INTRODUCTION
The filarial nematode Loa loa is a parasite of humans and several simian species that is found only in Central Africa. The microfilariae of the human strain show a diurnal periodicity in the peripheral blood, and the parasite is mostly transmitted by 2 ombrophilic tabanids: Chrysops dimidiata and C. silacea. The geographical distribution of L. loa is closely related to the distribution of its vectors and the prevalences of infection are particularly high in forested regions. However, high prevalences of loiasis have been reported from savanna areas of north Cameroon and of other countries (Boussinesq and Gardon, 1997; Kamgno and Boussinesq, 2001).
Clinical signs of human loiasis are usually mild (e.g. Calabar swellings, subconjunctival migration of adult worms) though severe complications such as nephropathy (Katner, Beyt and Krotoski, 1984; Pakasa, Nseka and Nyimi, 1997), and spontaneous encephalopathy (Kivits, 1952; Cauchie et al. 1965; Samé Ekobo et al. 1981) have also been associated with L. loa infection. In some parts of Africa, loiasis has been reported as a major health issue for the afflicted populations (Boulesteix and Carme, 1986), but concerns about loiasis have increased with the implementation, in forest areas, of large-scale ivermectin distribution programmes to control onchocerciasis. Ivermectin treatment may induce severe adverse reactions, including fatal encephalopathy, in individuals harbouring high L. loa microfilarial (mf) loads (Gardon et al. 1997; Boussinesq et al. 2003). Thus, in areas where L. loa and Onchocerca volvulus are co-endemic, the risk of post-ivermectin severe adverse events (SAEs) hampers the implementation of the African Programme for Onchocerciasis Control (APOC). Specific measures have been taken to reinforce the surveillance of SAEs during ivermectin distribution in communities where the prevalence of L. loa microfilaraemia is >20%. In these communities, more than 1% of the population may harbour more than 30000 microfilariae (mfs) per ml of blood and thus have an increased risk of neurological SAEs (Boussinesq et al. 2001).
At present, the within-individual processes influencing the mf status of a given host, and the immunological mechanisms regulating the L. loa mf loads are not completely understood. It has recently been shown that, in addition to a genetic predisposition to becoming microfilaraemic (Garcia et al. 1999), a high exposure to incoming infective larvae might play an essential role in the appearance of L. loa microfilaraemia (Pion et al. 2005). A detailed analysis of age- and sex-specific parasitological profiles of loiasis has shown that the mean mf loads among microfilaraemic individuals were fairly constant among age groups (Pion et al. 2004).
Increasing empirical evidence supports the notion that host infection with one helminth species can influence the outcome of infection with other helminths (Haswell-Elkins, Elkins and Anderson, 1987; Booth et al. 1998; Behnke et al. 2001; Cox, 2001; Howard, Donnelly and Chan, 2001; Lello et al. 2004; Faulkner et al. 2005), with recent theoretical studies investigating the possible nature of such interactions (Bottomley, Isham and Basáñez, 2005). Though human co-infection by L. loa and O. volvulus raises important public health concern, to our knowledge, the association at individual host level between these two species has not been adequately assessed.
In order to identify whether the co-infection with O. volvulus might explain the presence of high L. loa microfilaraemia in some individuals, we used statistical models to analyse the association between L. loa microfilaraemia and O. volvulus microfilaridermia. We based our analysis on data collected prior to the implementation of large-scale ivermectin treatment in a population living in central Cameroon, the area where most of the severe, and sometimes fatal, post-ivermectin adverse reactions have been reported (Twum-Danso, 2003).
PATIENTS AND METHODS
Study area, selection of subjects and parasitological examinations
The data analysed in the present paper were collected as part of epidemiological surveys of filarial infections, conducted in 1991–1993 in the Lékié Division of the Central Province of Cameroon. During these surveys, a total of 3244 consenting individuals aged ≥5 years (1447 males and 1797 females) were examined in 19 villages that had been selected after stratification according to the distance between their location and the Sanaga River. The Lékié Division is an area of degraded forest located on the left bank of the loop of the Sanaga River, near its confluence with the Mbam River. A number of sites presenting a series of rapids constitute particularly productive breeding sites for the Simulium species transmitting onchocerciasis in this area of Cameroon.
Diagnosis of Loa loa infection
A blood sample was collected from each individual by finger-prick, between 10:00 and 16:00 h, in a non-heparinized capillary tube, and calibrated thick blood films (1 per individual) were immediately prepared, using 30 μl of blood. Each Giemsa-stained smear was then examined under a low-power microscope and all the L. loa mfs present on the slide were counted.
Diagnosis of Onchocerca volvulus infection
Examinations were done using the methods recommended by the Onchocerciasis Control Programme in West Africa, OCP (Moreau, Prost and Prod'hon, 1978; Prost and Prod'hon, 1978): 2 skin snips (one from each iliac crest) were taken using a 2 mm Holth-type corneoscleral punch in 16 of the villages and a 1·5 mm punch in the remaining 3 villages, and left to incubate in normal saline for 24 h. The emerging mfs were counted and the individual mf loads were calculated as the arithmetic mean of the number of mfs/skin snip (mfs/ss). To account for the difference of size of the snips taken with the 1·5 mm and 2 mm Holth punches, the former were multiplied by 1·33.
All the participants were registered and information on their age, sex and date of any previous treatment with antifilarial therapy was recorded. Data from 54 individuals (18 males and 36 females) who had been treated during the last 5 years were discarded from the analyses presented here.
Statistical analysis
We investigated the associations between L. loa and O. volvulus in the human host using regression models to adjust the observed associations for exogenous individual- and community-level factors, that is, factors independently associated with the presence and density of mfs of one or the other species. Infection with O. volvulus has already been shown to be influenced by individual-level characteristics such as host sex and age (Basáñez and Boussinesq, 1999; Pion et al. 2004). At the community level, the degree of endemicity for L. loa and O. volvulus (measured in terms of mf prevalence or intensity), and the density of their respective vectors (measured in terms of biting rates), vary following distinct geographical and ecological factors (e.g. distance from the village to the breeding sites for onchocerciasis).
To account for ecological factors possibly acting at the community level, we defined endemicity variables for each filarial infection. For onchocerciasis, the level of endemicity was defined by the Community Microfilarial Load (CMFL), i.e. the geometric mean of the mf densities among the population (both sexes) aged ≥20 years (Remme et al. 1986). For loiasis, and following Boussinesq et al. (2001), the level of endemicity was defined according to the prevalence of microfilaraemia among adults aged ≥15 years (PMF). These prevalence values were standardized for age and sex using the OCP standard population (Moreau et al. 1978) modified for its application to a population ≥15 years old. We defined 3 classes of endemicity for each species: for onchocerciasis, we considered CMFLs <15, 15–49·9 and ≥50 mfs/ss and, for loiasis, PMFs <20%, 20–27·9% and ≥28%. These classes were chosen to represent the range of infection intensity and prevalence among villages while ensuring sufficient and similar numbers in each group.
The analysis was divided into 2 sections. Section 1 considered the L. loa microfilaraemia as a qualitative (presence/absence) outcome, and looked for factors associated with the presence or absence of L. loa mfs among individuals. Section 2 focused on quantitative association (mf loads) between the two species. We used the software package MLwiN (Rasbash et al. 2004) to fit multilevel (or hierarchical) logistic regression models. Multilevel models are an extension of standard regression models that use random effects to allow for unobserved factors at the community-level (or at higher clustering levels), in addition to observed individual- and community-level factors (Goldstein, 2003).
For the qualitative analysis, we used the logistic model (model A) to assess whether the presence/absence of L. loa microfilaraemia was associated with the presence of O. volvulus microfilaridermia. Two sets of estimates were obtained for model A. The first set contains the adjusted estimate of the effect of O. volvulus, where adjustments were made for individual-level (age and sex, where the former was treated as a continuous variable) and community-level factors (model Aa). The second set contains ‘interaction’ estimates of the effect of O. volvulus (model Ai). ‘Interaction’ refers to the regression interactions between two or more variables, and should not be confused with the within-host/biological interaction between L. loa and O. volvulus. Model Ai is an extension of model Aa, to allow separate O. volvulus effects for communities with the different levels of loiasis and onchocerciasis endemicity. There are 6 estimates of the effect of O. volvulus from model Ai, one for each of L. loa-O. volvulus endemicity combinations within which the sample villages were observed: (I) low L. loa and low O. volvulus; (II) medium L. loa and low O. volvulus; (III) high L. loa and low O. volvulus; (IV) low L. loa and medium O. volvulus; (V) medium L. loa and medium O. volvulus, and (VI) low L. loa and high O. volvulus (see Fig. 1). The total numbers of patients examined in each of these 6 situations can be found in Table 1.

Fig. 1. Distribution of the 19 villages surveyed in Central Cameroon according to their respective levels of endemicity for Loa loa and Onchocerca volvulus microfilarial infection: (I) low L. loa and low O. volvulus; (II) medium L. loa and low O. volvulus; (III) high L. loa and low O. volvulus; (IV) low L. loa and medium O. volvulus; (V) medium L. loa and medium O. volvulus, and (VI) low L. loa and high O. volvulus.

For the quantitative analysis, we looked for factors associated with the patients’ L. loa mf load, and, in the first instance, we assessed the relationship between the L. loa and O. volvulus mf loads using non-parametric Spearman's correlations (rS). As in previous studies (Basáñez et al. 1999; Pion et al. 2004), we found evidence of high overdispersion (with variance to mean ratio values ranging between 170 and 1800, see Table 1) in the distributions of both L. loa and O. volvulus. This motivated the use of negative binomial regression models. However, the sample had an excess of zero L. loa loads compared to the best fitting negative binomial distribution, because only 60% of the population are genetically predisposed to be microfilaraemic for L. loa (Garcia et al. 1999). Hence, as the negative binomial distribution fitted closely the distribution of the L. loa positive loads (with mean=377·4; k=0·26; chi2 goodness of fit test: P<0·31), we excluded zero loads and fitted negative binomial models to those positive L. loa loads only. Alternatively, we could have used a zero-truncated negative binomial model here, but such models cannot be fitted in MLwiN. We assessed whether this would have made a difference to our results by comparing the results from fitting negative binomial regression using MLwiN without random effects to those from a truncated negative binomial model using STATA. The resulting differences between estimates were small and did not affect the interpretation of the models. Therefore, and for the sake of consistency we used MlwiN throughout. As with the qualitative analysis, ‘adjusted’ (Ba) and ‘interaction’ (Bi) estimates were obtained for the negative binomial model. This analysis provides relative risk estimates, whose interpretation in our context, is more precisely the increase in L. loa mf load per unit increase in O. volvulus mf load. Therefore we use the term relative load (RL) to refer to the parameters we obtained. Thus, for quantitative variables (e.g. O. volvulus mf load, age) RL is the ratio of mean L. loa load when explanatory factor is [x+1] to mean L. loa load when the explanatory factor is [x] (holding all other factors constant). For qualitative variables (e.g. sex, L. loa endemicity) RL is the ratio of mean L. loa load for that category compared to the baseline category.
RESULTS
General information
Among the 3190 individuals examined in 19 villages, the overall prevalence of O. volvulus microfilaridermia was 65·8% (respectively 69·8% and 64·7% in L. loa positive and L. loa negative individuals). The overall prevalence of L. loa microfilaraemia was 21·9% (respectively 23·2% and 19·4% in O. volvulus positive and O. volvulus negative individuals). Some 15·3% of the individuals presented mfs of both species.
The levels of endemicity for each infection in the different villages are presented in Fig. 1. Amongst the 19 villages, the CMFL and the PMF were negatively and highly significantly correlated (rS=−0·895, P<10−5, n=19).
Qualitative analysis
A visual inspection of the L. loa prevalence age profiles suggested that for a given level of L. loa endemicity, such age profiles did not appear to be affected by the level for onchocerciasis endemicity (Fig. 2). Nonetheless, according to model Aa, the association between the presence of L. loa and the presence of O. volvulus was positive and significant (OR=1·790, 95% CI [1·431–2·238]). In addition, the presence of L. loa microfilaraemia was dependent on age and sex of individuals. When including interactions to allow the effect of O. volvulus to vary between communities of different endemicity class (model Ai), none of the estimates of association between the presence of L. loa and the presence of O. volvulus in the 6 endemicity groups were significant (for example in group I, OR=2·111 (95% CI [0·938–4·751]). These results also indicate that there is insufficient evidence to reject the hypothesis that the effect of O. volvulus on L. loa does not vary by endemicity group (Table 2).
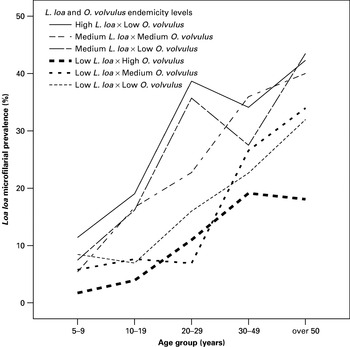
Fig. 2. Age profiles of Loa loa microfilarial prevalence in the different endemicity groups stratified according to the 3 L. loa endemicity levels and to the 3 onchocerciasis endemicity levels.
Table 2. Estimates (odd ratios and 95% confidence intervals) obtained by hierarchical logistic models to assess the association between the presence of L. loa microfilarial infection and O. volvulus microfilarial infection (qualitative analysis) (Two models were assessed: adjusted on individual factors and endemicity levels in the communities (Aa), and including interaction terms between endemicity levels and O. volvulus infection (Ai).)

The variance of the random effect distribution was very small in both the adjusted and interaction models (results not shown), indicating that much of the unobserved variation between villages had been accounted for by observed individual- and community-level factors. Thus, at this stage of the analysis, our results for model A indicate that (a) model Aa supports the hypothesis for a positive association between the presence of Loa microfilaraemia and O. volvulus infection and (b) there is insufficient evidence to support model Ai over model Aa.
Quantitative analysis
The L shape relationship observed between the individual L. loa and O. volvulus mf loads (Fig. 3) suggested a possible negative correlation between the loads. Using the log (x+1) transformation, with x being the microfilarial load (including zero counts), the correlation between the mf loads was positive but not significant (rS=0·020, P=0·255, n=3190). However, when grouping these individuals in their villages, classifying villages according to their respective endemicity levels for the two parasite species and considering the three following age-groups, 5–19, 20–49 and ≥50 y, we found significant positive correlations between the loads in those individuals: those aged 5–19 living in endemicity groups III or IV, those aged 20–49 living in endemicity group III, and those aged ≥50 living in endemicity groups I, II, III or VI (Fig. 4).

Fig. 3. Scatter plot of Loa loa and Onchocerca volvulus microfilarial loads amongst the 3190 individuals examined in the Lékié area (Central Cameroon).

Fig. 4. Scatter plots of Loa loa and Onchocerca volvulus microfilarial loads showing the relationship between the loads in different age- and endemicity-groups. The loads are represented as log (x+1) and expressed as number of microfilariae per skin snip for O. volvulus and as number of microfilariae per 30 μl blood for L. loa. The presence of a star in the graph indicates a significant correlation between the loads. The lines represent least squares estimates and, although error structure is clearly not normal, they are shown to help the reader judge the general direction of the trend. Estimates obtained with the more appropriate negative binomial regression were very similar (not shown).
The negative binomial regression results are shown in Table 3 and confirm the trend observed in the analysis of correlations. In the adjusted model (Ba) the association between the positive L. loa loads and the O. volvulus loads (including zero counts) was positive and significant, but the strength of this association was small when interpreted as a relative increase of L. loa mf load per O. volvulus microfilaria. However, the relative load of 1·001 (95% CI [1·000–1·002]) equates to an 11% increase in mean L. loa load for every 100 mfs/ss increase in O. volvulus load. (The increase from [x+100] to [x] is (1·001)100=1·11 or an 11% relative load.) Similarly to the qualitative analysis, the results from the interaction model (Bi) did not support the hypothesis that the effect of O. volvulus on L. loa varies by village endemicity level. In other words, the association between the mf loads was not specific to villages with particular levels of endemicity for one or the other infection.
Table 3. Estimates (relative loads* and 95% confidence intervals) of the hierarchical negative binomial models to assess the association between the positive L. loa microfilarial (mf) loads and the positive or negative O. volvulus mf loads (quantitative analysis) (Two models were assessed: adjusted on individual factors and endemicity levels in the communities (Ba) and including interaction terms between endemicity levels (Bi) and O. volvulus mf load.)

DISCUSSION
It is well known that mixed parasitic infections in general, and helminth infections in particular, are the rule rather than the exception in both human and non-human hosts (Christensen et al. 1987; Cox, 2001). Concerning filariases, the results of previous studies suggest the existence of a positive association between O. volvulus and Wuchereria bancrofti (Keita et al. 1981; Engelbrecht et al. 2003), and between O. volvulus and Mansonella perstans (Gbary et al. 1987). However, little is known about the association between O. volvulus and L. loa mf infections despite the medical importance of this association with respect to microfilaricidal treatment. To address this point, we have explored a large dataset obtained from an area of Cameroon known to be co-endemic for loiasis and onchocerciasis, and where many post-ivermectin serious adverse reactions have been reported (Twum-Danso, 2003).
At the time of surveys in 1991–1993, neither mass treatment with ivermectin or any other filaricidal drug nor any vector control had been implemented in the area. When some of the villages included in the present study were again visited in 1995–1996 (just before commencement of the first large-scale ivermectin distribution), the levels of L. loa mf infections were very similar to the ones recorded during the first surveys (Boussinesq et al. 2001); it is the results of the former surveys that are analysed in the present study. We can thus assume that both parasite populations were at endemic equilibrium among the host populations prior to control-induced perturbations.
At the village level, we found a negative correlation between the prevalence of L. loa in those aged ≥15 y and the O. volvulus community microfilarial load (in those aged ≥20 y). Despite the fact that the study area is endemic for both species, none of the villages presented high endemicity levels for L. loa and medium or high endemicity levels for onchocerciasis, neither did they exhibit medium endemicity levels for L. loa and high endemicity level for onchocerciasis.
This apparent antagonistic correlation between the two infections might be explained by ecological factors influencing the respective geographical distributions of the vectors that transmit the two filariases in the area: the densities of the Simulium vectors decrease gradually from the breeding sites in the Sanaga River towards the more distant forest areas, whereas the densities of Chrysops are probably lower nearest the Sanaga River, where plant cover is scarcer than in others parts of the Lékié area. As said in the results section, the variance of the random effect in models (Aa) and (Ai) was very small, indicating that the observed individual- and community-level factors considered in the analysis had already accounted for much of the unobserved variation between villages. Our results highlight the importance of adjusting not only for individual factors, but also for ecological factors, when assessing the strength and direction of an association between two parasite species.
The results of the ‘adjusted’ and ‘interactions’ logistic models confirmed that the presence of a L. loa microfilaraemia increases with age and is more frequent in the male than in the female population, as previously reported (Pion et al. 2004).
After adjusting for age and sex of individuals and for ecological variation between villages (model Aa), we found that the probability of harbouring L. loa mfs was significantly positively associated with the presence of O. volvulus microfilaridermia. The inclusion of interaction terms in the models makes the effect of O. volvulus on L. loa borderline non-significant within each of the endemicity groups (model Ai). The total numbers of individuals in each of the 6 village groups (I to VI) were fairly high, but it remains a possibility that the application of model Ai to a larger sample would have demonstrated clear-cut significant differences in the effect of O. volvulus on the presence of L. loa microfilaraemia by village endemicity level.
Considering all the individuals with positive L. loa loads, and after appropriate adjustment (models Ba and Bi), we found a slight but significant positive association between the positive L. loa loads and O. volvulus mf loads, these latter including zero counts. Again, these results underline that use of unadjusted correlation might lead to misleading trends.
Taken together, our results suggest that, overall in the studied human population, there is a trend towards a positive association between the presence of L. loa and of O. volvulus microfilariae and that, among people harbouring L. loa mfs, there is also a positive, yet slight association between the two species. The present findings differ from the results obtained in the southwest province of Cameroon by Wanji et al. (2003). In this latter study, no significant association was found between L. loa and O. volvulus infection (P-value for χ2 test: 0·074). However, in the study by Wanji et al. several factors might have led to an inaccurate estimation of the possible association between L. loa and O. volvulus mf infections. First, in the study area, the overall prevalence of L. loa microfilaraemia was rather low (8·6% versus 21·9% in the present study) and, on the other hand, O. volvulus infection had been determined by nodule palpation only, a rapid epidemiological assessment method good enough for operational purposes but clearly not suited to obtaining accurate estimates of mf prevalence. Low endemicity of loiasis and probably underestimated prevalence of onchocerciasis may have led to low statistical power when assessing the association between the two infections. Secondly, the statistical analysis, limited to qualitative aspects (absence/presence of L. loa mfs and O. volvulus nodules), was performed without any adjustment for individual factors or endemicity levels, which constitute an important source of variance between individual co-infections, as demonstrated in the present study. These differences in methodology may explain the different conclusions drawn from the two studies.
According to Bottomley et al. (2005), a feature of synergistic intra- and interspecific interactions is that they frequently lead to equilibrium correlations between species that are positive in sign for younger hosts but rapidly approach zero with host age, making their detection and measurement harder than in the case of antagonistic interactions. The effects that we describe here are slight yet positive after controlling statistically for the effects of age, sex, and endemicity. Also, we do not think that these positive effects are due to positively correlated exposures that may be masking underlying antagonistic interactions, as the densities of the simuliid and tabanid vectors vary in opposing ways with distance from the Sanaga river. We therefore proceed by distinguishing between 2 possible types of interaction mechanisms, (a) those acting directly on the parasites, and (b) those mediated through host immunity that may give rise to synergistic effects.
Concerning possible direct interactions on the one hand, the two species live in different compartments within the human body: O. volvulus adult worms live in subcutaneous nodules and their microfilariae are principally found in the lymphatic system, particularly in the dermal layers of the skin; by contrast, L. loa adults live freely in subcutaneous tissues and their microfilariae circulate in the blood. Although these differences may make a direct interaction between the microfilariae unlikely, they do not rule out the possibility that interactions may take place at other stages in the life-cycle, such as the point of establishment of incoming larvae, so that the infection by one species would facilitate infection by the other species.
On the other hand, it has been shown that filarial infections may provoke a profound immunosuppression of the host (Ottesen, Weller and Heck, 1977; Maizels and Yazdanbakhsh, 2003; Maizels et al. 2004) that can be parasite-specific (leading to synergistic intraspecific effects), or mediated through immunological cross-reactions, leading to interspecific effects (Wahl et al. 1998). In onchocerciasis, as well as in loiasis, the intensity of microfilarial infection has been found to be associated with high levels of IL-10 cytokine (Winkler et al. 1999; Hoerauf and Brattig, 2002) and for this reason, IL-10 cytokine has been suspected to play a key role in the immunosuppression process. Immune cross-reactions may also be cross-regulated by many different pathways and unfortunately, no experimental model supporting O. volvulus/L. loa co-infection is currently available for testing different hypotheses.
Besides, host immunosuppression can also be induced by components in the saliva of vectors, injected at the feeding site. It has been shown that saliva of Simulium vittatum affects immune cell responses and cytokine production (Cupp and Cupp, 1997). Some components of anthropophilic simuliids' saliva may also inhibit platelet aggregation, prevent coagulation and induce vasodilatation of capillaries. Repeated saliva injections by Simulium vectors into Loa infected, yet amicrofilaraemic individuals, could facilitate the appearance of L. loa microfilariae in the bloodstream. To test these hypotheses, studies on the effect of African Simulium species' saliva would be needed.
Finally, we cannot exclude the possibility that those individuals, presenting a high intensity of microfilarial infection for both species are people particularly exposed to the two infections. For instance, individuals with fishing and hunting activities might have an important contact rate with blackflies, when fishing on the banks of the river, and with Chrysops during daytime hunting in forested parts. Regarding onchocerciasis, this hypothesis is supported by a recent paper on the role of exposure to vectors on the level of mf infection at the individual level (Filipe et al. 2005). Regarding loiasis, to our knowledge, no studies have documented the role of individual exposure on the mf load.
In conclusion, a positive qualitative association between infection with O. volvulus and the presence of L. loa microfilaraemia has been found at the individual level. However, given the low strength of the quantitative association, it can be said that the two species can be considered as having separate epidemiologies. Lastly, the present study clearly demonstrated that co-infection with O. volvulus is not sufficient to explain why some individuals harbour high L. loa mf densities.
This work was supported by the Institut de Recherche pour le Développement. We wish to thank Dr Nathalie Gardon-Wendel, Dr Francis J. Louis, Tiburce Nyiama, Vincent Foumane and the staff of the Centre Pasteur du Cameroun for assistance in the field. S.D.S Pion thanks the Fondation pour la Recherche Médicale and the Fondation Singer-Polignac for financial support. J.A.N. Filipe and M.-G. Basáñez thank the Medical Research Council of the United Kingdom. We also thank Christian Bottomley and the referees whose comments contributed to improving an earlier version of the manuscript.









