Published online by Cambridge University Press: 06 October 2004
We monitored temporal changes in the magnitude of altered host behaviour in minnows (Pimephales promelas) experimentally infected with metacercariae of a brain-encysting trematode (Ornithodiplostomum ptychocheilus). This parasite develops and then encysts in a region of the brain that mediates the optomotor response (OMR), an innate behaviour that links visual stimuli with motor performance. The OMR of infected and uninfected minnows was evaluated between 0 and 10 weeks post-infection (p.i.), an interval spanning the development period of metacercariae to infectivity in birds. Trials involved monitoring the time an individual minnow spent following a spinning drum that had been painted with alternating black and white stripes. At 2 and 4 weeks p.i., infected minnows followed the drum 40% less often than controls. Differences between controls and infected fish declined thereafter, and were undetectable by 10 weeks p.i. Both control and infected fish habituated equally rapidly to the spinning drum. However, the difference in performance between controls and infected fish was 29% for experienced fish and 48% for fish that had never experienced the drum. Because maximum parasite-induced reduction in OMR coincided with the period of maximum parasite development, the behavioural effects are most likely due to unavoidable pathology in the brain associated with developing larvae.
Despite extensive interest in the phenomenon of parasite-induced alteration in host behaviour (reviews by Poulin, 2000; Moore, 2002), distinguishing those alterations that increase parasite reproduction from those that arise as side-effects of infection remains a difficult task. This is an important shortcoming, because it is central to our understanding of the adaptive nature of parasite induced changes in host behaviour. One problem is that ideally, distinguishing these two outcomes requires that comparisons in appropriate host behaviours be made between uninfected hosts and experimentally-infected ones (Moore, 2002). The problem is especially elusive for parasites with complex life-cycles that require transmission of infective stages into an appropriate final host. These types of parasites are notoriously difficult to rear under laboratory conditions, yet it is these types of parasites that provide some of the best examples of behavioural alterations that benefit parasite reproduction. This constraint probably best explains why so few of over 100 described cases of altered host behaviours can be attributed to a particular adaptive scenario (Poulin, 2000).
One indirect method that has been used to evaluate the adaptive nature of altered behaviours is to determine whether the timing and extent of the changes coincide with the period when the parasite is infective to its next host (Poulin, Brodeur & Moore, 1994; Moore, 2002). Compelling supportive evidence for a parasite adaptation exists if the onset of the behavioural change coincides with the onset of infectivity (Bethel & Holmes, 1974; Tierney, Huntingford & Crompton, 1993; Robb & Reid, 1996; Levri & Lively, 1996). If the behavioural change occurs prior to parasite infectivity, and does not persist, then the change in host behaviour is unlikely to be parasite adaptive. For example, altered behaviours may result from pathology or energy constraints associated with a developing parasite's requirement for specific types, or amounts, of host nutrients within particular sites (Barber, Hoare & Krause, 2000). In this case, altered behaviours may simply arise in moribund hosts, in which case they may not necessarily favour parasite transmission.
Fathead minnows (Pimephales promelas) are commonly infected by metacercariae of the trematode Ornithodiplostomum ptychocheilus, Faust. In most fathead populations in Alberta, Canada, particularly those associated with breeding populations of piscivorous birds (final host), most fish are exposed to larvae released from Physid snails (first intermediate host) when they are between 2 and 5 months of age (Sandland, Goater & Danylchuk, 2001). Thus, prevalence of O. ptychochelus infection in most minnow populations in Alberta is 100%, with individual adults harbouring up to 500 metacercariae (Sandland et al. 2001). The metacercariae are encysted immediately beneath the outer surface of the optic lobes (Hendrickson, 1979; Radabaugh, 1980; Sandland & Goater, 2000). This structure is the primary vision-processing centre in teleost fishes (Guthrie, 1986) and it is here where visual information concerning movement, shape, and colour are analysed and where stimuli from the lateral line system are received (Springer, Easter & Granoff, 1977; Kortrschal et al. 1991). Encystment of O. ptychocheilus in this site is associated with reduced survival of juvenile fish and with altered cranial morphology (Sandland & Goater, 2001).
Encystment in the optic lobes is also associated with a reduction in behaviours associated with host vision (Shirakashi & Goater, 2001, 2002). In our earlier studies we assessed visual performance by evaluating the optomotor response (OMR). This is an innate component of rheotaxis and is defined as a locomotory response to visual stimuli received as an animal moves relative to its background (Rock, Tauber & Heller, 1964; Rock & Smith, 1986; de Peyster & Long, 1993). The OMR of fish has been shown to play important roles in the detection of moving objects (Schaerer & Neumeyer, 1996), and in the recognition of conspecifics (Shaw & Tucker, 1965). Trematode-induced alterations in visual acuity and associated performance could therefore affect the ability of minnows to detect predators, and/or their ability to respond to them via alterations in features such as school cohesion. Individual parasites would benefit from such an alteration if it increased their probability of ingestion by final hosts.
In this experiment, we evaluated the extent of trematode-induced alteration in minnow behaviour relative to the development of the parasite. Larvae of this parasite undergo a 6–10 week period of development within the optic lobes prior to infectivity (Sandland & Goater, 2000). We also evaluated the role of host experience, by comparing OMR performance among fish that experienced optomotor trials over a period of time, with naïve fish that had not. Thus, the aim of this experiment is to monitor changes in OMR of minnows exposed to known numbers of O. ptychocheilus larvae relative to parasite development and host experience.
Uninfected juvenile fathead minnows (<20 mm total length) were collected from a pond (Sterling pond, located 30 km south of Lethbridge, Alberta, Canada) on 5 August 2000. The fish were transferred in groups of 100 into 9, 1200 litre outdoor artificial ponds for 8 weeks. The fish grew at rates equal to, or greater, than in natural populations and they were never exposed to O. ptychocheilus larvae. One week prior to the start of the optomotor trials, minnows were relocated to 9 indoor aquaria (60 cm long×30 cm wide×20 cm high) that were held at 20 °C and on a constant 16[ratio ]8 light[ratio ]dark photo-period. Each aquarium was provided with a constant ration of Tetramin fish flakes 3 times daily for the duration of the experiment.
Because of the complex life-cycle of this trematode, experimental infections required a source of infected minnows, snails (Physa gyrina) and an avian definitive host. Infected minnows were collected (17–20 July 2000) from a site in central Alberta (Rochester Lake; Lat. 54 °22′, Long. 113 °27′) that was known to contain minnows with high intensities of larval O. ptychocheilus. Adult snails were collected from the same lake on the same date. Day-old chickens purchased from a local hatchery were used as surrogate definitive hosts. For infections, we fed the brains of adult minnows to chickens; 5 days later their faeces were collected and sorted for parasite eggs (Sandland & Goater, 2000). The eggs were incubated in the dark for 12–14 days at 20 °C in tapwater. Miracidia hatched from the eggs and were exposed to F1 snails in groups of 5/snail for 3 h in 3 ml tubes. Exposed snails released larvae (cercariae) 28 days later.
Minnows were exposed to cercariae following methods described by Sandland & Goater (2000). On 27 (Block 1) and 28 (Block 2) September 2000, 2 to 3-h-old larvae from 3 infected snails were pooled together and their density in 500 ml of water estimated by dilution. From this cercariae/water solution, the estimated volume required to contain 120 cercariae, or an equal volume of water for controls, was pipetted into 300 ml plastic containers. Individual minnows were selected haphazardly from the 9 stock aquaria and assigned at random into the containers containing cercariae for 3 h. A total of 110 size-matched fish (19–22 mm) were exposed to cercariae on the 2 infection dates. The exposure dose of 120 was chosen based on the maximum O. ptychocheilus intensity in juvenile fathead minnows sampled between 1995 and 1999 from 2 north-central Alberta lakes (Sandland et al. 2001) and to parallel our earlier study (Shirakashi & Goater, 2001).
For fish, the OMR can be simulated in the laboratory by monitoring the duration that fish swim in the same direction, and with the same velocity, as a moving screen (Shaw & Tucker, 1965; Springer et al. 1977; Anstis, Hutahajan & Cavanagh, 1998). The optomotor apparatus was constructed based on the design by Shaw & Tucker (1965) and is detailed in Shirakashi & Goater (2001). It consisted of a cylindrical glass aquarium (30 cm diameter×30 cm height) surrounded by a rotating cylindrical drum (40 cm diameter×30 cm height), onto which alternating 25 mm black and white stripes were painted. A small rubber wheel attached to an electric motor rotated the drum in either direction at approximately 15 rpm while the aquarium remained stationary. Fish activity and behaviour were recorded with a 8-mm video camera, mounted 50 cm directly above the aquarium. A 60-watt incandescent light bulb was placed above the apparatus.
The first optomotor trials were performed prior to infection (27 and 28 September 2000) and then repeated at 2 week intervals for 10 weeks post-infection. We monitored the OMR of 12 size-matched infected and control fish following the general methods of Springer et al. (1977) and applied to fathead minnows by de Peyster & Long (1993). The 10-week period was selected to ensure that we encompassed the pre-encystment and post-encystment phases of O. ptychocheilus development within the optic lobes (Sandland & Goater, 2000). At each 2-week interval, individuals were selected at random from their container and placed into the apparatus with aged tapwater at 20 °C. First, fish were acclimatized to the OMR apparatus for 2 min. During the following 4 min, the drum remained stationary, during which the fish's general activity and directional preference was video-monitored. The drum was then rotated in a randomly selected direction for the following 2 min. Following this period of acclimation, the OMR was monitored for the following 2 min in one direction, and then in the other direction for the subsequent 2 min.
The effects of O. ptychocheilus were evaluated on two components of the OMR (Shirakashi & Goater, 2001). The first, ‘following time’, was defined as the time that minnows spent following in the direction of the spinning drum. The second, ‘latency time’, was defined as the time it took for fish to respond to a change in the drum's direction of spin. To evaluate ‘following time’, the total available time that a fish could potentially follow the stripes was calculated as 4 min minus latency time. This removed any potential covariation between the two response variables. Thus, ‘following time’ was represented as the proportion of time that a fish followed the stripes, relative to the total time that the stripes moved after they were first detected. As in our previous experiment (Shirakashi & Goater, 2001), it was important to distinguish parasite effects on OMR performance from those on host activity. The latter was evaluated as the proportion of time during the initial 4 min acclimation period that fish spent in motion and was used as a covariate in the subsequent analyses. Lastly, minnows were measured for standard length and returned to the stock aquaria. After the 10 week trial, the brain of each fish was removed from the braincase, squashed between 2 glass slides, and then observed under a dissecting microscope to obtain parasite counts.
In addition to parasite-induced effects on host vision and activity, it is possible also for the parasites to affect a minnow's ability to learn the optomotor apparatus. To test for an experience effect, the optomotor performance of an additional group of 24 naïve fish (12 infected and 12 uninfected) was evaluated at 10 weeks, and then compared with a 2-way (experience×infection) ANCOVA where host activity was the covariate. A significant experience×infection interaction would provide evidence for an effect of infection on the ability for minnows to learn to swim with the moving drum. The 24 unexperienced fish were reared under the same conditions as all other fish.
Parasite infectivity was assessed at 2, 4, and 10 weeks p.i. (post-infection). At each interval, 6 day-old chickens were each fed the brains of 3 minnows that had been exposed to 120 O. ptychocheilus larvae. Thus, each bird was exposed to approximately 360 infective metacercariae. At 120 h, the chickens were killed and dissected. The small intestine of each bird was removed, straightened lengthwise, and then cut into 20 equal sections. The mucosal surface of each section was scraped into a Petri dish, mixed with water, and then examined under a dissecting microscope. Parasites were counted and then examined under a compound microscope for the presence of eggs in the uterus.
OMR data were tested for normality using Shapiro-Wilkes tests. Analyses involving ‘following time’ used proportional data that were arcsin (square-root) transformed. Analyses involving ‘latency time’ were log-transformed. The effect of parasite intensity on ‘following time’ was analysed using a combined within-subject and between-subject ANCOVA, with direction being a within-subject factor, and parasite intensity and time (weeks) being between-subject factors. This is a repeated measures ANOVA since the same individuals were monitored during the first 2 min and in the subsequent 2 min after the change in direction of the screen. In this case, the repeated measure tests for an endurance effect; its interaction with ‘infection’ therefore tests for a potential effect of parasites on endurance. Host size and host activity were covariates in the analyses. Fisher's LSD multiple comparison tests were used to determine if there were differences in performance between infected and uninfected fish at each 2-week interval.
At 10 weeks p.i., all fish exposed to cercariae harboured fully-encysted metacercariae in their brains. Mean parasite intensity was 108±15 (N=12) representing approximately 90% parasite recovery. Metacercariae infectivity in chickens was zero at 2 and 4 weeks p.i. In contrast, the 6 birds dissected at 10 week contained 118±41 parasites, representing 36·5±12·7% metacercariae recovery.
The proportion of time that minnows spent active during the 4 min interval before the drum was spun was not affected by host length (F1,131=1·379, P=0·242), infection (F1,131=2·539, P=0·113), or the Time×Infection interaction (F5,131=0·562, P=0·729). A slightly significant effect of time on activity (F5,131=2·222, P=0·056) is due to the difference in the proportion of time minnows spent active during the pre-infection trial (mean=12·9±6·1) compared to the 4 weeks p.i. trial (mean=25·6±13·5). Mean minnow activity remained approximately constant thereafter (6 weeks=24·3±11·6; 8 weeks=20·9±11·5; 10 weeks=26·8±12·6). Because minnow activity was not affected by infection, activity was used as a covariate in subsequent analyses.
Following time was affected by host activity, time, and infection (Table 1). The significant Infection×Weeks interaction indicates that the manner in which optomotor performance changed over the 10-week period was affected by infection. Thus, although the following times of infected fish were always lower than controls (Fig. 1), maximum differences between control and infected fish occurred at 2 and 4 weeks p.i. During this period, the mean following time of controls exceeded mean following time of infected fish by 38·6 and 39·2%, respectively. Fisher's tests indicated that although uninfected minnows always outperformed infected minnows, only the differences at 2- and 4-weeks p.i. were significant.

Fig. 1. Proportion of time minnows spent swimming in the direction of moving stripes in performance trials run at 2-week intervals. Filled bars represent means (+S.E.) calculated for 10–12 uninfected minnows (controls). Empty bars represent means (+S.E.) calculated for 12 infected minnows. The L designation on the x-axis represents mean (+S.E.) following time of 12 minnows with 10-week-old infections that had not had prior experience with the optomotor apparatus. Dark and light stripes represent control, and infected minnows, respectively.
Table 1. Summary of repeated measure ANCOVA that tested for the effects of infection with larval Ornithodiplostomum ptychocheilus, time (weeks), and direction (the repeated measure) on the proportion of time minnows followed the direction of a spinning drum (Performance data were arcsin (square root) transformed. Host activity was evaluated prior to the spinning of the drum and was used as the covariate.)

Response latency was highly variable between treatments and between weeks. The mean times (in seconds) fish took to respond to the changes of direction of drum spin at the 2, 4, 6, 8, 10 week intervals were 8·3±5·2 (N=23), 9·6±12·0 (N=24), 8·1±5·4 (N=24), 7·7±5·2 (N=22), and 8·2±5·0 (N=22), respectively. Total activity (the covariate) had no effect on latency (F1,126=0·04, P=0·835), nor did infection (F1,126=1·57, P=0·212) or time (F4,126=1·08, P=0·342).
Analyses restricted to the optomotor trials at 10 weeks p.i. showed that following time was affected by host activity (F1,43=6·21, P=0·0166), infection (F1,48=6·25, P=0·0163), and prior experience with the apparatus (F1,48=21·88, P=0·0001; Fig. 1). The 2-way Experience×Dose interaction was also significant (F1,43=4·96, P=0·0312; Fig. 1). At 10 weeks p.i., the differences in following time between experienced and naïve fish were 28·6 and 48·2% for controls and infected fish, respectively. Following time was not affected by the repeated measure (direction of the drum) (F1,43=0·56, P=0·457) and none of the factor×repeated measure interactions were significant.
Maximum parasite-induced reduction in OMR occurred at 2 and 4 weeks p.i., when the parasites were not infective to birds. This period coincides with maximum rates of development of O. ptychocheilus metacercaria. Sandland & Goater (2000) described distinct growth, encystment, and consolidation phases of metacercariae development in the brains of minnows. Maximum growth occurred between 1 and 4 weeks p.i when larvae increased their length by approximately 5×. During this period, the entire body surface of the metacercaria is covered by countless microlamelli and villi that extend into adjacent brain tissue, indicating an absorptive function (Goater, unpublished observations). After 4 weeks p.i., the microlamelli disappear, the surface of the parasite becomes smooth, and growth apparently ceases (Sandland & Goater, 2000). It is at this time that the cyst wall is formed, following which the metacercariae enter their resting stage. Although nothing is known regarding the nutrient requirements of metacercariae, and we do not know the extent to which they feed directly on brain tissue, the growth phase coincides with distortion of the cranium and adjacent tissues and is also associated with increased host mortality (Sandland & Goater, 2001). Thus, the period of maximum reduction in OMR performance coincided with pathological damage imposed by O. ptychocheilus during its obligatory period of development within the optic lobes.
The most straightforward interpretation is that reduction in optomotor performance during the non-infective stage is due to unavoidable pathology (sensu Moore, 2002) associated with development in the optic lobes. Presumably, some pathology should be expected for larval parasites such as O. ptychocheilus and others that are associated with the host's nervous system (review by Barber et al. 2000). This is especially the case for those species that require an obligate period of growth prior to encystment and infectivity. If unavoidable pathology coincides with the non-infective stage of the parasite, then any altered behaviours that arise are possibly non-adaptive consequences of parasite development. For the O. ptychocheilus/minnow system, the best evidence for this interpretation lies in the temporal link between maximum reduction in OMR performance, and maximum parasite growth rate within a critical structure that determines the performance component of visual acuity.
Reductions in OMR prior to infectivity may be adaptive under some scenarios. One possibility is that altered OMR may reduce the risk of predation by potential definitive hosts, or by hosts that are not involved in the life-cycle. Levri (1998) showed that snails harbouring non-infective metacercariae altered their selection of microhabitats to reduce their rates of predation by non-hosts. Uninfected snails and those with mature metacercariae did not. However, we consider this adaptive scenario to be unlikely for fathead minnows, where any reductions in visual performance would presumably lead to a decrease in their ability to forage, and to detect and respond to a range of predators.
We do not know the relative importance of particular final hosts to the transmission of O. ptychocheilus in local lakes and ponds. Since we can utilize domestic chickens as surrogate final hosts, we presume that a subset of the large numbers of species of breeding piscivorous birds can act as final hosts in nature. In this case, natural selection may be weak for alterations to intermediate host behaviour, particularly under conditions of 100% prevalence when infection of the definitive host is possible with virtually every ingested minnow. We need more information on the precise role of potential definitive hosts to O. ptychocheilus transmission. Subsequent studies also need to evaluate the consequences of reduced OMR to rates of avian predation under field conditions (e.g. Lafferty & Morris, 1996).
Results from the performance trials that compared experienced and non-experienced fish provide preliminary evidence that infection may affect rates of habituation, and possibly learning. At 10 weeks p.i., when the parasites were infective to birds, infected naïve minnows did not improve their OMR to the same extent as infected experienced minnows. This result indicates that infection reduced the ability of minnows to detect, or respond to, the moving bars. The significance of this result to natural populations of minnows, and to parasite transmission, is difficult to judge. One possibility is that infection may lead to a reduction in the ability of minnows to detect novel stimuli. This could have widespread ecological consequences, particularly in the context of avoiding predators and feeding on novel prey. The influence of parasites on host learning and cognition has been demonstrated in other systems (Kvalsvig, 1988; Kavaliers, Colwell & Galea, 1995), although the adaptive nature of such changes have not been evaluated. For the minnow/brainworm interaction, a full test of parasite-induced effects on host learning require follow-up experiments on specific components of host learning throughout the parasite's development cycle.
Our results must be interpreted in the context of natural patterns of O. ptychocheilus transmission. The infection procedure in this study involved hosts exposed to a single dose of large numbers of infective larvae. Although Sandland et al. (2001) showed that juvenile minnows can enter their first winter with >100 O. ptychocheilus metacercariae in their brains, these parasites would have accumulated over a period of approximately 5 weeks at the end of summer. Thus, the single exposure to 120 cercariae used in our experiment is unlikely to reflect natural transmission. This is an important consideration in the interpretation of pathogenic consequences of infection, especially at 2 and 4 weeks p.i. when all larvae would be undergoing rapid, simultaneous development within the optic lobes. This likely contrasts with the natural situation, where individual minnows contain both pre-infective and infective metacercariae at most times of the year. In this experiment, all metacercariae developed simultaneously and the main effects at 2 and 4 weeks p.i. occurred in the absence of encysted (i.e. infective) stages. Because even small numbers of encysted worms affect optomotor behaviour (Shirakashi & Goater, 2001), interpretations that emphasise the importance of effects of pure infections of unencysted larvae should be treated with caution.
Moore (2002) discussed the notion of unavoidable pathology in the context of parasite-induced altered host behaviours. Examples of such pathology are common in parasite/host systems (limb alterations in trematode-infected frogs; distended abdomens in Schistosome-infected humans; grossly distended limbs in filarid-infected humans) and such pathology is often associated with altered host behaviours (Poulin & Thomas, 1999). However, our understanding of the association between pathology and parasite transmission is poor. For O. ptychocheilus, the magnitude of the difference between the OMR performance of infected fish and controls gradually declined as the infection aged. This pattern provides initial evidence that altered behaviours that could affect parasite transmission represent persistent carry-over effects of pathology that occurred shortly after infection. Under this persistent-pathology scenario, site-selection itself could be considered parasite adaptive, so long as unavoidable pathology within the site persists up to the time of parasite infectivity.
We thank Yukiyo Kojima for assistance with construction of the optomotor apparatus and with video-monitoring minnow behaviour. Thanks to J. Holmes, A. Hurly, and S. Pellis for comments on an earlier version of the manuscript. All minnows used in this study were collected and infected under permit. This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to C.P.G.
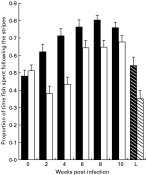
Fig. 1. Proportion of time minnows spent swimming in the direction of moving stripes in performance trials run at 2-week intervals. Filled bars represent means (+S.E.) calculated for 10–12 uninfected minnows (controls). Empty bars represent means (+S.E.) calculated for 12 infected minnows. The L designation on the x-axis represents mean (+S.E.) following time of 12 minnows with 10-week-old infections that had not had prior experience with the optomotor apparatus. Dark and light stripes represent control, and infected minnows, respectively.
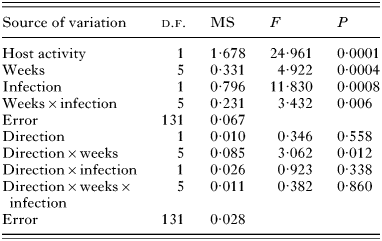
Table 1. Summary of repeated measure ANCOVA that tested for the effects of infection with larval Ornithodiplostomum ptychocheilus, time (weeks), and direction (the repeated measure) on the proportion of time minnows followed the direction of a spinning drum