Published online by Cambridge University Press: 28 November 2001
A cDNA encoding a Na,K-ATPase alpha subunit homologue, designated SNaK1, was isolated from an adult cDNA library of Schistosoma mansoni. The 3.8 kb DNA contained a 3021 bp open reading frame potentially encoding a 1007 amino acid protein that had an Mr of 111817 and a pI of 5.48. Homology searches for SNaK1 revealed approximately 70% sequence identity with a variety of Na,K-ATPases from evolutionarily diverse organisms. SNaK1 is predicted to contain 10 transmembrane regions typical of this protein family as well as other conserved domains, such as the phosphorylation site and ATP binding domain. Antibodies raised against an amino terminal peptide detected the protein in membrane preparations of eggs, cercariae and adult males and females, suggesting a general role for SNaK1. The mobility of the protein differed in various life-stages suggestive of post-transcriptional or post-translational modification. Immunolocalization of SNaK1 in sections of adult worms using epifluorescence and electron microscopy, revealed antibody labelling in the subtegumental and peripheral layers. Strong staining was discernible in the peripheral muscle band indicating that SNaK1 plays a central role in muscle contraction in adult parasites and may be the primary target of ouabain action. Staining was also detected in the secretory bodies in sections of ducts in this region and over the RER of the presumed gastrodermis. Immunogold labelling was also localized over neuronal vesicles in axons associated with the peripheral muscle layer.
Sodium potassium ATPases (Na,K-ATPases or Na pumps) are heterodimeric membrane proteins that couple the hydrolysis of ATP to the transport of sodium and potassium ions across the plasma membrane. In this way the protein generates the cationic gradients that are fundamental to a variety of cell functions such as the regulation of cell volume, pH and the uptake of organic compounds. Na,K-ATPases are comprised of alpha and beta subunits. The alpha subunit contains the ATP catalytic domain, the ion-transporting function as well as the binding site for cardiac glycosides such as ouabain. Na,K-ATPase alpha subunits belong to a larger protein family called the P-type ATPases which includes Ca-ATPases and H,K-ATPases. All members of this family share some sequence similarity and all are phosphorylated at a conserved aspartic acid residue.
In schistosomes, there is evidence for Na,K-ATPase activity both in the tegument and underlying tissues. The schistosome tegument is a syncytium that surrounds the worm and is bounded externally by 2 lipid bilayers and internally by a single lipid bilayer. It is through the tegument that schistosomes regulate water and ion levels, absorb nutrients and engage the immune defences of the host. Schistosomes maintain an electrical potential gradient across their outer tegument that is dominated by potassium ions (Fetterer, Pax & Bennett, 1980) suggesting the presence of proteins such as Na,K-ATPases at this site. Na,K-ATPase activity has been detected in schistosome homogenates (Nechay, Hillman & Dotson, 1980), has been localized cytochemically to the tegument (Shaw, 1987) and detected in tegumental membrane preparations (Podesta & McDiarmid, 1982; Taylor & Wells, 1984).
Na,K-ATPase activity is also present in the internal tissues of the parasite underlying the tegument (Noel & Soares de Moura, 1986). The enzyme activities of the tegumental and internal tissues are similar except that the tegumental ATPase is approximately 10 times less sensitive to ouabain (Noel & Soares de Moura, 1986). Ouabain-binding studies using whole worm homogenates suggest that there are at least 2 isoforms of Na,K-ATPases in the worms (Pardon & Noel, 1994).
Electrophysiological examination of the tegument and muscle membranes in adult schistosomes suggests a close electrical coupling between the two tissues (Bricker, Pax & Bennett, 1982). Microelectrode studies suggest that the electrical potential of both the tegument and the underlying muscle is K+ dependent (Bricker et al. 1982). Physiological data show that the active transport of Na and K is particularly important to schistosomes for the maintenance of normal ionic gradients. Specifically, inhibition of Na,K-ATPases using ouabain elicits a large (20 mV) depolarization of the tegumental cytoplasm and a dramatic contraction of the somatic musculature (Fetterer, Pax & Bennett, 1981). Given the close electrical coupling of the tegumental and muscle tissues it is difficult to determine if these physiological effects of ouabain are due to inhibition of Na,K-ATPases in the tegument or muscle (or both).
As schistosomula mature they become refractory to immune-mediated killing mechanisms (Sher & Moser, 1981; McLaren, 1984). Concurrently there is a change in the tegumental membrane potential (Pearce et al. 1986; Tarrab-Hazdai et al. 1986). This temporal correlation has led to the suggestion that developmental changes in the tegument involving proteins like Na,K-ATPases may be central to the acquired diminution of parasite vulnerability to immune elimination (Pearce et al. 1986). More recently a direct role for Na,K-ATPases in conferring refractoriness to complement-mediated damage of schistosomula has been reported (Tarrab-Hazdai et al. 1997). For this reason immunological or pharmacological inhibition of Na,K-ATPases has been proposed as an effective anti-schistosome therapy. In order to better understand the complex role of Na,K-ATPases in the biology of schistosomes, we have characterized a Schistosoma mansoni cDNA encoding an alpha subunit of a Na,K-ATPase homologue (designated SNaK1) and determined the in situ localization pattern of its encoded product.
A Puerto Rican strain of Schistosoma mansoni was maintained by passage through Biomphalaria glabrata snails and CBA/J mice. The parasite life-stages: eggs, cercariae and adult male and female worms were obtained as described (Hackett, 1993).
Two opposing primers, AP1001: 5′-ATCTGCAGCGACAAGAC(A/C/G/T)GG(A/C/G/T)AC-3′ and MNKA4: 5′-ATTGGATGATCACCTGT(A/C/G/T)ACCAT-3′, were synthesized that would anneal to conserved amino acid sequence motifs within Na,K-ATPases. A polymerase chain reaction (PCR) was performed using 1 ng of S. mansoni adult cDNA as template under the following conditions: 25 cycles, each having an annealing time of 60 sec at 50 °C, an extension time of 60 sec at 72 °C and denaturation for 60 sec at 94 °C. PCR products were resolved by agarose gel electrophoresis and a fragment of the expected size was identified and purified by glass affinity. The PCR product was radio-isotope labelled and used to probe approximately 120000 plaques of a lambda ZAP schistosomula cDNA library. One positively hybridizing plaque was purified and its cloned insert was excised and mapped. The insert comprised approximately 4 kb of DNA, and contained the complete coding sequence of an alpha subunit homologue of Na,K-ATPase. Synthetic oligonucleotides were used to obtain the complete overlapping sequence from both strands of the coding region by the method of Sanger, Nicklen & Coulson (1977).
A peptide was synthesized based on the first 18 amino acids of the full-length protein and with a terminal cysteine. The sequence is: NH2–MATEKKSKKNPKKDDLNEC–COOH, and corresponds to amino acids 1–18. The peptide was coupled to both bovine serum albumin (BSA) and ovalbumin with m-maleimidobenzoyl-N-hydroxysuccinimide ester (Harlow & Lane, 1988). A rabbit was immunized subcutaneously with 1 mg of BSA-coupled peptide in complete Freund's adjuvant and was boosted 3 times with 100 μg of the uncoupled peptide dissolved in PBS and mixed with Freund's incomplete adjuvant, at 3-week intervals.
Anti-peptide antibodies were purified by affinity to the immunizing peptide. First the ovalbumin-coupled peptide was coupled to an NHS-activated HiTrap column (Pharmacia, Inc) as recommended by the manufacturer. Then serum was loaded onto the column and washed extensively with phosphate-buffered saline (PBS). Bound antibody was eluted with 0.1 M glycine, pH 2.5, neutralized with NaOH and dialysed against PBS.
S. mansoni membrane fractions were prepared from the following life-stages: eggs, cercariae, adult males and females, as described (Zhong et al. 1995). Proteins were resolved by SDS–PAGE. Proteins were then transferred to PVDF membrane which was washed with PBS, 0.3% Tween 20 for 1 h prior to incubation for 1 h in primary, anti-Na,K-ATPase-peptide antibody (5 μg/ml in PBS, 0.3% Tween 20). The membrane was washed 3 times and incubated in a 1:3000 dilution of alkaline phosphatase-conjugated goat anti-rabbit IgG (Bio-Rad) and developed with BCIP/NBT (Kirkegarrd and Perry).
Adult worms were embedded in O.C.T. compound and frozen in liquid nitrogen. Sections of 7 μm thickness were air-dried on poly-L-lysine coated slides and fixed in acetone for 5 min. After air-drying, the sections were rehydrated in 1% normal goat serum in PBS for 30 min. Sections were incubated for 60 min with 50 μg/ml affinity-purified anti-Na,K-ATPase antiserum and were then washed 3 times for 10 min in PBS, 2% fetal calf serum. A 1:300 dilution of fluorescein-conjugated F(ab′)2 goat anti-rabbit IgG (Bio-Rad) was used to detect bound primary antibody. After washing as above the slides were mounted in 90% glycerol, PBS, 2% 1,4-diazabicyclo[2,2,2]octane. The sections were examined by conventional epifluorescence microscopy and by laser scanning confocal microscopy using a Bio-Rad MRC600 microscope.
Whole schistosomes were first fixed for 1 h at 4 °C in 2% double-distilled glutaraldehyde (DDG) in 0.1 M sodium cacodylate buffer (pH 7.2) containing 0.1 M sucrose, washed overnight in fresh sodium cacodylate buffer, then dehydrated rapidly through a graded ethanol series, infiltrated and embedded in ‘Agar 100’ resin; the resin was allowed to polymerize for 48 h at 60 °C. Sections (60–70 nm in thickness) were cut using an ultramicrotome, mounted on 200-mesh nickel grids, and then etched with 10% hydrogen peroxide for 10 min, rinsed with 20 mM Tris–HCl buffer (pH 8.2) containing 0.1% (w/v) BSA and Tween 20 (1:40 dilution), and finally exposed to normal goat serum for 30 min. All steps were carried out at room temperature. After washing (5×1 min) in Tris–HCl buffer, sections were incubated in anti-Na,K-ATPase antibody diluted to 1:3000 with Tris–HCl buffer for 24 h at room temperature, then washed in Tris–HCl buffer (5×1 min) and incubated in a 25 μl droplet of 10 nm-sized gold-conjugated goat anti-rabbit IgG (1:100). Following a wash in Tris–HCl buffer (5×1 min), sections were lightly fixed in 2% DDG for 3 min, washed in double-distilled water (5×1 min), stained with uranyl acetate (8 min) and lead citrate (8 min), and viewed in a JEOL 100CX transmission electron microscope operating at 100 kV.
A full length cDNA encoding a schistosome Na,K-ATPase homologue was obtained as described in the Materials and Methods section. The cloned DNA is 3782 bp and contains a 3021 bp open reading frame that encodes a 1007 amino acid protein (Fig. 1). The GenBank accession number for the SNaK1 nucleotide sequence reported here is AF303222. The protein is predicted to have an Mr of 111817 and a pI of 5.48. The protein has a similar size and has substantial sequence similarity with the alpha subunits of several Na/K ATPases and is therefore designated SNaK1 (Schistosome Na,K-ATPase 1). A fragment of this cDNA, encoding the 104 amino acids from M598 to G701, was previously reported by de Mendonca et al. (1995) during that group's characterization of cation transport ATPases from S. mansoni. In what likely reflects polymorphism in SNaK1 cDNAs, the previously reported 104 amino acid sequence differs from that described here at 2 positions; in our sequence S replaces F at position 621 and S replaces R at position 636. Fig. 1 shows a comparison of the full length amino acid sequence of SNaK1 with the Na,K-ATPase alpha isoforms of several other organisms. SNaK1 has approximately 70% sequence identity and approximately 80% sequence similarity with a variety of Na,K-ATPases from evolutionarily diverse sources including humans, the fruit fly, Drosophila melanogaster and Hydra vulgaris (Table 1) as determined by gapped BLASTP 2.0.4 analysis (Altschul et al. 1997). Note that the figures for identity and similarity are virtually identical when SNaK1 is compared with any of the 3 well-characterized human Na,K-ATPase alpha subunit isoforms. SNaK1 also exhibits substantial sequence identity (close to 60%) and similarity (over 70%) with a second class of P-type ATPases: the vertebrate H/K ATPases (Table 1). SNaK1 displays substantially less sequence similarity with a third class of P-type ATPases: the Ca-ATPases. In the examples shown in Table 1, the sequence similarity of the schistosome Na,K-ATPase, SNaK1, when compared with either a schistosome Ca-ATPase or a human Ca-ATPase, is virtually indistinguishable (45–46% similarity and 28% identity in both cases, Table 1). The family of Na,K-ATPases exhibit greatest sequence divergence at their amino termini (Fig. 1).

Fig. 1. Alignment of the Schistosoma mansoni Na,K-ATPase alpha subunit homologue, SNaK1, with members of the Na,K-ATPase protein family. Predicted transmembrane domains are underlined, the conserved phosphorylation site is indicated (P site) and the conserved, phosphorylated aspartic acid residue (D360) is in bold text. ‘[bull ]’ indicates identity with the SNaK1 sequence. Gaps (indicated by dashes) have been introduced to maximize homology. Sequence motifs used in PCR primer design are double underlined. HUMAN: isoform α 1, accession no. P05023; FLY: Drosophila melanogaster, accession no. P13607; HYDRA: Hydra vulgaris, accession no. P35317.

SNaK1 is predicted to have 10 hydrophobic transmembrane domains (underlined, Fig. 1) and a large cytoplasmic loop between transmembrane domains 4 and 5. The schistosome protein has many of the structural features of an ion-transporting P-ATPase (Horisberger et al. 1991; Fagan & Saier, 1994). These include several very highly conserved motifs such as a catalytic phosphorylation site (P site (Fig. 1), 357ICSDKTGTLT366, containing the predicted phosphorylated Asp360), several motifs that are proposed to encompass at least part of the ATP-binding domain (491KGAPE495, 577DPPR580 and 699TGDGVNDSPAL709) and a binding domain for the phosphate analogue, vanadate (202LTGES206) (Farley et al. 1984; Ovchinnikov et al. 1987; Fagan & Saier, 1994). The protein displays a conserved leucine zipper motif ((L(X)6L)n, where X indicates any amino acid) in the first transmembrane domain. Leucine zipper motifs have been described in a number of glucose and ion transporters and are indicative of molecular associations of the proteins within membranes (White & Weber, 1989). The presence of a conserved leucine zipper motif in SNaK1 is suggestive of an association of SNaK1 with other proteins in the cell membrane (for instance, the Na,K-ATPase beta subunit (Blanco, Koster & Mercer, 1994)). The binding site of ouabain – the inhibitor of the Na,K-ATPases of several organisms – has been mapped to the region joining transmembrane domains 1 and 2 (Price & Lingrel, 1988; Price, Rice & Lingrel, 1990). In this region the schistosome sequence differs substantially from the Na,K-ATPases of other species.
A rabbit was immunized with a peptide whose sequence was derived from the amino terminus of SNaK1. Anti-SNaK1-peptide antibodies were purified by antigen affinity purification. The crude serum, the flow-through from the affinity column and the purified acid-eluted antibodies were tested for immunoreactivity to proteins from a S. mansoni adult membrane preparation by Western analysis as shown in Fig. 2A. The crude serum (lane 1) and the flow through from the column (lane 2) contained antibodies that recognize multiple proteins in the membrane preparation. In contrast, the purified anti-SNaK1-peptide antibodies detected a prominent protein migrating at the expected size of the full-length SNaK1 protein, at approximately 110000 Mr (Fig. 2A, lane 3, arrowhead).

Fig. 2. Purification of anti-SNaK1 antibodies and developmental expression of SNaK1. (A) Western blot of adult schistosome membrane proteins probed using total serum from a rabbit immunized with a synthetic SNaK1 peptide (lane 1), the flow through after passing the same serum through an anti-SNaK1 peptide antigen affinity column (lane 2) and the purified anti-SNaK1 antibodies that were eluted with acid from the column (lane 3). The single dominant protein detected in lane 3 (SNaK1) is indicated (arrowhead). The positions of molecular mass markers (kDa) are shown, left. (B) Western blot of parasite membrane proteins isolated from eggs (E), cercariae (C), male (M) and female (F) adults probed with affinity purified anti-SNaK1 antibodies. The position of migration of SNaK1 in the egg extract (arrow) and the adult extract (arrowhead) is indicated.
Anti-SNaK1 antibodies, purified as described above, were used to examine the developmental expression of SNaK1 in the following life-stages: eggs, cercariae, adult male and female parasites (Fig. 2B). The protein was detected in all of these life-stages suggesting that it plays a general role creating ion gradients in schistosomes. In adult males and females the protein is detected as a single, sharp band at 110000 (arrowhead, Fig. 2B); in cercariae a broad band of reactivity is seen from 90000 to 110000 Mr and in eggs a band of approximately 125000 Mr is seen (arrow, Fig. 2B). We do not believe that the faster migrating SNaK1 species in cercarial extracts resulted from proteolytic degradation since these were found consistently in multiple experiments prepared in the presence of a cocktail of protease inhibitors (data not shown). Rather, the existence of the variants in cercariae and eggs suggests that the different SNaK1 products may result from alternative splicing or post-translational modifications.
In sections of adult parasites stained with anti-SNaK1 antibodies and detected by indirect fluorescence, immunoreactivity was seen in the subtegumental and peripheral layers (Fig. 3) Staining is most intense along the dorsal surface. Strong staining is detected just beneath the peripheral muscle bundles in what may be the subtegumental cyton network (Fig. 3, arrowheads). Staining is also clearly discernable in the peripheral muscle band. Immunological localization of SNaK1 by electron microscopy confirms clear staining by anti-SNaK1-antibodies in the subtegumental muscle (Fig. 4A) suggesting that SNaK1 plays a central role in muscle contraction in the adult parasite. Immunogold labelling is also detected in the secretory bodies in sections of ducts in this region and over the RER of the presumed gastrodermis (Fig. 4B). Staining is also well localized over neuronal vesicles in axons (Fig. 4C) associated with the peripheral muscle layer.

Fig. 3. Epifluorescence localization of SNaK1 in sections of adult worms. SNaK1 is detected in the peripheral, subtegumental region (arrowheads) in cross-section (A), and longitudinal section through the anterior sucker (B). Higher power magnification, (C). M shows the position of the peripheral musculature. The tegument, whose external boundary is indicated with arrows, does not stain with anti-SNaK1 antibody.

Fig. 4. Localization of SNaK1 in adult worms using electron microscopy. Immunogold labelling is noted in the peripheral musculature (A, arrowheads), in the secretory bodies in sections of ducts in the periphery (B, arrows), over the RER of the presumed gastrodermis (B, arrowheads) and over neuronal vesicles in axons (C, arrowheads) associated with the peripheral muscle layer.
The tegument proper, whose location is indicated by arrows in Fig. 3, stains poorly, if at all, with the antibodies. Immunoelectron microscopy also provides no evidence that SNaK1 is localized in the tegument although non-specific gold labelling is apparent over the spines. Since anti-SNaK1 antibodies do not stain the outer membranes of the tegument, this suggests that the protein does not directly function to help maintain an ionic gradient across the host-interactive worm surface. Nonetheless there is good evidence that the tegument does indeed contain a Na,K-ATPase based on cytochemical, electrophysiological and biochemical work (Noel & Soares de Moura, 1986; Pardon & Noel, 1994). The recent characterization of an amino acid transporter localizing to the apical tegumental surface, whose function shows some sodium dependency, further suggests that a Na,K-ATPase exists in the outer tegumental membranes (Skelly et al. 1999). Since SNaK1 is not detected in the tegument, a second Na,K-ATPase is likely present at this site.
Since the tegument and muscle tissue of adult schistosomes are highly electrically coupled (Fetterer et al. 1980), it is difficult to determine exactly where ouabain exerts its inhibitory effects in schistosomes – by acting directly on tegumental or muscle Na,K-ATPases or on both. We suggest that ouabain is acting on the SNaK1 in muscle since (1) the effect of ouabain on muscle contraction is maximal at 5 min while tegumental depolarization takes 10 min to reach maximum (Fetterer et al. 1981) and (2) non-tegumental Na,K-ATPase has been shown to be much more sensitive to ouabain (Noel & Soares de Moura, 1986). We hypothesize that through its inhibition of SNaK1, ouabain directly induces depolarization and contraction of muscle tissue and this effect is spread passively from the muscle to the tegumental cytoplasm resulting in tegumental membrane depolarization.
We thank Professor Donald Harn for his support, Professor David Halton for helpful comments on the EM images and Hae-Jin Kim for technical help. This work was supported in part by NIH grant AI28499.

Fig. 1. Alignment of the Schistosoma mansoni Na,K-ATPase alpha subunit homologue, SNaK1, with members of the Na,K-ATPase protein family. Predicted transmembrane domains are underlined, the conserved phosphorylation site is indicated (P site) and the conserved, phosphorylated aspartic acid residue (D360) is in bold text. ‘[bull ]’ indicates identity with the SNaK1 sequence. Gaps (indicated by dashes) have been introduced to maximize homology. Sequence motifs used in PCR primer design are double underlined. HUMAN: isoform α 1, accession no. P05023; FLY: Drosophila melanogaster, accession no. P13607; HYDRA: Hydra vulgaris, accession no. P35317.

Table 1. Comparison of Schistosoma mansoni SNaK1 sequence with other P-type ATPases
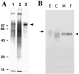
Fig. 2. Purification of anti-SNaK1 antibodies and developmental expression of SNaK1. (A) Western blot of adult schistosome membrane proteins probed using total serum from a rabbit immunized with a synthetic SNaK1 peptide (lane 1), the flow through after passing the same serum through an anti-SNaK1 peptide antigen affinity column (lane 2) and the purified anti-SNaK1 antibodies that were eluted with acid from the column (lane 3). The single dominant protein detected in lane 3 (SNaK1) is indicated (arrowhead). The positions of molecular mass markers (kDa) are shown, left. (B) Western blot of parasite membrane proteins isolated from eggs (E), cercariae (C), male (M) and female (F) adults probed with affinity purified anti-SNaK1 antibodies. The position of migration of SNaK1 in the egg extract (arrow) and the adult extract (arrowhead) is indicated.
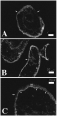
Fig. 3. Epifluorescence localization of SNaK1 in sections of adult worms. SNaK1 is detected in the peripheral, subtegumental region (arrowheads) in cross-section (A), and longitudinal section through the anterior sucker (B). Higher power magnification, (C). M shows the position of the peripheral musculature. The tegument, whose external boundary is indicated with arrows, does not stain with anti-SNaK1 antibody.

Fig. 4. Localization of SNaK1 in adult worms using electron microscopy. Immunogold labelling is noted in the peripheral musculature (A, arrowheads), in the secretory bodies in sections of ducts in the periphery (B, arrows), over the RER of the presumed gastrodermis (B, arrowheads) and over neuronal vesicles in axons (C, arrowheads) associated with the peripheral muscle layer.