INTRODUCTION
One of the most important determinants of congestive heart failure and sudden death in Latin America is Chagas disease, provoked by infection with the intracellular protozoan parasite Trypanosoma cruzi. It affects approximately 20 million people (World Health Organization, 2007; Moncayo and Silveira, Reference Moncayo and Silveira2009) and represents a serious public health problem in Central and South America (Biolo et al. Reference Biolo, Ribeiro and Clausell2010). Chagasic cardiopathy appears to carry the worst prognosis (Pereira et al. Reference Pereira Silva, Del Carlo, Tavares de Oliveira, Scipioni, Strunz Cassaro and Franchini Ramírez2009) and has become the most frequent cause of heart failure and sudden death, as well as the most common cause of cardio-embolic stroke in Latin America. Chagas disease also represents an increasing challenge for clinicians in the United States (Bern et al. Reference Bern, Montgomery, Herwaldt, Rassi, Marin-Neto, Dantas, Maguirre, Acquatella, Morillo, Kirchhoff, Gilman, Reyes, Salvatella and Moore2007) and some European countries (Reesink, Reference Reesink2005) due to the continuous immigration of people from disease-endemic countries (Polo- Romero et al. Reference Polo-Romero, Beato-Pérez and Romero-Portilla2011).
There are 3 stages in the evolution of Chagas disease: the acute phase with a local inflammatory lesion that appears at the site where the metacyclic trypomastigotes entered, and the parasite spreads throughout the host organism (Prata, Reference Prata2001; Umezawa et al. Reference Umezawa, Stolf, Corbett and Shikanai-Yasuda2000); the cardiac chronic phase in which the diversity and severity of the symptoms range from a mild electrocardiographic alteration to sudden death due to cardiac dysrhythmias, varying in different patients and in different regions (Storino and Milei, Reference Storino and Milei1994). Between the acute and the cardiac chronic phases exists a period called the chronic indeterminate stage, which is generally symptomless and may last for 10 to 20 years (Macedo, Reference Macedo1999; Ribeiro and Rocha, Reference Ribeiro and Rocha1998). More sensitive tests have demonstrated that patients in this stage may present significant abnormalities (Ribeiro and Rocha, Reference Ribeiro and Rocha1998). According to cardiologists, this period could be the key to recognizing which patients will develop the cardiac form of infection (Elizari, Reference Elizari1999).
Trypanosoma cruzi invasion and replication in the cardiomyocytes provoke cellular damage and a cytotoxic reaction with inflammatory cytokines and nitric oxide production, both of them being a source of reactive oxygen species (ROS) in the acute (Cardoni et al. Reference Cardoni, Antunez, Morales and Nantes1997) and cardiac chronic phases of the infection (Talvani et al. Reference Talvani, Rocha, Barcelos, Gomes, Ribeiro and Teixeira2004). A similar inflammatory response has been described for the chronic indeterminate phase. These responses are triggered to control parasite reproduction, but it may also have toxic effects upon host cellular components (Ueda et al. Reference Ueda, Masutani, Nakamura, Tanaka, Ueno and Yodoi2002). The myocyte response to oxidative stress involves the progression of cellular changes, primarily targeting the mitochondria (Long et al. Reference Long, Goldenthal, Wu and Marín-García2004) and modifying therefore the energy supply; this bioenergetic dysfunction could be involved in the genesis and progression of heart failure (Marin Garcia and Goldenthal, Reference Marin-García and Goldenthal2008; Tsutsui, Reference Tsutsi2006).
Previous work from our laboratory (Báez et al. Reference Báez, Lo Presti, Rivarola, Pons, Fretes and Paglini-Oliva2008, Reference Báez, Lo Presti, Rivarola, Guzman Mentesana, Pons, Fretes and Paglini-Oliva2011), and from other authors (Garg et al. Reference Garg, Popov and Papaconstantinou2003; Vyatkina et al. Reference Vyatkina, Bhatia, Gerstner, Papaconstantinou and Garg2004), have demonstrated different structural and functional alterations in cardiac mitochondria isolated from mice infected with different T. cruzi strains in the acute and cardiac chronic stages.
Positive serological reactions for T. cruzi, normal thoracic radiography and normal electrocardiographic tracings are the elements used for the characterization of the chronic indeterminate form of Chagas disease. But the study of this long silent period of the disease, with more sensitive, invasive or non-invasive, diagnostic methods, demonstrates that most chagasic patients with normal electrocardiograms have some type of anatomical or functional alteration. Taking this into account, our current study investigates whether the structure and function of cardiac mitochondria are altered as a result of the infection with different T. cruzi strains, in order to determine their involvement in the pathophysiological mechanism of chronic chagasic myocardiopathy. These studies could help to elucidate which patient will develop a chagasic cardiopathy.
MATERIALS AND METHODS
Infection
Albino Swiss female and male mice weighing 30 ± 1 g (n = 90) were used as follows: 30 mice were inoculated, by intraperitoneal injection, with 50 trypomastigote forms of T. cruzi, Tulahuen strain (Tulahuen), and 30 mice with 50 trypomastigote forms of the SGO Z12 isolate (SGO Z12), which was obtained from a patient from an endemic area. The number of parasites/ml of blood was determined in each group using a Neubauer haemocytometer. A non-infected group (n = 30) was also studied.
Parasitaemias in all groups were determined in a Neubauer haemocytometer using blood samples obtained from the tail of the mice, twice a week, beginning 7 days after the infection until 75 days post-infection (chronic indeterminate stage).
The investigation was carried out according to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health, NIH publication (No. 85–23, revised 1996).
Serology
Blood samples were collected 50, 90, and 135 days post-infection, representing the acute, intermediate, and chronic phases of the experimental infection, respectively. These samples were then assayed for antibodies to ‘cruzipain’ antigen (see below), by ELISA (Gea et al. Reference Gea, Gruppi, Cerbán, Pistoresi-Palencia and Vottero-Cima1992).
Trypanosoma cruzi antigen
Epimastigote forms of T. cruzi SGO Z12 isolate were grown at 26 °C in liver digest neutralized medium (Oxoid America, Columbia, USA) supplemented with 0·5% tryptose, 10% FCS, 200 mg/ml hemin, 100 U/ml streptomycin. Parasites were harvested at the exponential growth phase, centrifuged at 5000 g for 10 min, and washed with PBS. One millimolar N-ά-p-tosyl-L-lysine chloro methyl ketone (TLCK) was added as a cysteine protease inhibitor and the parasite pellet was lysed by 8 cycles of freezing and thawing. The parasite homogenate was centrifuged at 105 000 g and the supernatant was used for cruzipain purification. Cruzipain was purifed by affinity chromatography as previously described (Labriola et al. Reference Labriola, Sousa and Cazzulo1993). The absence of enzyme activity was controlled in gels containing 0·1% gelatin (Sigma) incubated after the PAGE run at pH 5·7 and stained with Coomassie blue R250; the samples were neither reduced nor boiled.
Mitochondria isolation
Hearts were removed on day 75 post-infection (p.i.), which corresponds to the chronic indeterminate phase of the experimental infection (Lo Presti et al. Reference Lo Presti, Rivarola, Bustamante, Fernández, Enders, Levin, Juaneda, Fretes, Triquell and Paglini-Oliva2008), obtaining both ventricles. They were washed and suspended in ice-cold isolation buffer (5 mm HEPES, pH 7·2, containing 210 mm mannitol, 70 mm sucrose, 1 mm EGTA, and 0·5% BSA (fatty acid-free), tissue/buffer ratio, 1:10 w/v) and immediately homogenized. Homogenates were centrifuged at 1500 g at 4 °C for 20 min and the supernatant transferred to a new tube. The pellet was re-suspended in isolation buffer, homogenized, and centrifuged again at 10 000 g, 4 °C for 5 min. The supernatant was discarded and the pellet was resuspended in buffer and centrifuged at 10 000 g at 4 °C for 10 min (twice = purification). The mitochondrial pellet was re-suspended in isolation buffer (tissue/buffer, 1:1 ratio, w/v), and the aliquots stored at −80 °C.
Respiratory complex and citrate synthase activity
The activities of the respiratory complexes (CI–CIV) and citrate synthase were monitored by spectrophotometric methods as previously described (Trounce et al. Reference Trounce, Kim, Jun and Wallace1996; Jarreta et al. Reference Jarreta, Orus, Barrientos, Miro, Roig, Heras, Moraes, Cardellach and Casademont2000; Vyatkina et al. Reference Vyatkina, Bhatia, Gerstner, Papaconstantinou and Garg2004; Báez et al. Reference Báez, Lo Presti, Rivarola, Pons, Fretes and Paglini-Oliva2008, Reference Báez, Lo Presti, Rivarola, Guzman Mentesana, Pons, Fretes and Paglini-Oliva2011) with slight modifications. Protein concentrations were determined by Bradford assay (Bradford, Reference Bradford1976).
All assays were performed in 1 ml final volume with 30–40 mg (for complexes I and II), 20–30 mg (for complex III) and 15 mg (for complex IV) of mitochondrial protein, and the linear change in absorbance was measured for 3 min.
CI (NADH-ubiquinone oxidoreductase)
The reaction mixture consisted of 10 mm Tris–HCl buffer, pH 8·0, 80 μ m 2,3-dimethoxy-5-methyl-6-decyl-1,4-benzoquinone (DB), 1 mg/ml BSA, 0·25 mm KCN. After incubating the mitochondria in the reaction mixture at 30 °C for 10 min., oxidation of NADH (200 μ m) was monitored at 340 mm (e 8 mm−1 cm−1).
CII (succinate-ubiquinone oxidoreductase)
Mitochondria were incubated in 1 m potassium phosphate buffer, pH 7·0, containing 0·1 ml succinate phosphate 0·1 m. After addition of assay mixture consisting of 50 μ m 2,6-dichlorophenolindophenol (DCPIP), 5 μl EDTA 1 mm, 10 μl of Triton X-100 1%; 50 μl of DB. All the components were mixed. The reduction of DCPIP in association with CII-catalysed DB reduction was measured at 600 nm (e 20·5 × 106 m−1cm−1).
CIII (ubiquinol-cytochrome c oxidoreductase)
Mitochondria were suspended in 50 mm Tris–HCl buffer, pH 7·4, containing 1 mm EDTA, 250 mm sucrose, 2 mm KCN and 50 μ m oxidized cytochrome c. After the addition of 80 μ m reduced DB (DBH2), the reduction of cytochrome c was measured at 550 nm (e 19·0 mm−1cm−1).
CIV (cytochrome c oxidase)
Mitochondria (2 μg protein) were permeabilized in 10 mm Tris–HCl, pH 7·0, 25 mm sucrose, 120 mm KCl, and 0·025% n-dodecyl-b-D-maltoside, and 50 μ m reduced cytochrome c added. The oxidation of cytochrome c was measured at 550 nm (e 19·0 mm−1 cm−1).
Citrate synthase
The mitochondrial pellet was added to 100 mm Tris–HCl buffer, pH 8, 0·3 mm acetyl CoA, 100 μ m 5,5-dithio-bis-2-nitrobenzoic acid (DTNB). The reaction was initiated by 0·5 mm oxaloacetate. Citrate synthase-catalysed reduction of acetyl CoA with oxaloacetate in conjunction with DTNB reduction was monitored at 412 nm (e 13·6 mm−1 cm−1).
Histopathological studies
The hearts were removed from the infected mice on day 75 p.i., fixed in buffered (pH 7·0) 10% formaldehyde, and embedded in paraffin. Each heart was cut horizontally into 5 μm sections from the apex to the auricles. The sections were stained with haematoxylin–eosin. In total 50 slices from each group were analysed with a 40 × objective.
Parasite detection
For parasite detection in cardiac tissues, polymerase chain reaction (PCR) was performed for 40 cycles, using T. cruzi-specific oligonucleotides (Tcz1: 5′-CGAGCTCTTGCCCACACGGGTGCT-3′ and Tcz2: 5′-CCTCCAAGCAGCGGATAGTTCAGG-3′) with 2·5 μl of the total DNA as template. Denaturation, annealing, and elongation steps were performed for 30 sec at 95 °C, 30 sec at 60 °C, and 30 sec at 72 °C, respectively. A 10 μl aliquot of each PCR reaction was resolved on a 1·2% agarose gel. The ethidium bromide-stained gels were visualized using long-wave UV light.
Electron microscopy studies
A 1 millimeter square section, from the tip of the left ventricle, was fixed immediately after extraction in a Karnovsky solution (4% formaldehyde and 1·5% glutaraldehyde in 0·1 m cacodylate buffer) for at least 2 h at room temperature. Then the tissues were washed 3 times in cacodylate buffer and post-fixed in 1% osmium tetraoxide for 1–2 h. After dehydration in a graded acetone solution (50%, 70% and 90%), the inclusion of samples was performed in an Epoxy composite mixture of Araldite 506 (48·5%), dodecenyl succinic anhydride (48·5%), dibuthyl phthalate (0·5%) and dimethyl aminobenzene accelerator (2·5%). Ultrathin cuts were stained with uranyl acetate and lead citrate and examined in a Zeiss electron microscope. In order to evaluate the changes on mitochondrial morphology observed in the different experimental groups (5 micrographs for each mouse), a 4-grade classification was used as follows. Grade 1: normal structure; Grade 2: normal size with dilated cristae; Grade 3: normal size and/or altered shape. Intact membrane with few cristae; Grade 4: mitochondrial swelling.
The 3-dimensional studies were carried out using the Femtoscan program. FemtoScan Online software analysed 5 micrographs for each mouse to build images from electron microscopy. The program runs on operating systems Windows XP, Vista, Windows 7, as well as their server analogues – Windows Server 2003, 2003 R2, 2008, 2008 R2.
Statistical analysis
Results are shown as mean ± standard error. The data obtained were analysed by ANOVA (Fisher test), MANOVA (Hotelling corrected by Bonferroni test) and Chi2 test for categorical variables. Axiovision 3.0 software was used to quantify mitochondria. The significance level was set at P < 0·05 for all cases.
RESULTS
Detection of parasites in blood and cardiac tissue
Parasitaemias were analysed to assess the infection rate. Both infected groups showed the highest parasitaemia levels by day 14 p.i., with the levels of the SGO Z12 group being significantly lower (P < 0·01) than those presented by the Tulahuen group (87·58 ± 7·64 and 225·39 ± 19·11 parasites/μl, respectively). Parasitaemias were negative from day 42 onwards in the Tulahuen-infected mice and from day 49 onwards in the SGO Z12-infected mice.
The PCR analyses carried out in cardiac tissues were positive in Tulahuen and SGO Z12 infected groups.
Serology
CI was positive at 50, 90 and 135 days post-infection.
Mitochondrial respiratory complexes and citrate synthase activity
In order to determine the mitochondrial function, we analysed the enzymatic activity of the mitochondrial respiratory chain complexes I to IV (cristae) and citrate synthase (matrix) in the myocardium from uninfected and Tulahuen- and SGO Z12-infected mice on day 75 p.i. As can be observed in Table 1, citrate synthase enzyme activity from Tulahuen and SGO Z12 mice was significantly decreased compared to the control group (P < 0·0001); there were no significant differences between parasite strains.
Table 1. Mitochondrial enzymatic activity (ηmoles min−1/mg of prot) of hearts obtained from uninfected (n = 10), Tulahuen infected (n = 10) and SGO Z12 infected mice (n = 10) at 75 days post-nfection from different groups under study

CI complex activity significantly decreased in both experimental groups, P < 0·05 when compared with the control. CII complex enzyme activity increased in both infected groups 75 days p.i. (P < 0·05) but the increment was higher in the SGO Z12-infected mice than in the Tulahuen mice. As shown in Table 1, CIII complex enzyme activity significantly diminished with infection with either parasite strain, but the Tulahuen group presented a greater reduction in its activity (P < 0·001). The CIV complex showed an increase in its specific activity in chronic indeterminate infection in the SGO Z12-infected group (P < 0·001).
Cardiac histopathological studies and mitochondrial ultrastructural analysis during chronic indeterminate T. cruzi infection
In the chronic indeterminate stage of the infection, the Tulahuen strain-infected group showed lympho-monocitary infiltrates (Fig. 1 D). Hearts from mice infected with SGO Z12 isolate presented few inflammatory infiltrates (Fig. 1 G). Sections with no cardiac alterations were also observed in this latter group.

Fig. 1 (A) Cardiac histological sections from non-infected mice, 200 × . (B) Cardiac ultrastructure from non-infected mice where sarcomere and mitochondria can be observed, 6000 × . (C) Cardiac ultrastructure from non-infected group where mitochondria can clearly be observed, 27 800 × , bar: 2·2 micrometer. (D) Cardiac histological sections from the Tulahuen-infected group (50 trypomastigotes of Trypanosoma cruzi/animal), 75 days post-infection showing cell infiltrate, 200 × . (E) Ultrastructure of cardiac mitochondria from the same group showing separate cristae with increase of matrix volume, 6000 × . (F) Ultrastructure of cardiac mitochondria from the Tulahuen-infected group, 75 days post-infection; increase of matrix can be observed (dilation), 27 800 × . (G) Hearts from the isolate SGO Z12-infected group (50 trypomastigotes of T. cruzi/animal), 75 days post-infection showing mononuclear cell infiltrates, 200 × . (H) Ultrastructure of cardiac tissue from the isolate SGO Z12-infected group, 75 days post-infection showing mitochondria 6000 × . (I) Ultrastructure of cardiac tissue from the same group showing mitochondria with different morphology and increase of matrix volume, 27 800 × .
At this time, 83% of the mitochondria from the Tulahuen-infected mice presented at least 1 significant abnormality such as an increase in their matrix or disorganization in their cristae (Fig. 1 E, F) when compared with the non-infected group (Fig. 1 B, C). The average area of mitochondria from this group was greater than the control (Table 2). Some alterations of different importance were detected in the 91% of the mitochondria from the SGO Z12-infected group, showing a considerable reduction in their area (P < 0·01); a greater number of mitochondria was also found (see Fig. 1H and I and Table 2); cristae dilatation and increase in mitochondrial matrix, can be observed in the figures (see Fig 1 E, F, H and I).
Table 2. Results of the ultrastructural analysis of cardiac mitochondria of uninfected (n = 5 micrographs/mouse), Tulahuen infected (n = 5 micrographs/mouse) and SGO Z12 infected (n = 5 micrographs/mouse) mice at 75 days post infection
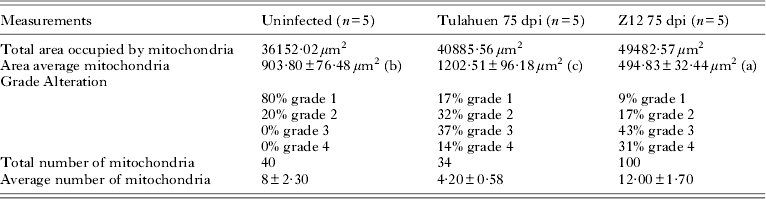
Figure 2 shows a 3-dimensional view of cardiac mitochondria obtained from non-infected and SGO Z12- and Tulahuen-infected mice. In this figure an increase in the matrix and disorganization of the cristae can be observed in both experimental groups.

Fig. 2. Three-dimensional view of cardiac mitochondria obtained from non-infected (A), SGOZ12- (B) and Tulahuen- (C) infected mice. We observed an increase both the size of the mitochondria of the infected groups and the matrix. Measurements were made in pixels.
DISCUSSION
The globalization of Chagas disease, due to migration from endemic countries to North America, Europe, Asia and Oceania has created a new epidemiological, economic, social and political concern in non-endemic countries (Schummis, Reference Schummis2007; Coura and Borgues-Pereira, Reference Coura and Borges-Pereira2010) The main issue in non-endemic countries is the risk of transfusion and congenital transmission, while infected triatomines are still the common way of transmission in endemic countries.
The determinants of Chagas disease come from several, complex and different factors: quantity of parasites that provoke the infection, T. cruzi lineages, specific histotropic receptors of the host and patient's initial immune response, among others (Coura, Reference Coura1988; Andrade et al. Reference Andrade, Campos, Sobral, Magahlanes, Guedes and Guerreiro2006; Bustamante et al. Reference Bustamante, Rivarola, Fernández, Enders, Fretes and Paglini Oliva2003; Zingales et al. Reference Zingales, Andrade, Briones, Campell, Chiari and Fernandez2009); despite this, all infected individuals present a symptom or symptomless acute phase that will evolve into the chronic indeterminate phase.
In this asymptomathic chronic indeterminate stage no anatomo-pathological manifestations are found, except for isolated inflammatory foci with mild reduction of cardiac neurons, which are not enough to provoke clinical manifestations. In the present paper, myocardium samples from Tulahuen- and SGO Z12-infected mice presented cardiac isolated inflammatory infiltrates and absence of amastigote nests. Even though these isolated inflammatory infiltrates described are the result of T. cruzi persistence in host tissues by successful evasive strategies used by the parasite: it releases proteases that activate TGF-β (Araujo Jorge et al. Reference Araujo Jorge, Waghabi, Soeiro, Keramidas, Bailly and Feige2008; Waghabi et al. Reference Waghabi, De Souza, De Oliveira, Keramidas, Feige, Araujo Jorge and Bailly2009; Maya et al. Reference Maya, Orellana, Ferreira, Kemmerling, Lopez Muñoz and Morello2010). Additionally, phagocytosis and apoptotic bodies originated from T cells or neutrophils induce a prostaglandin-dependent production of TGF-β in macrophages. All this provokes a diminished nitric oxide production and the INF-γ induced inflammatory response is attenuated; consequently, the parasite proliferates (Gutierrez et al. Reference Gutierrez, Mineo, Pavanelli, Guedes and Silva2009). This may be the cause of the low-grade of inflammatory response described in the chronic indeterminate phase, that we also demonstrated in our results. But all of these changes that have no clinical evidence are generating cellular structural damage that can affect cardiac mitochondria either at or near the inflammatory formation (Tsutsui, Reference Tsutsi2006).
In this paper we analysed the effect of the infection with 2 different strains of T. cruzi (Tulahuen and SGO Z12) in this ‘clinically silent’ stage, upon the structure and function of cardiac mitochondria. The structural results, using a 4-grade classification of mitochondrial damage, showed that at this time, 83% of the mitochondria from the Tulahuen-infected mice presented at least 1 significant abnormality such as an increase in their matrix or disorganization in their cristae (14% Grade 4) that are probably related to the enzymatic dysfunction.
Alterations of different importance were detected in the 91% of the mitochondria from the SGO Z12-infected group (31% Grade 4) (P < 0·05). The abnormalities described were corroborated using the 3-dimensional studies (Fig. 2).
The number of mitochondria in cardiac tissue was not modified by the T. cruzi infection in the Tulahuen group, showing a significant increase in the average area of the organelle (P < 0·01). These results are different from those previously obtained in the cardiac chronic phase (Báez et al. Reference Báez, Lo Presti, Rivarola, Guzman Mentesana, Pons, Fretes and Paglini-Oliva2011) when a clear reduction in the mitochondrial size was described. The SGO Z12-infected group showed a significantly diminished area occupied by mitochondria (P < 0·01). The greater number of mitochondria found in this group probably compensates the important number of them with several alterations.
Additionally, in this ‘silent’ stage of the T. cruzi infection we demonstrated functional changes in cardiac mitochondria of different involvement related to the strain that infected the host.
When we studied the Krebs cycle functionality through the measurement of the specific citrate synthase activity, we found it to be significantly diminished, in contrast to the increased activity found in the acute and cardiac chronic stages of the infection (Báez et al. Reference Báez, Lo Presti, Rivarola, Pons, Fretes and Paglini-Oliva2008, Reference Báez, Lo Presti, Rivarola, Guzman Mentesana, Pons, Fretes and Paglini-Oliva2011). When the mitochondrial respiratory chain was analysed in the chronic indeterminate stage of the infection through the measurement of the specific activity of complexes I to IV, we found that the activity of CI and CIII were altered in a similar manner in either infected group. CII presented a significant increase in both experimental groups, while CIV incremented its activity in the SGO Z12-infected mice (P < 0·0001) while the Tulahuen group presented a conserved activity not different from the control group; this last result may be related to the maintenance of the respiratory chain redox potential efficiency. Additionally, the dysfunction in the citrate synthase activity and respiratory complexes could be achieved via the matrix and cristae disorganization found in both infected groups.
It was recently demonstrated that oxidative stress-induced protein modifications occur in the myocardium of T. cruzi-infected experimental animals (Wen and Garg, Reference Wen and Garg2004; Wen et al. Reference Wen, Vyatkina and Garg2004; Dhiman et al. Reference Dhiman, Nakayasu, Madaiah, Reynolds and Wen2008) and in peripheral blood of seropositive chagasic patients (Wen et al. Reference Wen, Yachelini, Sembaj, Manzur and Garg2006; De Oliveira et al. Reference De Oliveira, Pedrosa and Filho2007). This peripheral oxidative stress was associated with a poor glutathione antioxidant defence and an increased myeloperoxidase activity and ROS production (Dhiman et al. Reference Dhiman, Zago, Nunez, Amoroso, Rementeria, Dousset, Nunez Burgos and Garg2012). Besides, T. cruzi infection provokes a significant fall in mitochondrial membrane potential; the ROS-induced oxidation of mitochondrial membranes may constitute a secondary signal affecting its potential, and consequently the respiratory chain efficiency (Gupta et al. Reference Gupta, Bhatia, Wen, Wu, Huang and Garg2009).
The inflammatory pathology is not very important during the chronic indeterminate stage by enhancing an antioxidant status, which is beneficial to preserve the cardiac function for a long time; but as it has been demonstrated that the mitochondrial ROS release due to electron transport chain dysfunction and enhanced release of electrons to molecular oxygen is the primary source of oxidative stress in the heart (Wen and Garg, Reference Wen and Garg2008). During this long stage there is a permanent aggression to the cardiac cells.
Previous studies from our laboratory have demonstrated that the chronic indeterminate phase is not so ‘silent’ as has been described: modifications of cardiac contractility, alterations of some components of the cardiac β adrenergic system (Enders et al. Reference Enders, Paglini, Fernández, Marco and Palma1995; Lo Presti et al. Reference Lo Presti, Rivarola, Bustamante, Fernández, Enders, Levin, Juaneda, Fretes, Triquell and Paglini-Oliva2008), and electrocardiographic alterations (Bustamante et al. Reference Bustamante, Rivarola, Fernández, Enders, Fretes and Paglini Oliva2003) were previously found. Now we have demonstrated that the responsible organelle for cardiac energy supply is also compromised both structurally and functionally.
The hypothesis that the indeterminate chronic phase is a stage of host-parasite equilibrium rather than a process of progressive damage (Prata, Reference Prata2001) explains the long-term and clinical silence of the chronic indeterminate phase; present results, however, point to the substantial importance of the parasite strain and the different damage that each of them is capable of inducing throughout this silent stage.
The mechanisms involved in the pathogenesis of Chagas disease and its progression to chronic cardiomyopathy are still under intense discussion. We have demonstrated in the present paper that cardiac mitochondria are clearly involved in the genesis and progression to the chronic chagasic cardiopathy when different factors alter the host-parasite equilibrium.






