Published online by Cambridge University Press: 11 November 2005
Activation of casein kinase II (CK2) was one of the first observations made on how Theileria parasites manipulate host cell signal transduction pathways and we argue that CK2 induction may in fact contribute to many of the different activation events that have been described since 1993 for Theileria-infected lymphocytes such as sustained activation of transcription factors c-Myc and NF-κB. CK2 also contributes to infected lymphocyte survival by inhibiting caspase activation and is probably behind constitutive PI3-K activation by phosphorylating PTEN. Finally, we also discuss how CK2A may act not only as a kinase, but also as a stimulatory subunit for the protein phosphatase PP2A, so dampening down the MEK/ERK and Akt/PKB pathways and for all these reasons we propose CK2 as a central player in Theileria-induced lymphocyte transformation.
Theileria parva is an obligate, intracellular, parasitic protozoan that causes East Coast fever, an acute leukaemia-like disease of cattle. T. parva and T. annulata (causative agent of Tropical Theileriosis) are unique among eukaryotes in that infection induces transformation of their host cells, namely, bovine leukocytes. One of the earliest observations on host cell activation induced by infection showed that T. parva-infected lymphocytes display a 4- to 12-fold increase in total protein phosphorylation and that this is largely due to a serine/threonine kinase called casein kinase II (CK2, (ole-MoiYoi et al. 1993)). Augmented kinase activity correlates with increased transcription, as there is a 4- to 6-fold increase in CK2a mRNA in the infected cells relative to controls and a marked increase in the amount of CK2 protein in infected cell lines. Bovine lymphocyte CK2 therefore, appears to be constitutively activated in Theileria-infected cells. Theileria parasites however, code for their own CK2 (ole-MoiYoi et al. 1992; Shayan & Ahmed, 1997), but the enzyme does not appear to be secreted into the host cell cytoplasm and it is unlikely therefore, that parasite kinase activity underlies the increased mammalian CK2 activity associated with infected leukocytes (Biermann et al. 2003).
CK2 is a heterodimeric enzyme made up of two catalytic alpha subunits in a complex with two regulatory beta subunits (Fig. 1). The complex is dynamic and the different subunits can associate with other complexes (Filhol, Martiel & Cochet 2004). Analysis of the human gene for CK2α showed promoter activity resides essentially in a region between positions -9 to 46 and binding sites for the transcription factors NF-κB and Sp1 were identified (Krehan et al. 2000). Interestingly, NF-κB is strongly activated by Theileria-infection (Heussler et al. 1999), so perhaps parasite-mediated activation of NF-κB might be responsible for the increased transcription of CK2α (ole-MoiYoi et al. 1993). However, cytokines such as TNF-α and growth factors can also stimulate CK2 activity (Sayed et al. 2000) and T. parva-infected B cells proliferate in part due to a TNF autocrine loop (Guergnon et al. 2003a), implying that TNF (and potentially other cytokines) might contribute to the sustained CK2 activity of infected cells. Whatever the origins of CK2 induction in Theileria-infected leukocytes, the potential consequences of sustained CK2A over-expression were elegantly demonstrated in a transgenic mouse model (Seldin & Leder, 1995) and the similarity with Theileria-provoked lymphoproliferation has been remarked upon (ole-MoiYoi, 1995). What was striking was that adult transgenic mice, like Theileria-infected lymphocytes, displayed a propensity to develop lymphomas, but when this occurred in the context of c-myc over-expression the mice developed neonatal leukemia. This is particularly striking, since Theileria-infected B cells have just been described as having constitutively high levels of c-Myc (Dessauge et al. 2005). Thus, the co-induction of CK2 and c-Myc may be the major driving force behind Theileria-induced lymphocyte transformation.

Fig. 1. Structure of the tetrameric CK2. A. High-resolution crystal structure of the CK2 holoenzyme (Niefind et al. 2001) was obtained with Cn3D program to generate a ribbon diagram illustrating the CK2 tetramer. The high-resolution structure of tetrameric CK2 demonstrated that the non-hydrolysable ATP analogue adenosine 5′-[β,γ-imido]triphosphate (AMPPNP) is present in the ATP binding site of only one of the catalytic CK2A subunits within the CK2 tetramer. Anti-parallel β strands are illustrated in blue and alpha helix strands are illustrated in red. B). Schematic structure of tetrameric CK2 with the two catalytic CK2A subunits and the two regulatory CK2B subunits.
Here, we argue that in addition to synergy with c-Myc, CK2 could influence a number of other host cell pathways induced by Theileria, such as activation of the lipid inositol 3-kinase (Baumgartner et al. 2000; Heussler et al. 2001) and we illustrate this with some experimental evidence that supports a pivotal role for CK2 in Theileria-induced lymphocyte transformation.
As stated, transgenic mice that over-express CK2a develop lymphomas particularly when co-expressed with a c-myc transgene and pharmacologic inhibition of CK2 activity in the T cell lymphomas reduced both their proliferation and the levels of c-Myc protein (Channavajhala & Seldin, 2002). This is due to proteasome-dependent accelerated turnover of c-Myc protein and inhibition of CK2 transcription with anti-sense CK2 constructs modulated c-Myc protein levels (Channavajhala & Seldin, 2002). Similarly, in T. parva-transformed bovine B cells pharmacological inhibition of CK2 with apigenin also reduced c-Myc activity (Dessauge et al. 2005) and over-expression of a kinase-dead mutant of CK2 also reduces c-myc-driven transcription (Fig. 2). In contrast, over-expression of active wild-type CK2 kinase stabilizes c-Myc and results in enhanced c-Myc-driven luciferase activity (Fig. 2). Thus in Theileria-infected B lymphocytes heightened CK2 kinase activity contributes to lymphocyte transformation via its ability to prolong the half-life of c-Myc by reducing degradation of the transcription factor by the proteasome.

Fig. 2. CK2 over-expression augments c-Myc transactivation in T. parva-infected B cells. T. parva-infected B cells have constitutive c-Myc activity (c-myc) compared to a control minimal promoter (pGL2) (Dessauge et al. 2005). C-Myc-driven luciferase activity is increased upon co-expression with wild type CK2 kinase (c-myc+CK2 wt) and is decreased when co-expressed with a kinase-dead mutant of CK2 (c-myc+CK2 mt). T. parva-infected B cells were transfected with a c-myc-luciferase reporter plasmid and constructs expressing wild type and kinase-dead CK2 (Penner et al. 1997) and luciferase activity estimated as described (Dessauge et al. 2005).
The c-Myc transcription factor in Theileria-infected B cells mediates its survival role via induction of the anti-apoptotic protein Mcl-1 (Dessauge et al. 2005). c-Myc however, can only bind to its specific sites called E-boxes (5′-CACGTG-3′) in the promoters of target genes with the help of a second factor called Max (Dang et al. 1999). During Fas-induced apoptosis Max is dephosphorylated and subsequently cleaved by caspase-5 and caspase-7, respectively (Krippner-Heidenreich et al. 2001). Thus, in Theileria-infected lymphocytes loss of Max due to caspase cleavage would prevent c-Myc from binding to the Mcl-1 promoter aggravating cell death. However, cleavage by caspase-5 is inhibited by CK2-mediated phosphorylation of Max (Krippner-Heidenreich et al. 2001). So, up-regulation of CK2 in Theileria-infected lymphocytes (ole-MoiYoi et al. 1993) not only slows down c-Myc degradation, but it could also prevent caspase cleavage of Max and combined these two events would assure sustained transcription of Mcl-1 and lymphocyte survival (Dessauge et al. 2005).
NF-κB is a transcription factor that is activated in response to proinflammatory stimuli, physical stress and in many infections, including those by Theileria, where it mediates a survival response (Heussler et al. 1999). Activation of NF-κB by many stimuli depends on the IκB kinase (IKK) complex, which phosphorylates IκB (Inhibitor of κB) at N-terminal sites provoking its degradation and in T. parva-infected T cells there is continuous phosphorylation and degradation of IκB (Palmer et al. 1997). Interestingly, Theileria hijacks IKK by sequestering it into a complex associated with the parasite surface (Heussler et al. 2002). In UV-induced, NF-κB activation phosphorylation of IκB occurs at a cluster of C-terminal sites that are recognized by CK2 (Kato et al. 2003) and CK2 has long been known to phosphorylate IκB (Barroga et al. 1995). Thus, it is possible that the high CK2 activity in T. parva-infected lymphocytes contributes to IκB phosphorylation, NF-κB activation and lymphocyte survival. To test this hypothesis, we transiently transfected T. parva-infected B cells with wild type and kinase-dead mutants of CK2, together with a luciferase expression construct under the control of NF-κB (Fig. 3). As a control, we independently transfected a phosphorylation resistant mutant of IκB (IBm) to demonstrate that reduced degradation of IκB by the proteasome leads to NF-κB inhibition (Van Antwerp and Verma, 1996). In T. parva-infected B cells, over-expression of a kinase-dead mutant of CK2 reduced NF-κB activity by 50%, consistent with a role for CK2 in Theileria-dependent NF-κB activation (Fig. 3). Over-expression of active CK2 kinase did not augment the NF-κB activity, most likely due to all the CK2 sensitive sites in IκB being already maximally phosphorylated by the high endogenous CK2 activity of Theileria-infected lymphocytes (ole-MoiYoi et al. 1993). Thus, CK2 contributes to NF-κB activation by promoting proteasome degradation of IκB and indeed, there could be a positive feedback loop, with activated NF-κB increasing CK2a transcription.

Fig. 3. CK2 contributes to NF-κB activation in T. parva-infected B cells. Similar to T. parva-infected T cells (Heussler et al. 1999), T. parva-infected B cells have constitutively high NF-κB-driven transcription (NF-κB-luc). Over-expression of a phosphorylation resistant mutant of IκB (Van Antwerp & Verma, 1996) decreases NF-κB activity (NF-κB-luc+IκBmt) and over-expression of kinase-dead CK2 also reduces NF-κB levels (NF-κB-luc+CK2mt). Over-expression of wild type CK2 kinase does not increase NF-κB (NF-κB-luc+CK2wt) most likely due to all CK2 sensitive sites being phosphorylated. All transfections were performed as described (Dessauge et al. 2005).
The effect of CK2 on c-Myc induction in Theileria-infected lymphocytes could also be operating at the transcription level, as NF-κB is known to contribute to c-myc transcription in other cell types (Grumont, Strrasser & Gerondakis, 2002). To validate this notion we co-transfected T. parva-infected B cells with the c-myc-luciferase reporter construct as previous described (Dessauge et al. 2005) and IκBm that resists proteasome degradation (Fig. 4). As a control, we again measured the ability of IκBm to inhibit NF-κB. One can see that IκBm induced inhibition in NF-κB results in a significant reduction in c-Myc activity. Thus, in T. parva-infected B cells NF-κB acts together with STAT3 (Dessauge et al. 2005) to induce c-myc transcription and CK2 is involved in augmenting c-Myc both at the transcriptional (via NF-κB) and post-translational (via inhibition of proteasome degradation) levels.

Fig. 4. Over-expression of IκB leads to a significant drop in NF-κB and a partial drop in c-Myc-driven luciferase activity. T. parva-infected B cells have constitutively high levels of both NF-κB- (NF-κB) and c-Myc- (c-myc) driven luciferase activities when compared to minimal promoter (pGL2). Over-expression of mutant IκB leads to a significant reduction in NF-κB-driven (NF-κB+IκBmt) and c-Myc-driven (c-myc-luc+IκBmt) luciferase activities demonstrating that NF-κB contributes to transcription of c-myc. All transfections were performed as described (Dessauge et al. 2005).
The PTEN tumour suppressor gene encodes a phosphatidylinositol 3′-phosphatase that is inactivated in a high percentage of human tumours and its inactivation leads to constitutive phosphatidylinositol 3′-kinase (PI3-K) activity. Theileria-infected lymphocytes are characterized by high constitutive levels of PI3-K (Baumgartner et al. 2000; Heussler et al. 2001) suggestive of inactivation of PTEN. However, upon drug-induced parasite death PI3-K activity drops implying that PTEN is not permanently inactivated by mutation. Previous studies in other cellular systems have shown that Ser(370), Ser(385) and Thr(366) of PTEN are phosphorylated by CK2 and their phosphorylation inhibits PTEN activity towards PIP3 produced by PI3-K (Miller et al. 2002).
PTEN can also be inactivated by caspase-3 cleavage at several sites located within the C terminus of the molecule and this increased when cells are stimulated with TNF-α, but caspase-3 cleavage is negatively regulated by phosphorylation of the C-terminal tail of PTEN by the protein kinase CK2 (Torres et al. 2003). This probably occurs in T. parva-infected B cells, as PTEN appears intact (see Fig. 5), even though they use a TNF-autocrine loop for proliferation (Guergnon et al. 2003a). The high CK2 activity of Theileria-infected lymphocytes therefore, appears to counterbalance any TNF-induced, caspase-3-mediated cleavage of PTEN. Thus, CK2 has both positive (phosphorylation blocking caspase 3 cleavage) and negative (phosphorylation inhibiting PTEN phosphates activity) effects on PTEN, the overall balance in Theileria-infected cells leading to a loss of phosphatase activity that results in the permanent PI3-K induction that characterizes both infected B and T cells (Baumgartner et al. 2000; Heussler et al. 2001).

Fig. 5. Pharmacological inhibition of CK2 by Apigenin leads to loss of PTEN phosphorylation. Infected B cells were treated for 6 hours with increasing doses of Apigenin (from 0 to 100 μM), as described in (Dessauge et al. 2005). Inhibition of CK2 ablates PTEN phosphorylation without effecting PTEN levels.
Interestingly, Pten heterozygous (Pten+/−) mutant mice that have reduced phosphatase activity develop a lethal polyclonal autoimmune disorder (homozygous Pten−/− mice are embryonic lethal) with features reminiscent of those observed in Fas-deficient mice (Di Cristofano et al. 1999). Fas-mediated apoptosis is impaired in Pten+/− mice and T cells show reduced activation-induced cell death and increased proliferation upon activation and inhibition of PI3-K restored Fas responsiveness in Pten+/− cells. In this context, it is worth pointing out that T. parva-infected T cells also show resistance to Fas-mediated apoptosis (Kuenzi, Schneider & Dobbelaene, 2003) and this observation is entirely consistent with the notion of CK2-mediated inhibition of PTEN.
As stated, T. parva-infected B cells use a TNF autocrine loop to proliferate and inhibition of TNF did not generate apoptosis (Guergnon et al. 2003a). Usually TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis via the death receptors DR4 and DR5 in transformed cells and inhibition of CK2 results in sensitization of tumour cells to TRAIL-induced apoptosis (Hilgard et al. 2004). Analysis of the death-inducing signaling complex (DISC) demonstrated that CK2 activation diminishes recruitment of procaspase-8 to the DISC and decreases degradation of X-linked inhibitor of apoptosis protein (XIAP). In T. parva-infected T cells XIAP is constitutively expressed and upon drug-induced parasite death XIAP levels drop (Kuenzi et al. 2003). This could be interpreted as being due to loss of CK2 activity that also decreases upon parasite death (ole-MoiYoi et al. 1993). In addition, resistance to TRAIL-mediated apoptosis in tumour cells is associated with a high Bcl-x(L)/tBID ratio and failure to activate caspase-9 (Ravi & Bedi, 2002). In T. parva-infected B cells and CD8+ T cells drug-induced parasite death leads to induction of caspase 9 and an increase in pro-apoptotic Bax, compared to anti-apoptotic Bcl-2 (Guergnon et al. 2003b). So, to determine whether, in infected T cells or B cells, parasite-dependent CK2-mediated phosphorylation appears to modulate caspase activity and to gain support for this idea we measured the activity of caspase 3 in T. parva-infected B cells that had been treated with increasing doses of two different CK2 inhibitors (Fig. 6). One can observe that even in the presence of live parasites inhibition of CK2 leads to induction of caspase 3. So, CK2 plays an anti-apoptotic role not only via induction of NF-κB and c-Myc, but also via suppression of caspase activation.

Fig. 6. Activation of caspase 3 increases following inhibition of CK2. Infected B cells were treated for 6 hours with increasing doses of Apigenin and Emodin (from 0 to 100 μM) and caspase-3 activity generated following cleavage of pro-caspase 3 was assayed by measuring cleavage of DEVD-AMC, as described in (Dessauge et al. 2005).
The dynamic process of signal transduction involves the concerted action of both protein kinases and protein phosphatases. Among serine/threonine phosphatases, PP1 and PP2A are key regulators of protein phosphorylation being responsible for more than 90% of protein dephosphorylation. PP2A is the predominant phosphatase activity detected in T. parva-infected B cells, whereas PP1 is specifically associated with the parasite (Cayla et al. 2000). Interestingly, PP2A can be activated by interaction with the alpha subunit of CK2 (Heriche et al. 1997) and activation of the phosphatase resulted in deactivation of mitogen-activated protein kinase kinase (MEK). Thus, CK2A acts as a stimulatory PP2A subunit, in contrast to the small t antigen of SV40 virus that behaves as an inhibitory subunit of PP2A (Sontag et al. 1993). In direct contrast to PP2A stimulation by CK2A, inhibition of the phosphatase by SV40 small t antigen leads to constitutive activation of the MEK/ERK pathways in virus-transformed cells (Sontag et al. 1993). In Theileria-infected lymphocytes the MEK/ERK pathway is constitutively switched off, consistent with CK2A stimulation of PP2A (Chaussepied & Langsley, 1996; Galley et al. 1997; Chaussepied et al. 1998). Therefore, it is attractive to imagine that in Theileria-infected lymphocytes there is an association between CK2A and PP2A that activates the phosphatase and leads to permanent dephosphorylation of MEK/ERK.
The MEK/ERK pathway may not be the only one down-regulated by a putative association between CK2A and PP2A, as P22A has also been described to function as a ser473 phosphatase of Akt/PKB (Liu et al. 2003) and lack of ser473 phosphorylation of Akt is observed in T. parva-infected B cells (Baumgartner et al. 2000). Therefore, the putative PP2A/CK2A phosphatase might be responsible for both lack of ERK activity and poor Akt/PKB activity associated by T. parva-infected B cells.
Activation of CK2 was one of the first observations made on how Theileria parasites manipulate host cell signal transduction pathways (ole-MoiYoi et al. 1993). In this review, we argue that CK2 induction may in fact contribute to many of the different activation events that have been described since 1993 for Theileria-infected lymphocytes and as such this makes CK2 a central player in lymphocyte transformation, a position that we have resumed graphically in Fig. 7. Most of our examples concern phosphorylation by CK2 and it can lead to sustained activation of transcription factors c-Myc (Dessauge et al. 2005) and NF-κB (Heussler et al. 1999), but by slightly different mechanisms. In the case of c-Myc, CK2 phosphorylation of a PEST sequence at the C-terminus reduces proteasome-mediated degradation of the transcription factor (Channavajhala & Seldin, 2002) and results in more robust transactivation of c-Myc-mediated transcription (Fig. 2). In the case of NF-κB, it is the CK2 phosphorylation of I-κB (Barroga et al. 1995) that promotes proteasome degradation of the inhibitor of NF-κB and enhanced NF-κB-mediated transcription (Fig. 3). Moreover, since diminished degradation of IκB also contributes to c-Myc induction via NF-κB activation (Fig. 4), CK2 increases c-Myc induction both directly and indirectly. As both c-Myc and NF-κB mediate anti-apoptotic responses of Theileria-infected lymphocytes, CK2 activation contributes to survival of infected host cells.

Fig. 7. Scheme showing central role that CK2 could play in Theileria-induced lymphocyte transformation. CK2 could dampen TNF/Fas death signalling by blocking caspase 8 cleavage of Bid and the release of the apoptosome from mitochondria. CK2 phosphorylation accelerates IκB degradation by the proteasome and hence augments NF-κB activation. CK2 phosphorylation ablates c-Myc degradation by the proteasome. CK2 phosphorylation of PTEN inhibits its lipid phosphatase activity and maintains PI3-K activity at high levels. CK2 could interact with PP2A and stimulate the protein phosphatase to dephosphorylate BAD and Akt. A combination of increased transcriptional transaction of NF-κB and c-Myc, combined with inhibition of apoptosis allows pro-survival signalling to dominate in Theileria-infected lymphocytes.
CK2 also contributes to infected lymphocyte survival by inhibiting caspase activation (Fig. 6) and this probably explains the resistance of T. parva-infected T cells to Fas-mediated apoptosis (Kuenzi et al. 2003). CK2 activation diminishes both recruitment of procaspase-8 to the DISC and decreases degradation of survival protein XIAP (Hilgard et al. 2004). CK2 activation could also explain why inhibition of TNF does not lead to apoptosis of T. parva-infected B cells (Guergnon et al. 2003a), via its role in dampening TRAIL/Apo2-mediated apoptosis (Ravi & Bedi, 2002; Farah et al. 2003).
CK2 is probably behind constitutive PI3-K activation that although this does not mediate an anti-apoptotic response, since it does not signal to NF-κB (Heussler et al. 2001), it does contribute to proliferation and AP-1 induction (Baumgartner et al. 2000). CK2 does this by phosphorylating PTEN (Fig. 5) an event that leads to a reduction in its lipid phosphatase activity and higher PIP3 levels, where PIP3 is the product of PI3-K (Miller et al. 2002). Permanently high PIP3 levels could explain how PI3-K contributes to proliferation, AP-1 and GM-CSF induction in T. parva-infected B cells (Baumgartner et al. 2000).
Finally, we give an example of where CK2A may be acting not only as a kinase, but also as a stimulatory subunit for the protein phosphatase PP2A (Heriche et al. 1997). In T. parva-infected B cells PP2A is the major protein phosphatase activity (Cayla et al. 2000) and CK2A activated PP2A could explain the lack MEK/ERK (Galley et al. 1997; Chaussepied et al. 1998) and reduced Akt/PKB activities associated with infected B cells (Baumgartner et al. 2000). Dampening down the MEK/ERK and Akt/PKB pathways may be as essential to successful lymphocyte survival as activating the c-Myc and NF-κB pathways and that is why we propose CK2 as a central player in Theileria-induced lymphocytes transformation.
We thank Volker Heussler for critical comment and David Litchfield for the gift of the wild type and mutants CK2 kinase constructs and Inder Verma for the phosphorylation resistant mutant of IκB. We acknowledge the support of the CNRS and INSERM.

Fig. 1. Structure of the tetrameric CK2. A. High-resolution crystal structure of the CK2 holoenzyme (Niefind et al. 2001) was obtained with Cn3D program to generate a ribbon diagram illustrating the CK2 tetramer. The high-resolution structure of tetrameric CK2 demonstrated that the non-hydrolysable ATP analogue adenosine 5′-[β,γ-imido]triphosphate (AMPPNP) is present in the ATP binding site of only one of the catalytic CK2A subunits within the CK2 tetramer. Anti-parallel β strands are illustrated in blue and alpha helix strands are illustrated in red. B). Schematic structure of tetrameric CK2 with the two catalytic CK2A subunits and the two regulatory CK2B subunits.
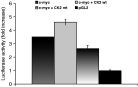
Fig. 2. CK2 over-expression augments c-Myc transactivation in T. parva-infected B cells. T. parva-infected B cells have constitutive c-Myc activity (c-myc) compared to a control minimal promoter (pGL2) (Dessauge et al. 2005). C-Myc-driven luciferase activity is increased upon co-expression with wild type CK2 kinase (c-myc+CK2 wt) and is decreased when co-expressed with a kinase-dead mutant of CK2 (c-myc+CK2 mt). T. parva-infected B cells were transfected with a c-myc-luciferase reporter plasmid and constructs expressing wild type and kinase-dead CK2 (Penner et al. 1997) and luciferase activity estimated as described (Dessauge et al. 2005).

Fig. 3. CK2 contributes to NF-κB activation in T. parva-infected B cells. Similar to T. parva-infected T cells (Heussler et al. 1999), T. parva-infected B cells have constitutively high NF-κB-driven transcription (NF-κB-luc). Over-expression of a phosphorylation resistant mutant of IκB (Van Antwerp & Verma, 1996) decreases NF-κB activity (NF-κB-luc+IκBmt) and over-expression of kinase-dead CK2 also reduces NF-κB levels (NF-κB-luc+CK2mt). Over-expression of wild type CK2 kinase does not increase NF-κB (NF-κB-luc+CK2wt) most likely due to all CK2 sensitive sites being phosphorylated. All transfections were performed as described (Dessauge et al. 2005).

Fig. 4. Over-expression of IκB leads to a significant drop in NF-κB and a partial drop in c-Myc-driven luciferase activity. T. parva-infected B cells have constitutively high levels of both NF-κB- (NF-κB) and c-Myc- (c-myc) driven luciferase activities when compared to minimal promoter (pGL2). Over-expression of mutant IκB leads to a significant reduction in NF-κB-driven (NF-κB+IκBmt) and c-Myc-driven (c-myc-luc+IκBmt) luciferase activities demonstrating that NF-κB contributes to transcription of c-myc. All transfections were performed as described (Dessauge et al. 2005).

Fig. 5. Pharmacological inhibition of CK2 by Apigenin leads to loss of PTEN phosphorylation. Infected B cells were treated for 6 hours with increasing doses of Apigenin (from 0 to 100 μM), as described in (Dessauge et al. 2005). Inhibition of CK2 ablates PTEN phosphorylation without effecting PTEN levels.
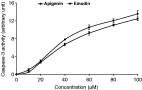
Fig. 6. Activation of caspase 3 increases following inhibition of CK2. Infected B cells were treated for 6 hours with increasing doses of Apigenin and Emodin (from 0 to 100 μM) and caspase-3 activity generated following cleavage of pro-caspase 3 was assayed by measuring cleavage of DEVD-AMC, as described in (Dessauge et al. 2005).

Fig. 7. Scheme showing central role that CK2 could play in Theileria-induced lymphocyte transformation. CK2 could dampen TNF/Fas death signalling by blocking caspase 8 cleavage of Bid and the release of the apoptosome from mitochondria. CK2 phosphorylation accelerates IκB degradation by the proteasome and hence augments NF-κB activation. CK2 phosphorylation ablates c-Myc degradation by the proteasome. CK2 phosphorylation of PTEN inhibits its lipid phosphatase activity and maintains PI3-K activity at high levels. CK2 could interact with PP2A and stimulate the protein phosphatase to dephosphorylate BAD and Akt. A combination of increased transcriptional transaction of NF-κB and c-Myc, combined with inhibition of apoptosis allows pro-survival signalling to dominate in Theileria-infected lymphocytes.