INTRODUCTION
Several parasitic nematodes have been reported in fish, but very few are responsible for seafood-associated infections in humans. Among the species responsible for the most frequent infections in humans are Anisakis simplex and Pseudoterranova decipiens (Adams et al. Reference Adams, Murrell and Cross1997; Butt et al. Reference Butt, Aldridge and Sanders2004; Karl, Reference Karl2008). Human anisakidosis is in most cases caused by eating raw or undercooked fish infected with third-stage larvae (L3) of A. simplex (anisakiasis) or P. decipiens (pseudoterranovosis) (Kassai et al. Reference Kassai, Delcampillo, Euzeby, Gaafar, Hiepe and Himonas1988; Audicana et al. Reference Audicana, Del Pozo, Iglesias, Ubeira, Milliotis and Bier2003). These parasites frequently cause acute gastric pain upon consumption of raw fish (Margolis, Reference Margolis1977), and can also cause allergic symptoms even after consumption of properly cooked fish (Audicana et al. Reference Audicana, Ansotegui, De Corres and Kennedy2002). Both species have marine mammals as their definitive host and a wide variety of fish may serve as primary and secondary hosts. Atlantic cod (Gadus morhua, L.), an example of the latter, is among the most commercially important species in the North Atlantic.
Despite the research efforts aimed at the development of non-destructive techniques for automatic detection of nematodes in whitefish in later decades (Arnarson et al. Reference Arnarson, Bengoetxea, Pau, Pau and Olafsson1991; Heia et al. Reference Heia, Lauritzen, Nilsen, Wold and Wedberg1997), detection and removal of nematodes are presently done by visual inspection on a light table (candling). This method is labour intensive and lacks the detection efficiency to render raw fish products safe for human consumption (Valdimarsson et al. Reference Valdimarsson, Einarsson and King1985; Bublitz and Choudhury, Reference Bublitz and Choudhury1992). Adequate freezing of fish and subsequent frozen storage have become the most important measure to control this potential health hazard. However, studies have shown that anisakid nematodes such as A. simplex and P. decipiens may survive in fish that have been stored frozen. This has brought to attention the importance of the freezing process and the length of the period of frozen storage (Gustafson, Reference Gustafson1953; Deardorff and Throm, Reference Deardorff and Throm1988).
Too little is known about the basic biology of these marine nematodes′ parasitic stages to understand the mechanisms by which they cope with a freezing environment. Available literature has focused on terrestrial nematodes experiencing subzero temperatures. The 3 main strategies of cold tolerance in nematodes in general are: (1) freeze avoidance, a supercooling strategy where the body fluid is protected from exogenous ice nucleation by a protective sheath and the nematode supercools in contact with external ice, (2) freeze tolerance, which makes the nematodes able to cope with ice formation in their bodies, and (3) cryoprotective dehydration, a strategy in which a supercooled nematode loses water when exposed to an icy environment (Wharton, Reference Wharton1995, Reference Wharton2003).
P. decipiens have a very similar life cycle compared to that of A. simplex. Whereas the definitive hosts differ between P. decipiens (seals and sea lions) and A. simplex (whales, dolphins and porpoises), they share several of the intermediate fish hosts that may transfer the parasites to humans. Variations in distribution and degree of parasite infection may differ between the species. In cod, P. decipiens L3 infects the whole fish muscle whereas A. simplex L3 are mainly encapsulated on the visceral organs and in the belly flaps (McClelland et al. Reference McClelland, Misra and Marcogliese1983; Jensen and Idas, Reference Jensen and Idas1992). Wharton and Aalders (Reference Wharton and Aalders2002) showed that A. simplex L3 have the ability to survive freezing, even if this species is not exposed to freezing in nature. For parasites embedded in the fish muscle which has been frozen, the probability of surviving the freezing process has to some extent been attributed to distribution in the fish. Increased survival is observed for parasites embedded in the thickest part of the muscle because of the longer freezing period needed for the surrounding fish muscle to reach lethal temperature (Deardorff and Throm, Reference Deardorff and Throm1988). As P. decipiens is the species predominantly found in fish muscle, the existence of cold tolerance features for its third stage larvae is therefore of interest.
The main objective of this study was to examine whether third-stage larvae of P. decipiens display cold tolerance. To do so, we investigated the nematodes' ability to supercool, both with and without a surrounding medium. In organisms that are able to prepare for cold conditions (cold hardening), a period of low temperature will induce physiological changes that increase the ability to survive freezing. In order to identify the occurrence of such adaptations, we investigated the influence of cold acclimation on (1) the supercooling point, (2) accumulation of the potential cryoprotectant trehalose and (3) changes in osmolality of the pseudocoelomic fluid.
MATERIALS AND METHODS
Parasite materials and acclimation
P. decipiens L3 were obtained from heavily infected Atlantic cod from a fish producer outside Tromsø, Norway. The fish were kept in a cold storage room (4°C) after capture and used within 4 days.
Cod fillets most heavily infected with P. decipiens L3 were attained from our local supplier and the parasites were collected from the muscle by the use of pepsin digestion (1 g pepsin per 200 g fillet) in a 1% salt solution (Instant Ocean, Aquarium Systems, Ohio, USA). Hydrochloric acid was used to maintain the pH in the range of 2–3 for approximately 24 h at room temperature (20°C). The parasites were subjectively grouped by size (small, medium and large) and kept in 1% IO (Instant Ocean) at room temperature for 2 days prior to acclimation.
Acclimation
Only viable nematodes (showing movement at room temperature) were used in the experiment. P. decipiens L3 were placed in 50 ml plastic tubes and cold acclimation at 3°C for 3 days in 45 ml of 1% IO prior to differential scanning calorimetry (DSC) and osmolality measurements. Nematodes kept at room temperature (20°C) served as control. A portion (~20 ml) of the IO medium was renewed every 24 h.
For the trehalose quantification nematodes were divided into 4 groups, in which 1 group served as control (20°C). Another group of nematodes were cold acclimated (3°C), whereas 2 were heat acclimated at 37°C and 45°C. Every temperature group consisted of more than 100 individuals and was acclimated for 3 days. The P. decipiens L3 were pooled according to temperature acclimation, then immersed in liquid nitrogen and freeze-dried prior to homogenization in a mortar.
Osmolality measurements
Pseudocoelomic fluid was collected from individual nematodes following the 3-day cold acclimation (3°C) and control temperature (20°C). The nematodes were submersed in water-free paraffin oil, and a hole was pierced into the cuticle using a sapphire glass scalpel (World Precision Instruments, Sarasota, FL, USA). The fluid in the pseudocoelom was then collected by an oil-filled capillary micropipette and loaded directly into the oil-filled wells of the sample holder in a Clifton Nanolitre Osmometer cryoscope (Clifton Technical Physics, Hartford, NY, USA) for determination of osmolality. The sample size was approximately one-third of the well size. At least 3 replicate samples were loaded from each individual nematode together with a 290 mOsm standard solution (Wescor Inc., Logan, UT, USA). The samples were then quickly (<15 sec) cooled to −40°C and the temperature raised at a rate of 0·10°C min−1 until the last ice crystal disappeared. This was taken to be the melting point. The melting point was determined 4 times for each individual and corrected against the measured osmolality of a standard solution. The true zero, i.e. the osmolality of double-distilled water, was also determined and found not to vary more than the given accuracy of the osmometer. A sample of the acclimation media was also measured.
To estimate whether cold acclimation or individual nematode size had any effect on the osmolality of the pseudocoelomic fluid, the measurements were tested through one-way ANOVA using a significance level of P⩽0·05. Values are given as mean±s.e.m.
Differential scanning calorimetry measurements
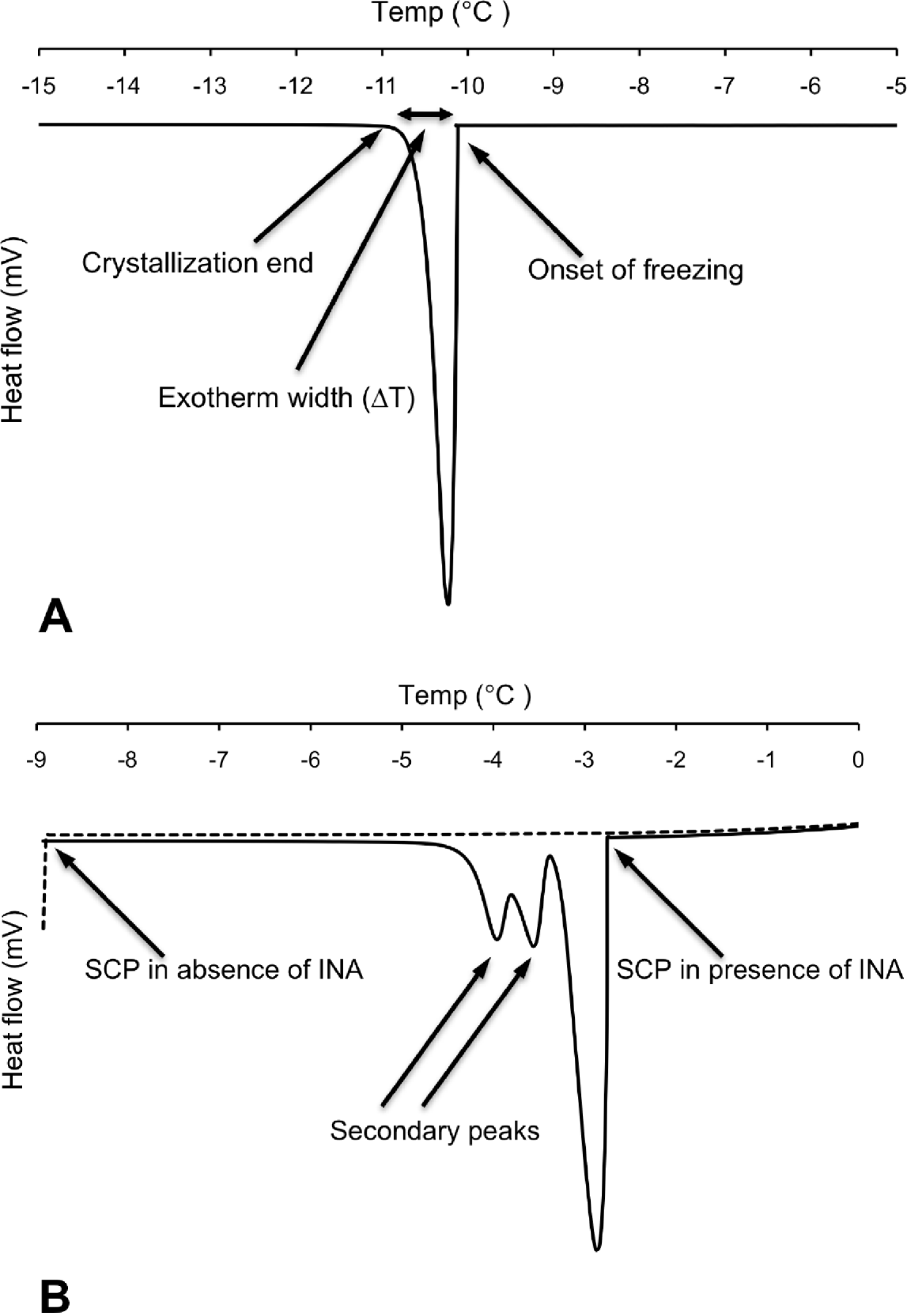
Differential scanning calorimetry (DSC) measurements were carried out on individual nematodes in the temperature interval from 5 to −25°C using a Perkin Elmer Pyris 1 DSC differential scanning calorimeter (Perkin Elmer Analytical Instruments, Norwalk, Connecticut, USA) and Pyris software for Windows NT. DSC runs were performed in an atmosphere of flowing (20 ml min−1) dry nitrogen gas of high purity (99·999%). Surface water was removed from the nematode to prevent inoculative freezing by placing the nematode in a cell strainer (nylon filter) on top of a soft paper cloth. The sample was sealed using standard aluminium sample pans, and an empty pan was used as reference. The DSC was calibrated with water and indium. Tests running several cooling scans using the same sample (P. decipiens L3) showed that thermal runs with a scan rate of −10°C min−1 did not influence the supercooling points (SCP), but the standard deviation of the SCP were higher than ±1·5°C. Scan rates of −5°C min−1 or slower resulted in a decreased standard deviation of the SCP (S.D.<±0·15°C). Thus, to ensure accuracy of the measured variables, the DSC-experiments were carried out using a scan rate of −2°C min−1. Samples were weighed prior to the DSC run. After the DSC run was completed, the top of the aluminium pan was punctured and the sample was freeze dried for 48 h, re-weighed, and the dry mass and total water content (Wtot) calculated. The supercooling point was taken as the lowest temperature reached prior to the release of the latent heat of fusion caused by the freezing of the body water. The amount of frozen water was calculated based of the latent heat of fusion, which for water is 333·55 J g−1. The latent heat of fusion (mJ) released was obtained through integration of the peak area of the exotherm (Fig. 1A) and was assumed to originate from freezing of extracellular water (Wext) in the pseudocoelom. The amount of unfrozen water (Wliq), comprising mainly intracellular water but also pockets of water with high osmolality, was calculated as follows: Wliq=Wtot−Wext.
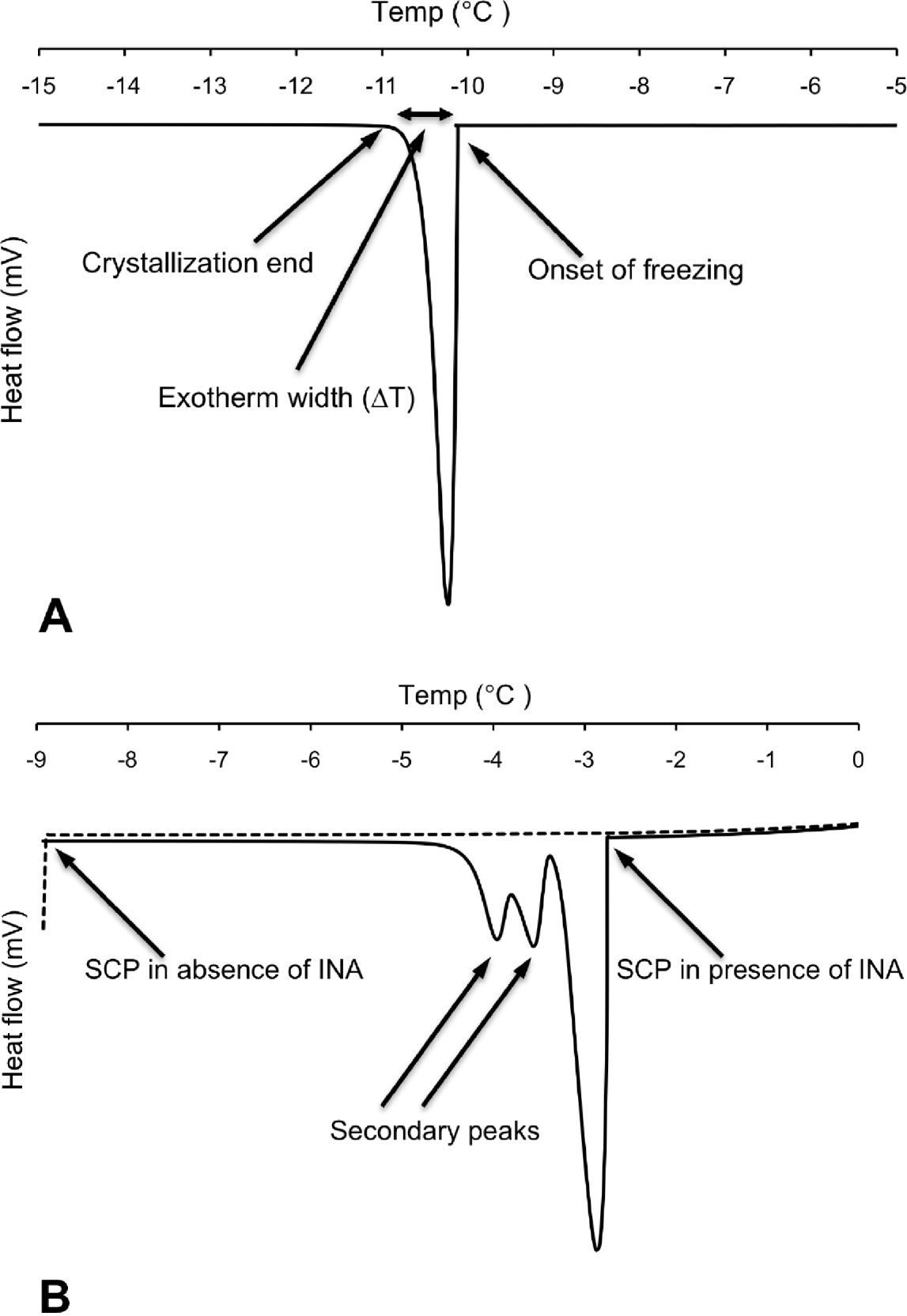
Fig. 1. (A) Differential scanning calorimetry (DSC) trace showing heat flow during cooling of a surface-dry third-stage larva of P. decipiens where onset of freezing occurred at its supercooling point (SCP). (B) Corresponding DSC trace of a different individual (surface-dry third-stage larva of P. decipiens) where cooling was stopped when SCP occurred (broken line). Solid line represents the thermal events observed when cooling was repeated using the same individual, but with added ice-nucleating agent (INA) solution.
The exotherm width was defined as the change in temperature (ΔT) from the onset of freezing (crystallization; Conset ) until no latent heat was detected, marking the end of the phase transition from liquid to solid (Cend). The exotherm width (ΔT=Cend−Conset) was acquired by measuring the exotherm peak width (°C) at the baseline of the DSC trace (Fig. 1A). Because the cooling scan rate was fixed for all the DSC runs (−2°C min−1), the exotherm width provides a relative measure of the speed of crystallization.
The variance within each group of variables was tested using F-tests. Pair-wise comparisons were tested for statistical significance at the P⩽0·05 level by two-tailed Student's t-tests. One-way ANOVAs were applied to determine the effects of cold acclimation, size of the individuals and Wliq on the SCP. The effect of size on the SCP was first tested for the 3 size groups using a univariate ANOVA applying a Tukey post-hoc test. The medium and large sized individuals showed non-significant difference in SCP and the two groups were pooled to increase statistical power in the subsequent analyses, giving size-groups small and large. To estimate the effects of weight, Wliq, and SCP on exotherm width (speed of crystallization), a step wise, leave-one-out, ANOVA was performed. In all comparisons and tests values of P⩽0·05 were considered significant. Values are given as mean±s.e.m.
Freezing and survival
Individual P. decipiens L3 were subjected to a thermal run (DSC) equivalent to the method described above, except for the scan rate, which was adjusted to −5°C min−1. At the onset of freezing the temperature was allowed to lower additionally −0·5°C to ensure that the nematodes froze. Next, this temperature (−0·5°C lower than the SCP) was held constant through different periods of time (0, 1, 5, 10, 20 and 60 min). After being frozen the nematodes were thawed and removed from the sample pans, transferred to 1% IO and incubated at 37°C for 18 h. P. decipiens L3 that moved spontaneously or moved continuously for more than 1 min in response to physical stimulation (needle) were considered to be alive. P. decipiens L3 normally exhibits vigorous movements at 37°C, thus the larvae that showed no movement or movement for only a brief period after stimulation were considered dead/moribund. A total of 30 P. decipiens L3 were used in the experiment (5 per time-period). Equivalent studies were done with P. decipiens L3 (N=30) using an ice-nucleating agent (INA) to facilitate inoculative freezing. A water solution containing Pseudomonas syringae (SnoMax, York Int., York, USA) was used as INA and 1 drop (~10 μl of 1 mg/ml) of solution was added to the sample pan along with the P. decipiens L3.
Trehalose extraction and quantification
Approximately 25 mg freeze-dried P. decipiens L3 material was added to 1 ml of 70% ethanol, vigorously vortexed and extracted in a shaker for 30 min at room temperature. Samples were then spun for 5 min at 10 000 g on a bench top centrifuge prior to HPLC analysis. Trehalose content was subsequently determined in the supernatant by quantitative HPLC analyses using a Waters 2695 Separations Module (Milford, MA, USA) equipped with an Eurosep DDL 31 evaporative light-scattering detector (Eurosep Instruments, Cergy St Christophe, France), and a Shodex Asahipak NH2P-50 4E column (250×4·6 mm id, Showa Denko, Tokyo, Japan). Trehalose was eluated using a 30 min linear gradient of 90–50% acetonitrile in water, at a flow rate of 1 ml min−1. The concentration of trehalose in the samples was calculated with a 4-point calibration curve with external standard in the range 10–1 μg trehalose.
RESULTS
DSC measurements and acclimation
Exothermic events were detected for all samples of P. decipiens L3 during cooling from 5 to −25°C. In all the thermograms of P. decipiens L3 a single exotherm were found (Fig. 1A). Cold acclimation at 3°C did not have any significant effect (P=0·52) on the SCP (−9·23±0·46°C, N=15; range −7·0 to −13·2°C) for P. decipiens L3 when compared to that of room temperature (−9·71±0·58°C, N=16; range −7·0 to −14·9°C). Neither percentual Wtot nor Wliq changed significantly through cold acclimation (data not shown).
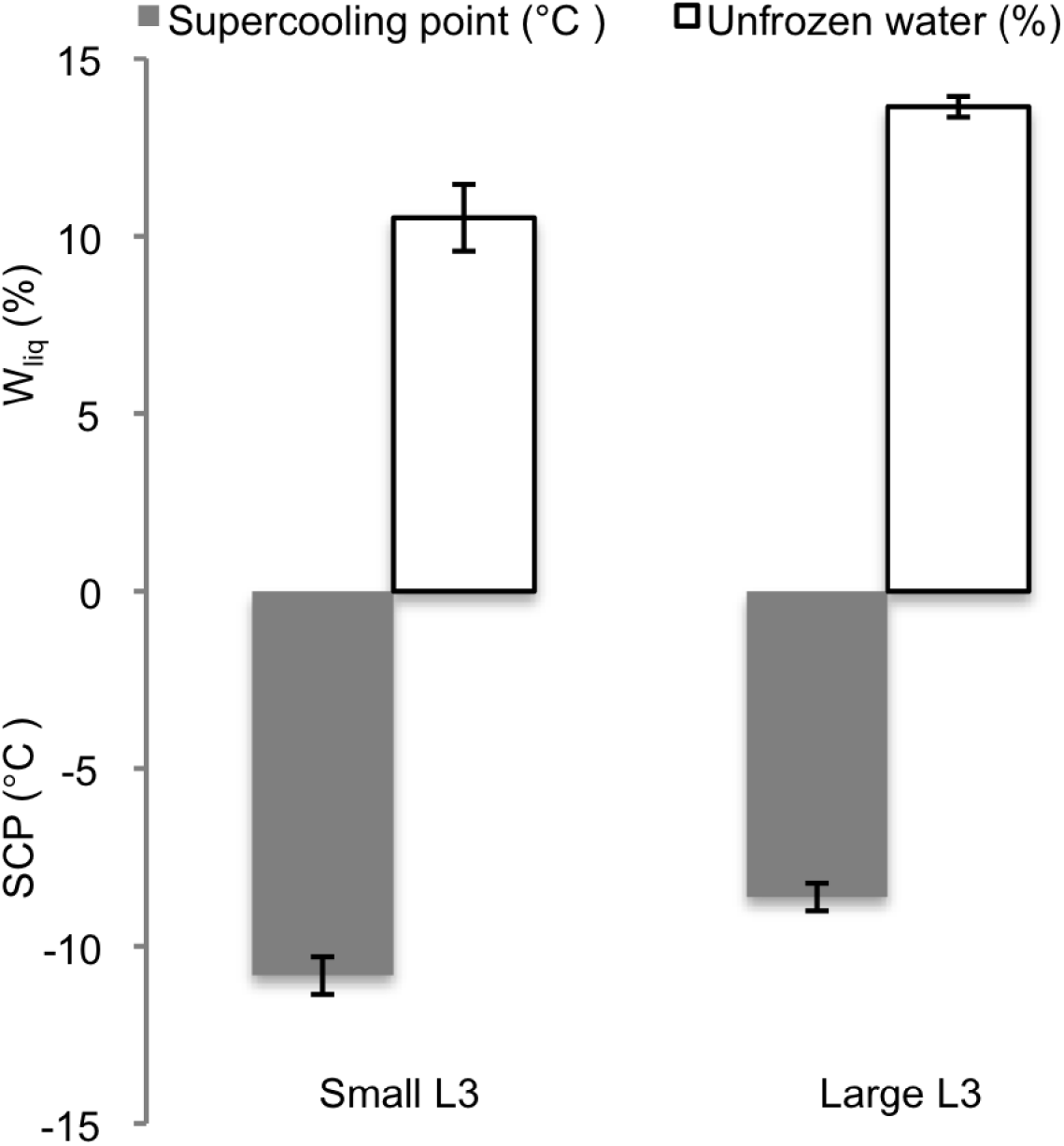
Pooling the SCP results in 2 groups according to size (small: 5·11±0·49 mg, N=12 and large: 15·93±2·04 mg, N=19) showed that differences in size between P. decipiens L3 of the two groups significantly affected the SCP (P=0·002) and the percentual Wliq (P=0·007) (Fig. 2). Total water content (percent Wtot) did not differ significantly between small and large nematodes (data not shown).

Fig. 2. Supercooling points (SCP) and content of unfrozen water (Wliq) in two pooled groups (small and large) of third-stage larvae of P. decipiens.
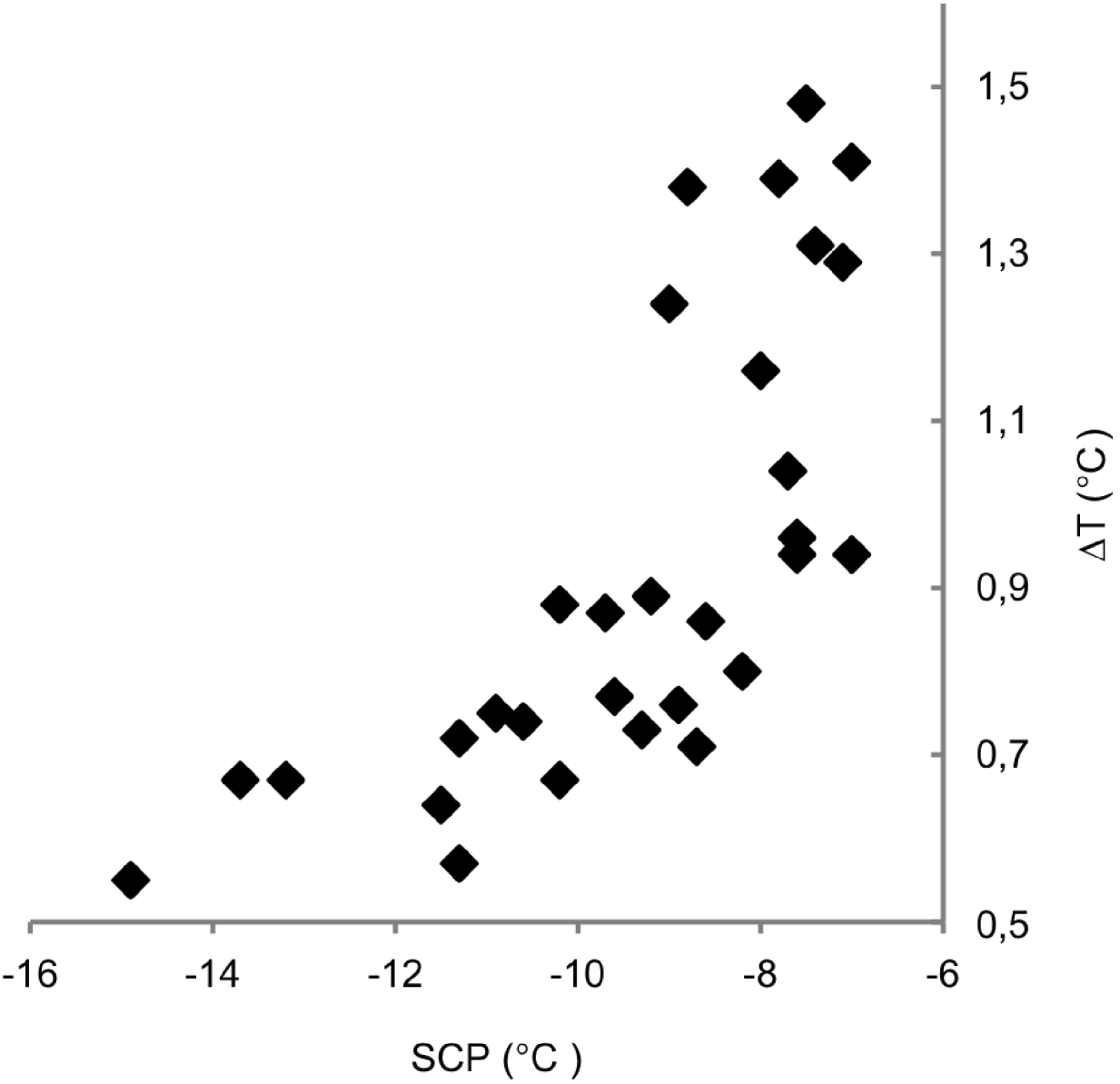
The exotherm width, i.e. the difference in temperature from the onset of freezing until no latent heat was detected, was found to be significantly (P<0·001) dependent on SCP (Fig. 3) but not on the size of the nematodes or their percentual Wliq.

Fig. 3. Plot showing supercooling points (SCP, x-axis) in relation to the exotherm width (ΔT, y-axis) measured as the peak width at the baseline of the differential scanning calorimetry trace.
Broader exotherms at higher temperatures with 1 or 2 secondary shoulder peaks were observed in samples where INA was added (Fig. 1B). For the first exotherm, resulting from the freezing of the added INA solution, the onset of freezing was observed at −3·00±0·10°C (N=5). This is close to the onset of freezing at −3·1±0·00°C (N=5), which was observed in tests using solely the INA solution. The 2 shoulder peaks show the freezing of the nematode, and the way in which freezing was inoculated in different parts of the larva at different temperatures.
Freezing and survival
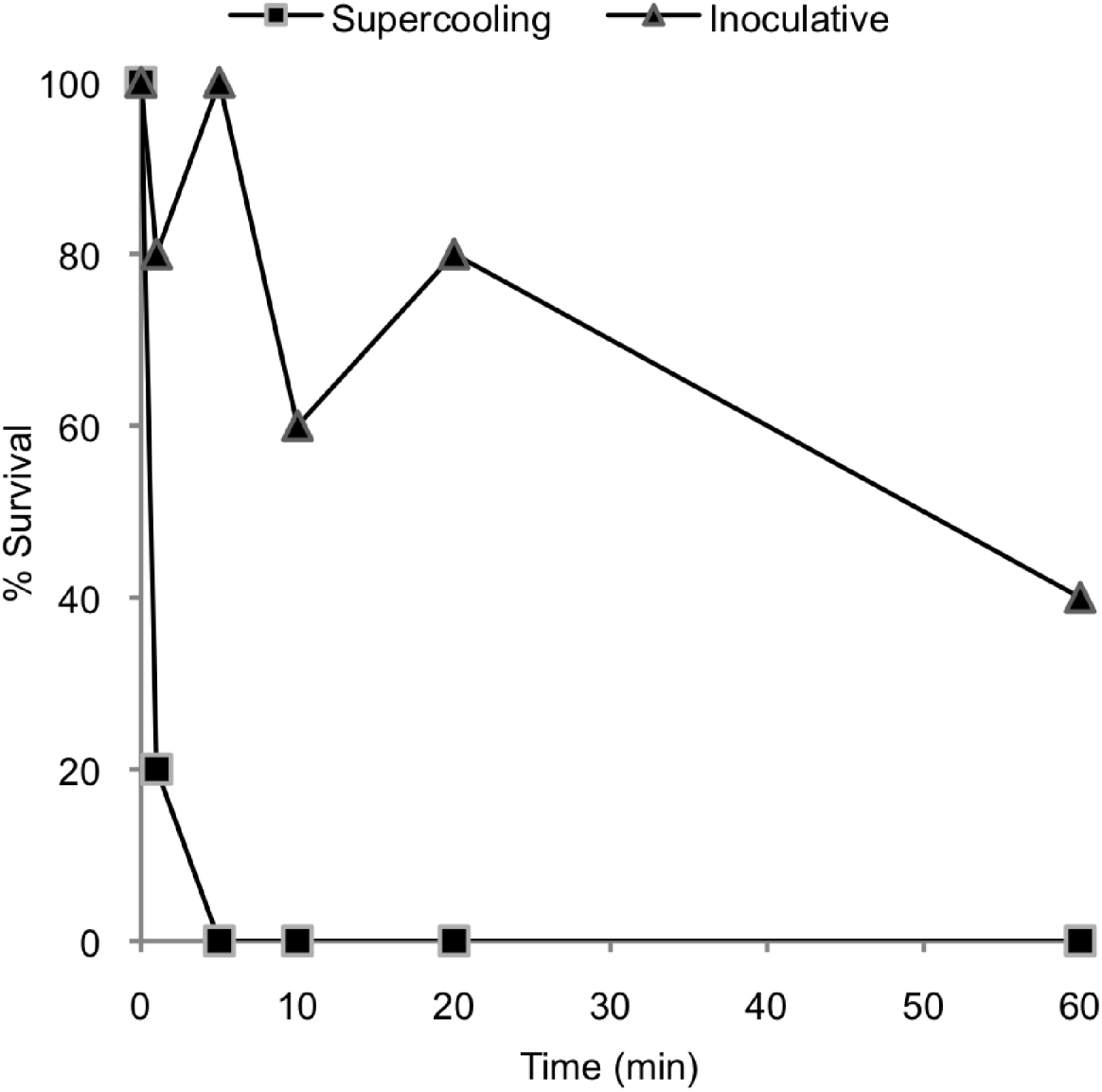
The P. decipiens L3 survived the onset of freezing independent of the temperature of the SCP when samples were thawed immediately after the freezing commenced. However, survival declined dramatically with time spent below the SCP. Survival was not observed when P. decipiens L3 were kept below the SCP for 5 min, and 80% of the L3 died after a period of 1 min below the SCP. The nematodes inoculated by INA, which froze at higher subzero temperatures, showed increased survival compared to the nematodes in which freezing occurred at their SCP (Fig. 4). For the former, even being in the frozen state for 5 min seemed to have little effect on survival, but survival had a falling trend on a 60 min time-span.

Fig. 4. Effect of the duration of freezing on the survival of third-stage larvae of P. decipiens when freezing occurred at the supercooling point and when freezing was inoculated by an ice-nucleating agent.
Effect of acclimation temperature on trehalose accumulation
Both warm and cold acclimation increased the trehalose level in P. decipiens L3 compared to the control kept at room temperature. The initial level of trehalose at room temperature was 8·4 μg mg−1 dry weight. Cold adapted (3°C) nematodes showed a trehalose level of 19·7 μg mg−1 dry weight (135% increase), whereas warmer regimes at 37°C and 45°C showed even higher levels of trehalose: 25·2 μg mg−1 (200% increase) and 48·3 μg mg−1 (475% increase) dry weight respectively. All P. decipiens L3 acclimated at 45°C were killed within 6 h.
Osmolality
The osmolality of the pseudocoelomic fluid was tested in individuals kept at 3°C (cold acclimation) and at 20°C (control). Both the cold acclimated P. decipiens L3 (316±7 mOsm) as well as the control group (296±13 mOsm) were significantly (20°C, P<0·05; 3°C, P<0·05) hyper-osmotic to the incubation media (237±3 mOsm). Significant differences in size were not observed between the 2 test groups (P=0·20). Neither cold acclimation (P=0·13) nor size (P=0·22) had any effect on the osmolality of the pseudocoelomic fluid when tested in ANOVA.
DISCUSSION
Third-stage larvae of P. decipiens are able to supercool, and variations in their SCPs are correlated with the size of the nematodes. It has been suggested that smaller nematodes display greater supercooling ability due to differences in feeding patterns between juveniles and adults, which in turn influences the level of ice-nucleators in the pseudocoelomic fluid (Pickup, Reference Pickup1990a, Reference Pickupb, Reference Pickupc). No difference in developmental stage is expected for P. decipiens used in this study, because they hatch as infective L3 before being transferred to the cod (Koie et al. Reference Koie, Berland and Burt1995) and do not develop to the adult stage outside mammals. Consequently, variations in the level of ice-nucleators due to differences in developmental stages do not explain the differences in SCP between small and large P. decipiens L3. A correlation between the size of the nematodes and the amount of water they contain is, however, apparent. According to the nucleation theory, supercooled water is metastable because ice-embryos need to reach a critical size to grow steadily. Ice-embryos that do not reach this threshold size easily dissolve. When the critical threshold size is reached, ice will spread rapidly in the supercooled water. For cylinders containing water it has been shown that water in large volume cylinders have higher SCP than water in cylinders of smaller volumes (Chen and Lee, Reference Chen and Lee1998; Chen and Chen, Reference Chen and Chen1999). This is mainly due to higher probabilities of formation of stable crystal nuclei. Hence, as larger volumes contain a greater number of water molecules, the number of large size ice-embryos is relatively increased (Vali, Reference Vali, Lee, Warren and Gusta1995). This explanation may not shed light on any potential adaptive trait attributed to P. decipiens L3, but may serve as a general reflection on the importance of size on SCPs applicable to P. decipiens L3 and similar organisms.
At the infective (L3) stage, parasites are exposed to the same temperatures as their fish hosts, and so it is readily assumed that the parasites' response to fluctuations in temperature would be similar to that of their hosts. In Atlantic cod a change in plasma osmolality due to temperature acclimation has been observed (Audet et al. Reference Audet, Besner, Munro and Dutil1993; Magill and Sayer, Reference Magill and Sayer2004). We show that a short cold acclimation does not yield significant changes in the osmolality of the P. decipiens L3 pseudocoelomic fluid. Even if P. decipiens L3 have the ability to osmoregulate for a short period of time (Fuse et al. Reference Fuse, Davey and Sommerville1993a, Reference Fuse, Davey and Sommervilleb; Davey, Reference Davey1995), it is more likely that this trait exists as an adaptation to habitat variations in osmotic pressure rather than as a response to temperature variations. P. decipiens L3 lose this capacity over time, and handling stress disrupts the osmoregulation (Davey et al. Reference Davey, Sommerville and Fuse1993). It is therefore reassuring, at least from a methodical perspective, that the acclimation regime used in this study did not induce handling stress to an extent that rendered the P. decipiens L3 osmoconform to the incubation medium. If our handling had rendered the nematodes osmoconform, the level of stress inflicted on them could easily be presumed to have influenced other parameters.
The amount of unfrozen water observed for the smallest P. decipiens L3 decreased when the nematodes froze according to their SCPs. The smallest nematodes showed the lowest SCPs, and low temperatures allow formation of ice crystals with small enough radii to penetrate pores in the cell membranes. Consequently, the lower amount of unfrozen water may be explained by an increased occurrence of intracellular freezing. Moreover, the presented results show that the speed of crystallization also increases with lower SCP, leaving cellular rearrangement less likely. Thus, rapid formation of small ice crystals seeding intracellular freezing may explain the high lethality observed during extensive supercooling.
The fact that freezing of the nematodes immediately follows the freezing of the surrounding medium (the INA solution) suggests that the L3 have very little potential for freeze avoidance by supercooling. Cold tolerant nematodes can prevent inoculative freezing if a protective sheath acts as a barrier to the ice in the surroundings (Wharton, Reference Wharton1995). P. decipiens L3 and A. simplex L3 may be surrounded in a capsule of connective tissue, but (Wharton and Aalders, Reference Wharton and Aalders2002) dismissed the idea that A. simplex L3 experience protection from inoculative freezing by host tissue. Because host response to infection of both species is comparable, a similar conclusion may be drawn for P. decipiens L3. Our results show that survival decreases dramatically over time when freezing occurs at the SCPs. Much higher survival rates were observed when freezing was initiated by INAs at high subzero temperatures. These results suggest that P. decipiens L3 posses the ability to survive freezing and thus display a moderate freeze tolerance. This demonstrated freeze tolerance is surprising as the nematodes in nature experience no selective pressure for this trait.
Based on the results presented in this study, it can be assumed that the frost tolerance mechanisms in P. decipiens resemble that of A. simplex. The two anisakid nematode species have a very similar life cycle and neither species experience freezing conditions in nature. Understandably, Wharton and Aalders (Reference Wharton and Aalders2002) questioned the adaptive significance of such freezing tolerance in their study of A. simplex. After all, the most significant temperature fluctuation these organisms experience is the increase in temperature occurring upon the entering of their definitive hosts. The supercooling ability displayed by P. decipiens L3 may be of secondary importance as far as the nematode's capacity to tolerate frost is concerned, because the pseudocoelomic fluid is not protected from exogenous ice nucleation. As the nematode's life cycle does not include a stage in which it is surface-dry, it can not avoid inoculative freezing. Many of the northern Gadoid species may experience temperatures as low as −1·8°C during winter without freezing due to antifreeze glycoproteins (Goddard and Fletcher, Reference Goddard and Fletcher1994; DeVries and Cheng, Reference Devries, Cheng, Farrel and Steffensen2005). The observed osmolality in the P. decipiens L3 of this study (~300 mOsm) correspond to a freezing point of −0·6°C. Thus, the nematode might take advantage of its supercooling capacity in the temperature range from −0·6°C to −1·8°C. Because such conditions are rare for P. decipiens L3, which is consistent with the results of this study showing that cold acclimation does not affect the SCP, it is questionable whether this capacity is a result of an adaptation. Temperature acclimation did, however, have an effect on the trehalose level of P. decipiens. Levels more than doubled for the cold acclimated (3°C) nematodes compared to the control (20°C). An even higher increase was observed for the high temperature acclimated nematodes, where a 3- and 6-fold increase was observed for those adapted to 37°C and 45°C respectively. In nematodes, accumulation of trehalose has been shown to play an important role in freeze tolerance (Behm, Reference Behm1997) by protecting membranes and proteins from desiccation damage and stabilizing cell contents (Crowe et al. Reference Crowe, Hoekstra and Crowe1992). A relationship between cold-shock and heat-shock responses have been established in entomopathogenic nematodes (Jagdale and Grewal, Reference Jagdale and Grewal2003; Jagdale et al. Reference Jagdale, Grewal and Salminen2005). In addition to the synthesis of heat-shock proteins, these nematodes synthesize trehalose as a response to both heat- and cold-shock. Our study shows that P. decipiens L3 accumulates trehalose as a response to both cold- and heat-shock treatments, possibly utilizing the trehalose as a general desiccation protectant. Both warm acclimations, the acclimation comparable to the temperature the P. decipiens L3 experience by entering their definitive host (37°C) and the acclimation which ultimately killed the nematodes (45°C), produced higher trehalose levels than was observed through cold acclimation. The fact that trehalose accumulation is most pronounced at high temperatures suggests that this part of a general thermal stress response may originate from adaptations to an increase in temperature. Both cold and warm events may precede desiccation stress, and an increased level of trehalose for both occurrences suggests that trehalose may serve as a general response to thermal stress. Consequently, in the case of P. decipiens L3, a moderate freeze tolerance may be acquired on account of adaptation to heat rather than coldness.
Our study further emphasizes the importance of adequate freezing conditions for fish commonly infected by anisakid nematodes. We confirm the assumption that nematode lethality increases with freezing at low temperatures and longer frozen storage time. The general recommendations regarding freezing and frozen storage of fish should be based on the temperature needed to kill the parasites, ensuring that this lethal temperature is reached in all parts of the fish. Earlier studies of A. simplex (Gustafson, Reference Gustafson1953; Deardorff and Throm, Reference Deardorff and Throm1988; Wharton and Aalders, Reference Wharton and Aalders2002) have shown that A. simplex L3 do not survive core fish temperatures lower than −15°C. Because of the similarity of the frost tolerance between the two species, a corresponding lethality for P. decipiens L3 is to be expected for this temperature.
In summary, this study demonstrates that even if P. decipiens L3 have the ability to supercool to temperatures as low as −15°C, they are not able to withstand inoculative freezing at higher subzero temperatures. This excludes the potential for freezing avoidance by supercooling. On the other hand, P. decipiens L3 do possess the ability to survive freezing when ice is inoculated at higher subzero temperatures. We show that temperature acclimation increases the level of trehalose, a possible cryoprotectant. Our findings suggest that the P. decipiens L3 hold a moderate freeze tolerance similar to that of A. simplex L3, which has a corresponding life cycle. Because they do not experience freezing in nature, it is unlikely that these marine nematodes are adapted to freezing conditions. They are, however, adapted to a significant temperature increase when entering their definitive host. This rise in temperature triggers the production of trehalose to a greater extent than cold acclimation. Based on the assumption that a connection between cold- and heat-shock responses exists, the freeze tolerance in P. decipiens L3 may originate from a more general thermal stress response. Thus, their freeze tolerance may be an additional effect of the adaptation to heat.
We thank Martin Brandl for access to the DSC and Merete Skar for skilful training. We would also like to thank Sigfrid Kjeldaas and Espen Hansen for insightful comments on this manuscript.