Introduction
Paragonimus kellicotti is a trematode and one of over 50 known species of Paragonimus (Blair, Reference Blair2019; Li et al., Reference Li, Li, Li, Li, Xu, Chang, Yang, Wang, Zhang, Ma, He, Wang and Huang2020). Paragonimus species typically infect hosts through ingestion of infected crustaceans, although the potential for infection also exists through ingestion of raw mammalian meat (Blair, Reference Blair2019). In 2005, it was estimated that over 23 million human infections due to Paragonimus occurred, among whom over 5 million were estimated to be heavily infected and over 165 000 had central nervous system infections (Fürst et al., Reference Fürst, Keiser and Utzinger2012). Paragonimus westermani complex and Paragonimus heterotremus (among other species) cause significant human diseases in East and Southeast Asia (Blair, Reference Blair2019). In some of these locations, culinary practices of consuming raw or pickled crabs or crayfish increase the population's exposure to infection (Defrain and Hooker, Reference Defrain and Hooker2002). There are also species of Paragonimus endemic to parts of India (including P. skrjabini and P. heterotremus complex), Africa (including P. Africanus), and Central and South America (including P. Mexicanus) (Procop, Reference Procop2009; Blair, Reference Blair2019).
Paragonimus kellicotti was first named as a species by Henry Ward in 1908 when he described it as an American form that was distinct but closely related to P. westermani (Ward, Reference Ward1908). The lifecycle of P. kellicotti was fully described by Ameel in 1934, and crayfish in Michigan were noted to be infected with P. kellicotti in 1959 (Ameel, Reference Ameel1934; Basch, Reference Basch1959). A 1964 case report was the first to describe North American autochthonous Paragonimus infection in a patient in Montreal who had never been outside of Quebec (Béland et al., Reference Béland, Boone, Donevan and Mankiewicz1969). Although not clear at the time, contemporary knowledge makes it seem most likely that this was P. kellicotti. This phenomenon is not uncommon in the Paragonimus literature, wherein the species is not always identified despite the fact that clinical manifestations may in fact vary by species (Blair, Reference Blair2019). Veterinary literature began reporting infections of dogs and cats by P. kellicotti in the 1980s (Johnson et al., Reference Johnson, Kazacos, Blevins and Cantwell1981; Kirkpatrick and Shelly, Reference Kirkpatrick and Shelly1985; Harrus et al., Reference Harrus, Nyska, Colorni and Markovics1997), and the first human autochthonous case in the USA was reported in 1984 (Pachucki et al., Reference Pachucki, Levandowski, Brown, Sonnenkalb and Vruno1984).
To date, 21 cases of human infection by P. kellicotti have been reported in the USA and Canada with manifestations including eosinophilic pneumonia, cavitary mass lesions, pleural effusion, rash and most recently eosinophilic meningitis (Béland et al., Reference Béland, Boone, Donevan and Mankiewicz1969; Mariano et al., Reference Mariano, Borja and Vruno1986; Defrain and Hooker, Reference Defrain and Hooker2002; Castilla et al., Reference Castilla, Jessen, Sheck and Procop2003; Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009, Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012; Centers for Disease Control and Prevention, 2010; Fischer and Weil, Reference Fischer and Weil2015; Horn et al., Reference Horn, Patel, Hawasli and Edwards2016; Johannesen and Nguyen, Reference Johannesen and Nguyen2016; Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017). Diagnosis is limited by an absence of commercial tests, although diagnosis via histological or microscopic examination is possible and speciality laboratories have serologic tests available. In addition, the syndromes caused by P. kellicotti may appear similar to those due to other infectious or non-infectious causes, particularly if eosinophilia is not recognized. These factors, combined with the relatively small number of human case reports, result in low physician awareness of this parasite although it should be noted that targeted public health campaigns may have improved awareness among physicians and parts of the general public in some areas. Regardless of whether awareness has improved in some groups, overall awareness likely remains low and the incidence of P. kellicotti infection in the USA and Canada may be higher than what is currently recognized. This review aims to summarize the existing literature pertaining to P. kellicotti in order to increase clinician awareness of this pathogen and to highlight areas where further study is needed.
Epidemiology
The global epidemiology of paragonimiasis has been well described and will not be repeated here in full (Madariaga et al., Reference Madariaga, Ruma and Theis2007; Procop, Reference Procop2009; Chai, Reference Chai2013; Diaz, Reference Diaz2013; Fischer and Weil, Reference Fischer and Weil2015; Blair, Reference Blair2019). Over 50 species have been described though many are not known to cause human disease (Blair, Reference Blair2019; Li et al., Reference Li, Li, Li, Li, Xu, Chang, Yang, Wang, Zhang, Ma, He, Wang and Huang2020). Briefly, P. westermani complex is endemic to large parts of East and Southeast Asia, as are P. heterotremus and P. skrjabini which are also present in parts of India (Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009; Chai, Reference Chai2013). Though less commonly described, P. Africanus has caused disease in parts of Africa and P. Mexicanus in parts of Central and South America (Procop, Reference Procop2009; Chai, Reference Chai2013).
Table 1 summarizes the geographic and demographic features of the 21 case reports of autochthonous human infection with P. kellicotti in the USA and Canada (Béland et al., Reference Béland, Boone, Donevan and Mankiewicz1969; Pachucki et al., Reference Pachucki, Levandowski, Brown, Sonnenkalb and Vruno1984; Mariano et al., Reference Mariano, Borja and Vruno1986; Procop et al., Reference Procop, Marty, Scheck, Mease and Maw2000; Defrain and Hooker, Reference Defrain and Hooker2002; Castilla et al., Reference Castilla, Jessen, Sheck and Procop2003; Boé and Schwarz, Reference Boé and Schwarz2007; Madariaga et al., Reference Madariaga, Ruma and Theis2007; Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009, Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012; Horn et al., Reference Horn, Patel, Hawasli and Edwards2016; Johannesen and Nguyen, Reference Johannesen and Nguyen2016; Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017). Paragonimus kellicotti has most commonly been reported in Missouri (n = 15) in recent years (Pachucki et al., Reference Pachucki, Levandowski, Brown, Sonnenkalb and Vruno1984; Mariano et al., Reference Mariano, Borja and Vruno1986; Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009, Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012; Horn et al., Reference Horn, Patel, Hawasli and Edwards2016; Johannesen and Nguyen, Reference Johannesen and Nguyen2016; Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017). Although human disease due to P. kellicotti has not been described in the following locations, it has been described in Lynx rufus and bobcats in Illinois, a skunk in Minnesota, in minks, skunks, foxes and coyotes in Ontario, felines in Louisiana, West Virginia, Arkansas and Georgia and racoons in Kentucky and thus may infect humans in these locations as well (Bemrick and Schlotthauer, Reference Bemrick and Schlotthauer1971; Ramsden and Presidente, Reference Ramsden and Presidente1975; Bech-Nielsen et al., Reference Bech-Nielsen, Fulton, Cox, Hoskins, Malone and Mcgrath1980; Watson et al., Reference Watson, Nettles and Davidson1981; Cole and Shoop, Reference Cole and Shoop1987; Snyder et al., Reference Snyder, Hamir, Nettles and Rupprecht1991; Hiestand et al., Reference Hiestand, Nielsen and Jiménez2014). It would also be reasonable to infer that infection may be possible in the states surrounding those where either human or non-human mammal cases have been described as well.
Table 1. Summary of case reports of P. kellicotti infections originating in North America

MO, Missouri; OK, Oklahoma; MI, Michigan; CO, Colorado; NE, Nebraska; M, male, F, female.
a Patient denied, stated last had sushi 2 years prior.
Among reported cases, humans infected by P. kellicotti range in age from 10 to 71 years old. The majority of cases occurred in young adults, with 10 of the 21 cases occurring in persons <30 years old and another six being between 30–39 years old. All but one case occurred in males, with the single exception being a 26-year-old female (Lane et al., Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012).
Risk factors
In addition to demographic and geographic risk factors, other notable factors were present in many human cases. All but two cases clearly identified the consumption of raw or undercooked crayfish from rivers in the central USA and Canada preceding the infection. Crustaceans in general and crayfish, in particular, are common intermediate hosts of Paragonimus species with crayfish being particularly common for P. kellicotti in North America (Procop, Reference Procop2009). Fifteen of the 21 reported infections were acquired in Missouri, where 37–69% of crayfish in three Missouri rivers are infected with P. kellicotti (Fischer et al., Reference Fischer, Curtis, Marcos and Weil2011). It has been noted that the infective metacercariae of P. kellicotti localize to the heart and pericardium of the crayfish (Procop, Reference Procop2009). Thus, risk of infection may depend on the portion of the crayfish that is consumed. Animal studies suggest that >50% of metacercariae develop into adults (which cause the majority of the pathophysiology) in infected hosts and so eating the portions of the crayfish with the largest concentration of metacercariae makes it quite likely that adult worms will eventually develop in a human who consumes raw or undercooked crayfish (Fischer et al., Reference Fischer, Curtis, Marcos and Weil2011).
Of the 19 patients who clearly consumed raw crayfish, 15 noted this consumption in the context of alcohol intoxication while on river float trips (Procop et al., Reference Procop, Marty, Scheck, Mease and Maw2000; Defrain and Hooker, Reference Defrain and Hooker2002; Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009, Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012; Horn et al., Reference Horn, Patel, Hawasli and Edwards2016; Johannesen and Nguyen, Reference Johannesen and Nguyen2016; Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017). Alcohol intoxication is a major risk factor in that it appears to lead to disinhibition in eating raw or undercooked crayfish. The majority of patients were previously healthy with no known medical conditions with the exception of a 51-year-old patient with asthma and COPD (Béland et al., Reference Béland, Boone, Donevan and Mankiewicz1969), and a 29-year-old patient who had a history of xanthoastrocytoma treated with surgery and radiation 14 years prior (Johannesen and Nguyen, Reference Johannesen and Nguyen2016). Importantly, natural definitive hosts for P. kellicotti include didelphids (such as opossums), canids (such as dogs), felids (such as cats), mustelids (such as weasels), procyonids (such as racoons), suids (such as pigs), bovids (such as cows) and murids (such as mice) (Hiestand et al., Reference Hiestand, Nielsen and Jiménez2014; Blair, Reference Blair2019). In addition to this information being useful simply to increase awareness of the disease in a geographical area when outbreaks occur in these animals, these hosts may present an alternative pathway of infection in humans as paragonimiasis in general can occur due to ingestion of raw meat from a mammalian host (Blair, Reference Blair2019). Furthermore, as part of a sylvatic cycle, these wild mammals are the principal definitive hosts for P. kellicotti (Fischer et al., Reference Fischer, Curtis, Marcos and Weil2011).
Human clinical syndromes
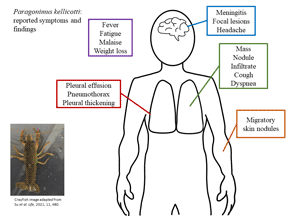
Table 2 summarizes the clinical syndromes described in the 21 human case reports of P. kellicotti infection in the USA and Canada (Béland et al., Reference Béland, Boone, Donevan and Mankiewicz1969; Pachucki et al., Reference Pachucki, Levandowski, Brown, Sonnenkalb and Vruno1984; Mariano et al., Reference Mariano, Borja and Vruno1986; Procop et al., Reference Procop, Marty, Scheck, Mease and Maw2000; Defrain and Hooker, Reference Defrain and Hooker2002; Castilla et al., Reference Castilla, Jessen, Sheck and Procop2003; Boé and Schwarz, Reference Boé and Schwarz2007; Madariaga et al., Reference Madariaga, Ruma and Theis2007; Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009, Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012; Horn et al., Reference Horn, Patel, Hawasli and Edwards2016; Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017). Of note, regardless of symptoms, all reported cases demonstrated clinical signs and all patients sought medical treatment – meaning a certain degree of severity was present in all cases that may infer the presence of less severe cases of human infection that do not present for care. Time from consumption of raw crayfish to development of symptoms ranged from 14 to 75 days (although the majority of reports do not specify an incubation period). Nineteen of the 21 reports describe primarily pulmonary disease. The most common symptoms were cough (n = 17), fever (n = 11), fatigue/malaise (n = 11) and weight loss (n = 11). Headache was reported in five cases, but additional neurological deficits were only reported in the two cases of eosinophilic meningitis (Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017) and one additional case that presented with blurry vision and intermittent blind spots (Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009).
Table 2. Summary of clinical presentations of P. kellicotti infections in North America
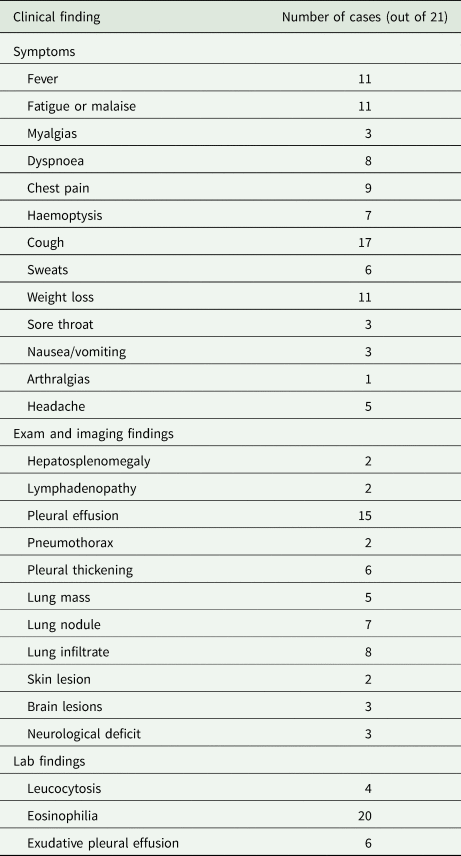
Imaging showed pleural effusions in 15 cases and pleural thickening in six for which two underwent thoracoscopic debridement. Other lung imaging findings included nodules in seven cases, masses in five cases and infiltrates in eight cases. Tender, migratory skin nodules were reported in two cases (Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009). In the two cases of eosinophilic meningitis, non-enhancing brain lesions were found on MRI but were not visible on CT (Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017). In the case describing a person with blurry vision, an initial head CT was also normal, but a later brain MRI showed an enhancing right occipital lobe lesion (Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009).
Leucocytosis was present in only four cases with the highest reported white blood cell count (WBC count) being 12 000 cells μL−1 (Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009). However, 20 of the 21 cases described peripheral eosinophilia ranging from 13 to 38% eosinophils on the WBC differential with absolute eosinophil counts of 849–3600 cells μL−1. The single case that did not report eosinophilia occurred in a man who had been symptomatic for many years and suffered from severe asthma and COPD requiring multiple hospitalizations (Béland et al., Reference Béland, Boone, Donevan and Mankiewicz1969). Six of the reports described diagnostic thoracentesis, all of which revealed exudative pleural effusions with eosinophilia ranging from 18 to 93% of the WBC differential and absolute eosinophil counts ranging from 296 to 2697 cells μL−1 (Pachucki et al., Reference Pachucki, Levandowski, Brown, Sonnenkalb and Vruno1984; Mariano et al., Reference Mariano, Borja and Vruno1986; Defrain and Hooker, Reference Defrain and Hooker2002). Lumbar puncture was performed in the three cases with neurological symptoms. The case by Lane et al. in 2009 had a normal cerebrospinal fluid (CSF) analysis. The two cases of eosinophilic meningitis revealed CSF pleocytosis with 42% eosinophils on WBC differential in one case (absolute count 302 cells μL−1) and 91% (absolute count 2100 cells μL−1) in the other (Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017).
Diagnosis
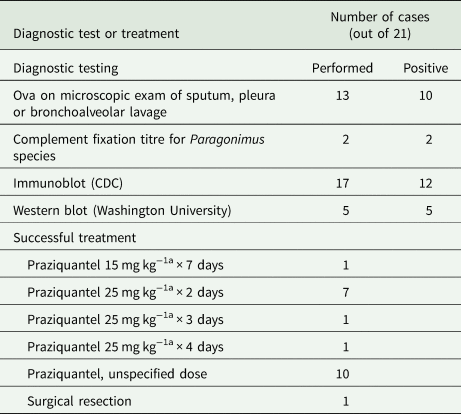
Table 3 summarizes the diagnostic tests used in the 21 human case reports. Previously, complement fixation techniques were often used along with microscopic identification of ova (Béland et al., Reference Béland, Boone, Donevan and Mankiewicz1969; Pachucki et al., Reference Pachucki, Levandowski, Brown, Sonnenkalb and Vruno1984; Mariano et al., Reference Mariano, Borja and Vruno1986). Currently, two additional methods of diagnosis are available in the USA: immunoblot performed by the US Centers for Disease Control and Prevention (CDC) and IgG Western blot performed at Washington University in St. Louis, Missouri (Centers for Disease Control and Prevention, 2010; Lane et al., Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012). Neither of these are commercial tests and are available only by special request. The CDC test reports 96% sensitivity and 99% specificity in diagnosing paragonimiasis, but does not differentiate P. kellicotti from other species of Paragonimus (Johannesen and Nguyen, Reference Johannesen and Nguyen2016). Similarly, though the Western blot is directed against P. kellicotti antigen, it may cross-react with other Paragonimus species and diagnostic accuracy measures are not available, although a 2013 report on serologic diagnosis via Western blot using the P. kellicotti adult worm antigen noted that samples from all 11 proven cases of P. kellicotti and two samples from suspected cases contained antibodies to antigens at 34 kDa and 21/23 kDa (Fischer et al., Reference Fischer, Curtis, Folk, Wilkins, Marcos and Weil2013). All seven sera from P. westermani contained antibodies to the 34 kDa antigen but only two of seven had antibodies to the 21/23 kDa antigen (Fischer et al., Reference Fischer, Curtis, Folk, Wilkins, Marcos and Weil2013). McNulty et al. subsequently identified possible candidate proteins that may correspond to these antigens by utilizing IgG from sera of humans infected with P. kellicotti using mass spectroscopy and transcriptome assembly as well as a worm proteome, although without being able to determine with certainty which candidates correspond to the antigens of interest (McNulty et al., Reference Mcnulty, Fischer, Townsend, Curtis, Weil and Mitreva2014). Among the 21 reported human cases of P. kellicotti infection, 17 report using the CDC immunoblot to aid in diagnosis, which returned positive for 12 patients (Mariano et al., Reference Mariano, Borja and Vruno1986; Defrain and Hooker, Reference Defrain and Hooker2002; Madariaga et al., Reference Madariaga, Ruma and Theis2007; Boé and Schwarz, Reference Boé and Schwarz2007; Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009, Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012; Horn et al., Reference Horn, Patel, Hawasli and Edwards2016; Johannesen and Nguyen, Reference Johannesen and Nguyen2016; Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017). Only five of the cases reported using the Western blot, which was positive in each case (Lane et al., Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012; Horn et al., Reference Horn, Patel, Hawasli and Edwards2016; Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017). Interestingly, Lane et al. report two cases in which the CDC immunoblot results were negative but the Washington University Western blot results were positive for samples from the same patient (Lane et al., Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012).
Table 3. Diagnostic and treatment modalities utilized in the published literature of Paragonimus kellicotti case reports
a Dosed three times daily.
Other diagnostic tests have been studied, including antigen detection and transcriptome sequencing, which both had difficulty differentiating between specific Paragonimus species (Fischer and Weil, Reference Fischer and Weil2015). In 2020, Rosa et al. successfully assembled the P. kellicotti genome and compared it to the genomes of P. westermani, P. heterotremus and P. miyazakii (Rosa et al., Reference Rosa, Choi, Mcnulty, Jung, Martin, Agatsuma, Sugiyama, Le, Doanh and Maleewong2020). They were able to match genes to previously identified antigen targets for potential diagnostic research focused on targets present across these four Paragonimus species, making these antigens attractive targets to detect infection regardless of the species of Paragonimus (Rosa et al., Reference Rosa, Choi, Mcnulty, Jung, Martin, Agatsuma, Sugiyama, Le, Doanh and Maleewong2020). Furthermore, Curtis et al. tested five proteins as potential diagnostic antigens and found that patient IgG4 against cysteine protease-6 and IgG against myoglobin-1 were both 100% sensitive and 100% specific for Paragonimus in relation to controls and other helminth infections, but did not differentiate between P. kellicotti and P. westermani infection (Curtis et al., Reference Curtis, Fischer, Choi, Mitreva, Weil and Fischer2021). IgG against cystatin-2 was 100% specific for P. kellicotti, but was poorly sensitive at 24% (Curtis et al., Reference Curtis, Fischer, Choi, Mitreva, Weil and Fischer2021). Nanocomposix Incorporated has successfully completed a Phase I study of a nanoparticle-based rapid diagnostic test for Paragonimus based on the cysteine protease-6 and myoglobin-1 antigens (Small Business Innovation Research, 2019). The company has applied for funding of a Phase II study to bring their assay to commercialization.
Thirteen of the cases report microscopic examination of sputum, bronchoalveolar lavage, pleural tissue or pleural fluid; 10 of these positively identified ova were characteristic of P. kellicotti (Béland et al., Reference Béland, Boone, Donevan and Mankiewicz1969; Pachucki et al., Reference Pachucki, Levandowski, Brown, Sonnenkalb and Vruno1984; Mariano et al., Reference Mariano, Borja and Vruno1986; Procop et al., Reference Procop, Marty, Scheck, Mease and Maw2000; Castilla et al., Reference Castilla, Jessen, Sheck and Procop2003; Boé and Schwarz, Reference Boé and Schwarz2007; Madariaga et al., Reference Madariaga, Ruma and Theis2007; Lane et al., Reference Lane, Marcos, Onen, Demertzis, Hayes, Davila, Nurutdinova, Bailey and Weil2012; Horn et al., Reference Horn, Patel, Hawasli and Edwards2016; Johannesen and Nguyen, Reference Johannesen and Nguyen2016). The ova are collectively described as operculated, birefringent, golden brown, regularly ovoidal, filled with amorphous material, measuring approximately 85 × 55 μm. Another report described birefringent material in oval-shaped granulomas, although no ova were reported (Defrain and Hooker, Reference Defrain and Hooker2002). Importantly, the period of time necessary for P. kellicotti to produce eggs in a mammalian host ranges from 6 to 8 weeks, so microscopic diagnosis has poor sensitivity in early phases of clinical illness (Fischer and Weil, Reference Fischer and Weil2015).
Treatment
As shown in Table 3, praziquantel is the typical treatment for human infection with P. kellicotti. The most common dosing regimen of praziquantel described with subsequent cure of P. kellicotti infection was 25 mg kg−1 three times daily for 2 days. One patient was treated for 3 days with the same dose due to a concern about possible cerebral involvement (Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009). Another patient was treated for 4 days; the reason for extended treatment duration was not specified (Horn et al., Reference Horn, Patel, Hawasli and Edwards2016). One patient was treated with 15 mg kg−1 dosed three times daily for 2 days but subsequently relapsed after 1 month and required repeat treatment (Defrain and Hooker, Reference Defrain and Hooker2002). These clinical outcomes are consistent with Japanese studies of P. westermani and Paragonimus miyazakii infection, which report 86–100% cure rates with a 2-day course of praziquantel 25 mg kg−1 three times daily and 100% cure rates with a 3-day course (Uchiyama, Reference Uchiyama1998). Veterinary literature describes the successful use of albendazole to treat P. kellicotti infection of felines, but this usage has not been reported in humans (Johnson et al., Reference Johnson, Kazacos, Blevins and Cantwell1981). A possible additional treatment option is triclabendazole, which has been successfully used as an alternative to praziquantel in South America to treat Paragonimus infections (Keiser et al., Reference Keiser, Engels, Büscher and Utzinger2005). In 2019, the FDA approved triclabendazole for the treatment of fascioliasis, but not paragonimiasis (Cox, Reference Cox2019).
Prior to diagnosis with P. kellicotti, many patients were misdiagnosed with autoimmune inflammatory diseases of the lung and thus treated with corticosteroids with temporary relief of symptoms but subsequent relapse after steroid taper (Pachucki et al., Reference Pachucki, Levandowski, Brown, Sonnenkalb and Vruno1984; Mariano et al., Reference Mariano, Borja and Vruno1986; Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009). One of the patients described by Bahr et al. in 2017 was treated with corticosteroid therapy in addition to praziquantel in the setting of severe critical illness due to concern for increased intracranial inflammation secondary to anti-parasitic treatment as is seen in other parasitic causes of eosinophilic meningitis.
Discussion
Paragonimus kellicotti is an increasingly reported infection in the central USA with three reported cases over 30 years from 1969 to 1999, eight cases over 10 years from 2000 to 2009, and 10 cases over 8 years from 2010 to 2017 (Table 1). However, these numbers do not necessarily reflect the true incidence of exposure nor infection (as unrecognized, less symptomatic or otherwise unreported cases exist but are not published) although they do give us a general sense of increasing awareness of the disease. Many characteristics of human infection with P. kellicotti remain unknown, including the infectious dose, the range of possible clinical syndromes, the rate of spontaneous cure and the best treatment regimens.
To date, there have not been any studies of antibody prevalence on a population basis to study the true geographic distribution of P. kellicotti human infection in North America. Such studies could also provide evidence of more benign clinical syndromes if indeed more individuals have been infected than previously thought without requiring medical attention. If that is the case, then the severe cases reported thus far deserve deeper investigation to identify potential rare risk factors that predispose individuals to such severe disease. Conversely, if the prevalence of P. kellicotti antibodies truly is rare, that would suggest that the severe clinical syndromes reported thus far are accurate representations of typical P. kellicotti infection. Additionally, many types of mammals serve as definitive hosts (including among domestic pets) (Blair, Reference Blair2019). Mammalian infection patterns could be utilized to improve our understanding of geographic areas with the potential for human exposure as well.
The lack of commercial testing also limits our understanding of the true spectrum of disease due to P. kellicotti. Currently, only severe infections among individuals with access to medical care are detected and reported. While most reports have described pulmonary disease, the two cases of eosinophilic meningitis reported by Bahr et al. in 2017 along with the dermatologic and neurologic findings reported by Lane et al. in 2009 suggest that P. kellicotti could be responsible for a wider array of clinical syndromes than previously thought – similar to its more reported relative P. westermani.
Furthermore, this lack of rapid commercial testing for P. kellicotti also makes definitively diagnosing this infection difficult, although characterizations of the ova by pathologists in the right epidemiologic and clinical settings are highly suggestive. Both currently available serologic tests are only available at speciality laboratories where approval to submit specimens is required and shipping to these laboratories can cause delays. Thus, a definitive diagnosis of P. kellicotti infection may not occur rapidly enough to inform clinical decisions. Indeed, empiric treatment with praziquantel is effective against many parasitic infections and, as reported in many of the case reports, can result in clinical remission before the Paragonimus serologic results return. Commercial testing options are needed to lower the barrier that currently impedes timely definitive diagnosis of P. kellicotti infection. Despite the helpful nature of the laboratories that do testing for Paragonimus in the USA, these are not commercial laboratories designed to streamline throughput rapidly for clinical care. A direct correlation has been previously demonstrated between the lack of specific diagnostic testing availability and clinician unawareness of the specific infectious aetiology (Zhang et al., Reference Zhang, Mnzava, Mitchell, Melubo, Kibona, Cleaveland, Kazwala, Crump, Sharp and Halliday2016). Thus, improving the availability of specific testing for P. kellicotti could positively impact clinician awareness of this parasite in North America, leading to quicker diagnosis and treatment. Various types of tests have been developed for other Paragonimus species including polymerase chain reaction, next-generation DNA sequencing, transcriptome sequencing, antigen-detection assays, intradermal skin tests, haemagglutination assays, enzyme-linked immunosorbent assays and immunochromatographic strip tests (Fischer and Weil, Reference Fischer and Weil2015). More funding and research are needed to further develop and adapt these testing modalities for specific detection of P. kellicotti infection with logistics conducive to clinical diagnostics. The rapid diagnostic test in development by Nanocomposix Incorporated appears to be one of the most advanced clinical diagnostic candidates in terms of reaching commercialization but requires further funding and research to obtain Food and Drug Administration approval in the USA.
The small number of P. kellicotti infections reported to date also limits the ability to study the best treatment regimens for these infections. The successful praziquantel treatment regimens in the 21 cases reported in North America suggest that praziquantel 25 mg kg−1 three times daily for 2 days is adequate for most infections although whether a longer duration might be considered for infection in certain anatomic locations is unclear. Whether triclabendazole or albendazole might have a role is unknown. Treatment trials would be difficult due to the small number of identified cases unless increased disease awareness and test access lead to increased incidence of significant infections. Even if prevalence studies are performed and identify a larger number of sub-clinical or less severe infections, if the disease is self-limited, therapeutical trials may not be necessary given spontaneous remission, which has been reported in infections from other Paragonimus species (Kimura et al., Reference Kimura, Kikui, Tsuyuguchi and Kishimoto1993).
Five of the reported cases describe the use of corticosteroids with subsequent clinical improvement of symptoms (Pachucki et al., Reference Pachucki, Levandowski, Brown, Sonnenkalb and Vruno1984; Mariano et al., Reference Mariano, Borja and Vruno1986; Lane et al., Reference Lane, Barsanti, Santos, Yeung, Lubner and Weil2009; Bahr et al., Reference Bahr, Trotman, Samman, Jung, Rosterman, Weil and Hinthorn2017). Reports of infections by other Paragonimus species also specify clinical improvement after corticosteroids (Kimura et al., Reference Kimura, Kikui, Tsuyuguchi and Kishimoto1993; Ravin and Loy, Reference Ravin and Loy2016; Xia et al., Reference Xia, Chen and Chen2019). However, corticosteroids have long been known to decrease eosinophil counts (Shoenfeld et al., Reference Shoenfeld, Gurewich, Gallant and Pinkhas1981), which are essential to the immune system's defence against parasitic infection (Ravin and Loy, Reference Ravin and Loy2016). Thus, further study is needed to elucidate the potential benefits and harms of corticosteroids in the treatment of infection with P. kellicotti.
Conclusion
Paragonimus kellicotti is an increasingly reported human infection in North America that is almost always associated with the consumption of raw crayfish in the setting of alcohol intoxication. Most cases occur in young men, and although commonly reported in Missouri, human cases have been reported elsewhere. Additionally, reports of veterinary infections throughout larger portions of the southern USA and Ontario, Canada provide clues to the possibility of unrecognized human disease in those regions as well. Severe infections most commonly manifest as eosinophilic pneumonia and pleuritis in otherwise healthy young adults, but more recent case reports have also demonstrated central nervous system disease. Human infections with P. kellicotti are likely underrecognized and underreported due to low awareness of this pathogen among physicians outside of areas where this disease is commonly reported, the similarity of the syndromes caused by P. kellicotti and other human pathogens, and the absence of commercially available diagnostic tests. Population prevalence studies and improved access to species-specific diagnostic testing are needed to better characterize the geographic distribution, define the clinical spectrum, increase clinician awareness, and determine the best treatment regimen for this increasingly threatening infectious disease. Molecular and microbiologic research in this area has advanced in recent years and holds promise for patient care, particularly in relation to diagnostic testing, but requires steady attention and funding from private and public sources to move clinical practice. As with many zoonotic conditions, physicians caring for humans would be well served to be aware of rises in veterinary cases in their areas to alert them to the possibility of human disease as well.
Acknowledgements
The authors acknowledge and appreciate institutional support from Dr Matthias Salathe.
Author contributions
B.C., S.S. and N.B. conducted literature searching and data gathering. B.C. wrote the manuscript. N.B. and S.S. provided editing. N.B. supervised the project and is its guarantor.
Financial support
Dr Bahr receives research support from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health, K23NS 110470.
Conflict of interest
None.
Ethical standards
Not applicable.