) and whether the most parsimonious model was more informative than the null model. In the second analysis, we assessed whether infection status was explained by variables related to the individual: sex (binary categorical variable), fat score, wing length and body mass (all continuous variables). This analysis was performed on a dataset that excluded missing values for the biometrical parameters (hence the retained sample size for the second analysis was N = 111). Each of the four candidate model sets consisted of five models, with four models allowing the infection status to vary with (1) sex, (2) fat score, (3) wing length, (4) body mass and (5) a null model, which assumed a constant infection status. We reported support for the models with the evidence ratio and Akaike weight (ωAICc).
Results
Description of blood parasites
The blood parasites found in breeding Aquatic Warblers belonged to three genera (Plasmodium, Trypanosoma and Leucocytozoon; no Haemoproteus infections were detected) and 12 lineages (Table 2). One out of the five first-year individuals was infected (with Trypanosoma culicavium). Six lineages were found in the Biebrza and ten lineages were found in the Polesie population. In both populations, the most prevalent lineage was SW2 (Plasmodium homonucleophilum). One individual was co-infected by two different Plasmodium lineages (SW2 and SW5) and eight individuals were infected by both Plasmodium and Trypanosoma. None of the identified lineages were specific to the Acrocephalus genus. Prevalence did not differ between the populations, however, lineage diversity was higher in Polesie than in the Biebrza Valley, in the case of all the parasites pooled and Plasmodium, but not Trypanosoma (Table 3).
Table 2. Blood parasites identified in breeding Aquatic Warblers. For Plasmodium and Leucocytozoon, information on the species, host range and transmission was obtained from the MalAvi database (Bensch et al., Reference Bensch, Hellgren and Pérez-TRIS2009), as of December 2018, with the transmission of SYAT24, SW5 and SYBOR02 additionally checked in other available literature. A lineage was considered to be transmitted in Europe if it has previously been found in juvenile birds of a migratory species or adult/juvenile birds of a resident species. For Trypanosoma, data were obtained from GenBank (features of deposited sequences) and through screening of published literature

a Ventim et al., Reference Ventim, Morais, Pardal, Mendes, Ramos and Pérez-Tris2012.
b Shurulinkov et al., Reference Shurulinkov, Spasov, Stoyanov and Chakarov2018.
Table 3. Diversity and prevalence of blood parasites in the two studied breeding populations of the Aquatic Warbler. Differences significant at the 0.05 level are marked in bold

a Yates correction applied.
Association of patch and landscape metrics with infection status
There was no clear support for the correlation of a patch or landscape metric with infection by any parasite or by Plasmodium, as none of the models ranked better than the null model and all the odds ratio (OR) estimates spanned 1 (Table 4a). The probability of infection by the most frequent Plasmodium lineage, SW2, appeared to increase with the relative amount of non-breeding habitat within a patch, however, the confidence interval of its OR reached 1 (Table 4a). In the case of Trypanosoma, we found support for two patch metrics, core area index (CAI) and edge density (ED), to affect infection status. The ORs of CAI and ED were both different from one, the models with these variables were the only ones within ΔAICc ⩽ 2 and they were about three times more probable than the null model. The probability of infection by trypanosomes decreased 0.94 times with one unit change in CAI and increased 1.07 times with one unit change in ED (Table 4a, Fig. 2). These two metrics, CAI and ED were correlated (Table S1), which explains their similar support and exactly opposite mutual effects.
Table 4. Model selection results and estimates of the fixed variables converted to odds ratios (OR) from the analysis relating a) landscape metrics and b) individual variables to infection status. Models within ΔAICc⩾2 (i.e. with strong relative support) and ORs different from 1, based on their 95% confidence intervals (CIs), are marked in bold. K – number of estimable parameters. AICc – Akaike information criterion corrected for small sample size, ΔAICc – difference of AICc relative to the top model, ωAICc – Akaike weight (probability of a model)

a Males relative to females.
b Model did not converge.

Fig. 2. Probability of infection of Aquatic Warblers by genus Trypanosoma in relation to (a) core area index and (b) edge density of breeding habitat patches. The lines were fitted based on the estimates from the respective top models in Table 4a and the bands show confidence intervals. Circles show infected (y = 1) and uninfected (y = 0) individuals, with the circle area proportional to the number of individuals (n) for a given value of the metric.
Effects of sex, wing length, fat deposits and body mass
Infection status was not clearly related to sex or biometrics variables, whether for all the parasites pooled, Plasmodium, SW2 lineage, or Trypanosoma. Constant infection models always ranked best and for each of the explanatory variables the confidence intervals of its estimated odds ratio spanned one (Table 4b).
Discussion
Our study is the first account of blood parasites in the Aquatic Warbler from a range of its breeding locations. We showed population differences in lineage diversity but not in prevalence. While we did not find support for infection status to vary with body mass, fat reserves, wing length and sex, we demonstrated, apparently for the first time in an open habitat bird, that infection probability by a blood parasite is affected by metrics of habitat edge.
Diversity and prevalence of blood parasites
The prevalence of blood parasites in the Aquatic Warbler in our study is difficult to compare with that of other birds due to high variation in prevalence attributable to location, habitat, year, age or sample size. Overall, considering studies carried out during the breeding season that inspected more than 20 individuals, Plasmodium prevalence in the Aquatic Warbler appears to be higher than in at least four out of seven other Acrocephalus species breeding in Europe, and is the closest to that found in the Great Reed Warbler (A. arundinaceus), 24–27% (Bensch et al., Reference Bensch, Stjernman, Hasselquist, Ostman, Hansson, Westerdahl and Pinheiro2000, Reference Bensch, Waldenström, Jonzén, Westerdahl, Hansson, Sejberg and Hasselquist2007; Zehtindjiev et al., Reference Zehtindjiev, Ilieva, Westerdahl, Hansson, Valkiunas and Bensch2008, Reference Zehtindjiev, Ilieva, Krizanauskiene, Oparina, Oparin and Bensch2009; Dimitrov et al., Reference Dimitrov, Zehtindjiev and Bensch2010, Reference Dimitrov, Ilieva, Ivanova, Brlik and Zehtindjiev2018; Biedrzycka et al., Reference Biedrzycka, Migalska and Bielański2015). Although absent in Aquatic Warblers, also during migration and wintering (Neto et al., Reference Neto, Pérez-Rodríguez, Haase, Flade and Bensch2015), Haemoproteus was detected in other acrocephalids breeding in Europe, with a high variation in prevalence within and across species. Studies that examined at least 20 individuals during the breeding season reported prevalences of 10–96% in the Great Reed Warbler, 9% in the Common Reed Warbler (A. scirpaceus), 4–54% in the Sedge Warbler (A. schoenobaenus) and 21–24% in the Paddyfield Warbler (A. agricola) (Bensch et al., Reference Bensch, Stjernman, Hasselquist, Ostman, Hansson, Westerdahl and Pinheiro2000, Reference Bensch, Waldenström, Jonzén, Westerdahl, Hansson, Sejberg and Hasselquist2007; Zehtindjiev et al., Reference Zehtindjiev, Ilieva, Westerdahl, Hansson, Valkiunas and Bensch2008, Reference Zehtindjiev, Ilieva, Krizanauskiene, Oparina, Oparin and Bensch2009; Dimitrov et al., Reference Dimitrov, Zehtindjiev and Bensch2010, Reference Dimitrov, Ilieva, Ivanova, Brlik and Zehtindjiev2018; Fernández et al., Reference Fernández, Rojo, Casanueva, Carrión, Hernández and Campos2010; Biedrzycka et al., Reference Biedrzycka, Migalska and Bielański2015). The prevalence of Leucocytozoon in our study was remarkably low, and so it is in other European Acrocephalus species, ranging between 0 and 1% during the breeding season (Shurulinkov and Chakarov, Reference Shurulinkov and Chakarov2006; Svoboda et al., Reference Svoboda, Marthinsen, Turčoková, Lifjeld and Johnsen2009; Fernández et al., Reference Fernández, Rojo, Casanueva, Carrión, Hernández and Campos2010; Biedrzycka et al., Reference Biedrzycka, Migalska and Bielański2015; Dimitrov et al., Reference Dimitrov, Ilieva, Ivanova, Brlik and Zehtindjiev2018). The lack of Haemoproteus and the low prevalence of Leucocytozoon in our study can be due to lack of vectors in the breeding habitat of Aquatic Warblers. Trypanosoma was found in Aquatic Warblers previously in one of the locations that we studied, the Biebrza Valley (Dyrcz et al., Reference Dyrcz, Wink, Kruszewicz and Leisler2005), with 48.5% breeding males being infected. This is a high prevalence compared to the one that we found in that population (12.5%), which could stem from the change in the occurrence of vectors, for example, due to different weather and humidity conditions in the year of sampling, relative to Dyrcz et al. (Reference Dyrcz, Wink, Kruszewicz and Leisler2005).
The three genera of hemosporidians that we targeted in our molecular analysis were previously studied in Aquatic Warblers during migration and wintering (Neto et al., Reference Neto, Pérez-Rodríguez, Haase, Flade and Bensch2015). In that study, only three Plasmodium lineages were found, with a prevalence of 26.9% on the migration stop-over site in Portugal and 5.5% on a wintering site in Senegal. In our study, we identified seven lineages of Plasmodium, with the 29.9% prevalence being similar to the one during migration but clearly higher than in wintering quarters. Aquatic Warblers could have higher infection rates during the breeding period due to the spring relapse of blood parasites, associated with higher vector activity (Beaudoin et al., Reference Beaudoin, Applegate, Davis and McLean1971; Valkiūnas, Reference Valkiūnas2005; Hellgren et al., Reference Hellgren, Wood, Waldenström, Hasselquist, Ottosson, Stervander and Bensch2013), but also because infected birds die before reaching their wintering sites. In addition, Neto et al. (Reference Neto, Pérez-Rodríguez, Haase, Flade and Bensch2015) established that two threatened acrocephalids, the Seychelles Warbler (A. seychellensis) and the Aquatic Warbler, had the lowest number of Plasmodium and Haemoproteus lineages found in relation to host samples screened, compared to common acrocephalids. They hypothesized that this is due to their rarity and habitat specialisation. Our results refute this hypothesis and demonstrate that the Aquatic Warbler shows an average number of lineages per number of analysed samples, relative to other acrocephalids listed in Neto et al. (Reference Neto, Pérez-Rodríguez, Haase, Flade and Bensch2015), which is likely attributable to our study being conducted on breeding birds.
As parasites affect survival and breeding success of birds, studying population differences is important to inform conservation management of threatened species. The comparable prevalence rates in both populations indicate that blood parasitaemia is of similar importance in terms of population viability in both locations. The higher diversity of lineages in Polesie than in the Biebrza Valley is likely a result of different sample sizes (90 and 54, respectively), as the number of lineages identified grows asymptotically with the number of screened individuals (e.g. Neto et al., Reference Neto, Pérez-Rodríguez, Haase, Flade and Bensch2015). Another possibility is the variation in vector diversity between the two regions, especially that Polesie consists of several patches, scattered over a larger area, which are more diverse than the fairly uniform habitat in the Biebrza Valley. The higher heterogeneity of the Polesie sites also likely translates to a more diverse bird community, which could be another factor affecting parasite lineage diversity, due to cross-species infections (Waldenström et al., Reference Waldenström, Bensch, Kiboi, Hasselquist and Ottosson2002; Reullier et al., Reference Reullier, Pérez-Tris, Bensch and Secondi2006; Sehgal, Reference Sehgal2015). Host community and species are known to be powerful predictors of parasite diversity, including Plasmodium (Scordato and Kardish, Reference Scordato and Kardish2014; Fecchio et al., Reference Fecchio, Pinheiro, Felix, Faria, Pinho, Lacorte, Braga, Farias, Aleixo, Tkach, Collins, Bell and Weckstein2018; Ferraguti et al., Reference Ferraguti, Martínez-de la Puente, Bensch, Roiz, Ruiz, Viana, Soriguer and Figuerola2018).
Effects of patch and landscape metrics on infection
Aquatic Warblers breeding in patches with a relatively larger core area (CAI) and lower amount of habitat edge per area unit (ED) were less likely to be infected by trypanosomes. The CAI is a function of patch size and total edge length, while ED is affected by edge length (Table 1). These two metrics are correlated (Table S1) and it is difficult to conclude which landscape feature specifically determines the probability of infection. Nevertheless, our results show that the known factors of habitat change and loss in peatlands, such as succession, fragmentation, drainage or eutrophication, could exacerbate the risk of infection by trypanosomes in the Aquatic Warbler, through reduction of the size of a patch and/or increase in habitat heterogeneity and edge length. This could translate into a lower breeding success, survival and population growth. We are not aware of other studies linking landscape features associated with edge to blood parasite infection in birds of open habitats. Core area of habitat (positively) and edge density (negatively) were found to be related to bird abundance in a grassland passerine (Herse et al., Reference Herse, With and Boyle2018). Within-species research on blood parasite infection in relation to habitat edge and fragmentation all concerned forest habitats and showed negative (Pérez-Rodríguez et al., Reference Pérez-Rodríguez, Khimoun, Ollivier, Eraud, Faivre and Garnier2018), positive (Laurance et al., Reference Laurance, Jones, Westcott, Mckeown, Harrington and Hilbert2013) or no associations (Sebaio et al., Reference Sebaio, Braga, Branquinho, Manica and Marini2010; Gudex-Cross et al., Reference Gudex-Cross, Barraclough, Brunton and Derraik2015). This area clearly requires more research, especially in birds of open habitats.
Our results should be given some reservation since the study covered a limited number of patches (11) and because we did not know whether all of the identified parasite lineages are transmitted specifically in the studied area. For instance, although the blood parasites identified in our study areas are all most likely transmitted in Europe (Table 2), some of them could be transmitted to Aquatic Warblers only during migration or on African wintering grounds, e.g. due to the absence of lineage-specific vectors in fen mires. In our study, removing the unexplained variance caused by individuals infected only by lineages not transmitted in Central-European fens could have revealed additional relationships with the landscape and patch metrics. For a more robust result, the association that we found requires further study in more breeding sites and fine-scale determination of transmission.
A range of mechanisms can underlie the relationship between trypanosome infection, CAI and ED. There is some evidence for increased parasitism near habitat edges for birds, mammals and amphibians (Ries et al., Reference Ries, Fletcher, Battin and Sisk2004). In the case of arthropod-borne blood parasites, this effect could be due to exposure of birds to more (and different) vectors and/or presence of different parasite lineages in the adjacent habitat, infecting the birds utilising this habitat. Hematozoan vectors are more abundant in the forest than in treeless habitats (Bennett et al., Reference Bennett, Montgomerie and Seutin1992) and forest bird species have higher prevalences of blood parasites than species from open habitats (Tella et al., Reference Tella, Blanco, Forero, Gajon, Donazar and Hiraldo1999). One reason for the low vector abundance in open habitats could be greater wind speeds, which are known to reduce host-seeking activity of vectors such as biting midges and black flies (Lillie et al., Reference Lillie, Kline and Hall1988; Fredeen and Mason, Reference Fredeen and Mason1991; Martin et al., Reference Martin, McCreadie and Colbo1994; Martínez-De La Puente et al., Reference Martínez-De La Puente, Merino, Lobato, Rivero-De Aguilar, Del Cerro, Ruiz-De-Castañeda and Moreno2009). In addition, increased heterogeneity of habitat reduces its quality for open-marshland specialist birds and might attract avian predators occupying overgrown or fragmented areas (Thomson et al., Reference Thomson, Tomás, Forsman, Broggi and Mönkkönen2010). This can lead to social and physiological stress, which is known to compromise immune function (Lyles and Dobson, Reference Lyles and Dobson1993; Oppliger et al., Reference Oppliger, Clobert, Lecomte, Lorenzon, Boudjemadi and John-Alder1998). On the other hand, if social or physiological stress was elevated and affected immunity of individuals in the patches with more edge in our study, we would have observed this effect also in Plasmodium.
Individual traits and infection status
In our study, sex, wing length, fat score or body mass had no discernible effect on the probability of infection of Aquatic Warblers by blood parasites. This was true both for overall parasite infection and when the analysis was performed separately for Plasmodium, the SW2 lineage and Trypanosoma. In an earlier study, breeding Aquatic Warbler males infected by trypanosomes were lighter than uninfected males but their wing length and fat deposits did not differ (Dyrcz et al., Reference Dyrcz, Wink, Kruszewicz and Leisler2005). In other bird species, experimental studies demonstrated negative effects of blood parasite infection on body mass (Atkinson et al., Reference Atkinson, Forrester and Greiner1988; Merino et al., Reference Merino, Moreno, Sanz and Arriero2000; Valkiūnas et al., Reference Valkiūnas, Z̆ic̆kus, Shapoval and Iezhova2006) and females of lower body condition had higher parasitaemia (Shutler et al., Reference Shutler, Clark, Rutherford and Mullie1999; Dawson and Bortolotti, Reference Dawson and Bortolotti2001; Hatchwell et al., Reference Hatchwell, Wood, Anwar, Chamberlain and Perrins2001). Sex effects have also been observed, for example, breeding females are more often infected by Haemoproteus than breeding males (McCurdy et al., Reference McCurdy, Shutler, Mullie and Forbes1998). It is possible that in our study variation arising due to differences in capture date or age confounded potential associations between blood parasite infection and body mass, fat score, wing length and sex.
Concluding remarks
In this study, we describe the diversity and prevalence of blood parasites in the threatened Aquatic Warbler, a breeder of fen mires, and demonstrate that infection risk by the genus Trypanosoma increases with habitat edge density and decreases with the amount of core area in a patch. This relationship indicates that habitat changes causing reduction of breeding area or increase in edge length could negatively affect the fitness of Aquatic Warblers through higher parasitism by trypanosomes. This could also be true for other avian habitat specialists breeding in open habitats, such as marshland and grassland, but to date research in this area is lacking. Our result contributes to informed conservation management of the Aquatic Warbler, as well as other bird species of the globally shrinking marshland ecosystems.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182019000350
Author ORCIDs
Justyna Kubacka, 0000-0003-1211-8324
Acknowledgements
We greatly thank Marine Boucaux, Mathilde Gely, Nicolas Hillier, Paul Lenrume, Piotr Marczakiewicz, Liza Olson, Zuzanna Pestka and Joanna Wołoszkiewicz for help in the field. Anna Dubiec and three anonymous reviewers provided extensive comments on earlier drafts of the manuscript. Borys Jurgiel kindly supported QGIS analysis.
Financial support
The study was supported by British Ornithologists' Union (J.K., Career Development Bursary); Evolutionary Biology and Animal Ecology lab at the University of Freiburg; and the National Science Centre, Poland (E.P., grant number 2011/03/N/NZ8/02106), (J.K., grant number 2016/20/S/NZ8/00434).
Ethical standards
The study was conformed to the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes and its appendix. The procedure of blood-sampling was approved of by the First Ethical Committee in Lublin, Poland. Capture and ringing of the birds were performed under permits from the Polish Ministry of the Environment, the Biebrza National Park, the Poleski National Park and the Regional Directorate for Environmental Protection in Lublin, in line with the requirements of the Polish Nature Protection Act.
Conflicts of interest
None.