Published online by Cambridge University Press: 09 October 2003
Chemotactic responses of Strongyloides stercoralis infective larvae (L3) to sodium chloride (NaCl) were investigated by recording larval tracks on a saline gradient in agarose. On agarose, larvae migrated randomly, whereas when placed at 0·01 M NaCl larvae moved to approximately 1·1 M NaCl where they turned, headed down the gradient and eventually remained circling at a favoured salinity (0·03–0·07 M). Conversely, when placed at 2·85 M NaCl, the L3 larvae moved unidirectionally to lower, more favoured salt concentrations. Here they circled, changing directions frequently while making ‘loop-like’ tracks. Larvae were immobilized within 5 min at salt concentrations exceeding 3 M NaCl. When placed at 0·01 M NaCl, 51·1%±26·9 migrated to 1·1 M NaCl after 2 min, and 80%±18·7 did so after 8 min, at an average velocity of 4·1±1·4 mm/min. Larvae (53·6%±21·6) were repelled from 2·85 M NaCl to lower concentrations after 2 min. After 8 min, 95%±11·1 were repelled, moving at an average velocity of 6·2±1·1 mm/min. Using this bioassay, the influence of neuronal control over chemotactic behaviour of S. stercoralis and other parasitic nematodes can be elucidated.
Strongyloides stercoralis is a medically important, skin-penetrating, intestinal nematode that infects canines and primates, including man. The parasite is capable of reproducing within its host frequently causing exceedingly long-enduring, low-grade internal infections. However, these mild infections may be exacerbated by immunosuppressive therapy or disease, poor host nutrition or intercurrent infections (Schad, Hellman & Muncey, 1984; Genta, Schad & Hellman, 1986). The resulting hyperinfective or disseminated strongyloidiasis may be fatal if appropriate treatment is not forthcoming (Grove, 1989). There is a need for better chemotherapeutic agents as well as prophylactic strategies. Thus, it is important that the parameters that control the infective process are investigated, and include host-seeking and recognition behaviour, skin penetration, initiation of development, migration and further development within the host. These parameters of the infective process are governed, in part, by tactic responses that are elicited when there is exposure to a variety of stimuli, for example, heat, humidity, gravity, gases like carbon dioxide and oxygen, and chemicals. Investigations showing such responses have been well documented.
Parker & Haley (1960), showed that the infective stage of Nippostrongylus muris was positively thermotactic, while Granzer & Haas (1991) showed that Ancylostoma caninum, in the presence of a heat source, described snake-like, undirected movements. Also, on a thermal gradient A. caninum infective larvae (L3) migrated relatively slowly but directly to the warm end (Bhopale et al. 2001), and Caenorhabditis elegans migrated towards its cultivation temperature (Mori & Ohshima, 1995; Bargmann & Mori, 1997). Sasa et al. (1960), demonstrated that as the concentration of CO2 increased in the environment, the infective larvae of 3 hookworm species, which normally adopt a resting pose on gypsum-charcoal substrate, reacted immediately by nictitating (rhythmic or waving movement of the heads). Granzer & Haas (1991), confirmed this stimulatory effect of CO2 on A. caninum in a humid environment. Recently, it was shown that the infective 3rd-stage larvae of A. caninum and S. stercoralis, in response to CO2, crawled actively but non-directionally, while those of H. contortus ceased crawling, coiled and moved within the coil (Sciacca et al. 2002 a). A geotactic study on these 3 parasites indicated that the former, that is A. caninum and S. stercoralis, were negatively geotactic, while H. contortus migrated randomly (Sciacca et al. 2002 b). A negative geotactic response was also characteristic of N. muris 2nd-stage larvae (Africa, 1931).
However, based on the nature of this study, the chemotactic response of nematodes to chemicals is of particular interest. C. elegans is positively chemotactic to some amino acids, cyclic nucleotides, vitamins, anions, cations and alkaline pH values but chemo-repellent to some other water-soluble chemicals, acids, compounds of high osmotic strength and a few volatile molecules (Ward, 1973; Zuckerman & Jansson, 1984; Bargmann & Mori, 1997). Ma (1987), showed that Ancylostoma braziliense exhibited a chemo-attractive response to rat plasma, mouse plasma, rat plasma diffusate, concentrated rat plasma dialysate and some salts, notably sodium chloride (NaCl). A. canimum also displayed a positive chemotactic response to dog hydrophilic skin surface extracts (Granzer & Haas, 1991), while S. ratti infective larvae were positively chemotactic toward a preferred zone of salt concentration on a NaCl gradient, avoiding high salinity (Tobata-Kudo et al. 2000 a, b). The direction of movement by the larvae depended on salinity at the release point on the gradient. L3 placed at concentrations that were less than 0·02 M NaCl dispersed randomly, but did not enter that part of the agarose plate where concentration exceeded approximately 80 mM NaCl (Tobata-Kudo et al. 2000 a). When they were placed between 0·23 and 0·37 M NaCl, the larvae migrated unidirectionally to lower concentrations (Tobata-Kudo et al. 2000 a). A study by Tada et al. (1997) also showed an accumulating response of the larvae toward sodium ions (Na+) instead of potassium (K+), calcium (Ca2+) and magnesium (Mg2+) ions, thereby indicating that chemoattraction to NaCl could be due to the presence of Na+ ions. Sweat occurring on the surface of mammalian hosts (Patterson, Galloway & Nimmo, 2000) could play a role in the invasive behaviour by skin-penetrating parasitic nematodes. Thus, the above findings showing the chemotactic behaviour of S. ratti L3 toward NaCl gradients in agarose, along with the fact that this had not been previously investigated using S. stercoralis L3, prompted the ensuing study.
Infective larvae (L3) of a dog strain of S. stercoralis, which has been maintained in a series of laboratory canines at the University of Pennsylvania School of Veterinary Medicine for over 10 years, were used for all studies. L3 were harvested from 5-day-old charcoal copro-cultures by use of the Baermann technique.
Sodium chloride gradients in agarose were established in rectangular polystyrene boxes (193×104×27 mm plastic boxes; Daigger & Co., Middlefield, OH, USA) by methods modified from Tobata-Kudo et al. (2000 a). These contained 10 ml of 1·5% (w/v) agarose (Sigma, Louis, MO, USA) and 0·25% (v/v) Tween 20 (Fisher Scientific, NJ, USA). Each box was rocked gently to distribute the liquid agarose in a layer approximately 1 mm thick. This was allowed to solidify (10 min) at room temperature (23–26 °C) before applying a line of crystalline NaCl to initiate the desired gradient.
Opposed, linear, saline gradients were established by depositing a thin line of NaCl crystals (0·21 g) across the long axis of the agarose substratum at its midpoint. After 20 h to allow the opposed gradients to develop and equilibrate, a multimeter (Model number 2B 0673, Type3201, Yokogawa Electric Works, Ltd, Tokyo, Japan) in series with a 9-volt battery was used to measure current in milliamperes (mA) at 20 mm intervals for 80 mm, on each side of the saline midline of each agarose plate (Fig. 1A and B). For the duration of each 30 min assay and, in fact to 40 min, the concentration of NaCl in the closed system at any point along the gradient remained essentially constant (Table 1).


Fig. 1. (A) Polystyrene box showing NaCl crystals (0·21 g) deposited in a line across the midpoint of the long axis of an agarose substratum to establish opposed, linear NaCl gradients. (B) NaCl concentrations (M) along opposed, linear gradients developed on agarose after 20 h.
A standard curve had been constructed previously by measuring the current (mA) on plates of known, uniform NaCl concentrations (0·02, 0·05, 0·1, 0·2, 0·3, 0·4, 0·5, 1·0, 2·0, 3·0, 4·0, 5·0 and 6·0 M) in agarose at 3 equidistant points along the midline, and at similar points 30 mm away on either side of the midline, with platinum electrodes. The plates were similar to those used for establishing NaCl gradients. The mean currents with standard deviations are shown (Table 2). Salt concentration in moles (M) was plotted against mean current. The statistical software STATA, Release 7, 2001 (Stata-Corp, College Station, TX, Stata Press) was used for analysis (procedure used: fractional polynomial regression), and a standard curve was fitted to the distribution. The model explained 99·9% of the variation. The NaCl gradient was then calibrated. By measuring current at any point along the gradient, the concentrations of NaCl (M) could be determined. For example, a mean current of 10·6±0·9 mA corresponded with 3·01 M NaCl, and 0·56±0·07 mA with 0·05 M NaCl (Fig. 2A and B).
Table 2. Mean current (mA) with standard deviations (S.D.) measured on agarose plates with uniform concentrations of NaCl (M) (A multimeter in series with a 9-volt battery was used to measure current in milliamperes (mA).)
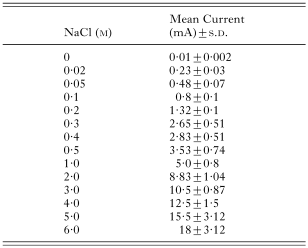

Fig. 2. Calibration of sodium chloride gradient using fractional polynomial regression where (A) 10·6 mA=3·01 M NaCl; (B) 0·56 mA=0·05 M NaCl.
Infective larvae (15–20) were placed at different points along the NaCl gradients and tracks left on the agarose substratum were recorded pictorially at 10-min intervals for 30 min with the aid of a Durst D 659 photographic enlarger. This was fitted with a 105 mm f:5.6 Schneider Componon enlarging lens. Each polystyrene box was placed on top of a 203·2 mm×254 mm sheet of Kodak Kodolith Ortho film on the enlarger's baseboard. Exposures were made with the enlarger head positioned 565 mm above the baseboard. The lens was set at f:22 for a 6 sec exposure, or at f:32 for a 12 sec exposure. The pictures gave a preliminary idea of the most distant points ‘up and down’ the gradient that evoked a definite migratory response. Measurements of current at these extremes where there was either an attractive or repulsive response were noted and used as reference points for further qualitative and quantitative work. All experiments, including controls (no salt added), were done in triplicate.
To investigate the precise larval responses with respect to time, this experiment was repeated as above using 4–6 L3. These were placed at designated points along the gradient at room temperature and contact prints of larval tracks were made at 2 min intervals for 8 min. With fewer larvae migrating, the individual tracking responses at different points along saline gradients could be more clearly seen.
The number of S. stercoralis L3 that migrated to either higher or lower concentrations of NaCl along the saline gradient, depending on where they were released, was counted at 2 min intervals for 8 min. The distance (mm) covered was noted and the average velocity of migration with time could then be determined. This allowed for a quantitative assessment of larval migratory behaviour. Again, control plates (polystyrene boxes) were constituted of the agarose base only.
S. stercoralis L3 migrated randomly on control plates without salt gradients (Fig. 3). In sharp contrast, when placed at a low NaCl concentration (0·01 M) the larvae generally migrated up the gradient to approximately 1·1 M where they turned around and headed back down the gradient (Fig. 4A). After approximately 20 min, this turn-around-level became well defined and indicated the upper limit of salt tolerance for S. stercoralis L3 (Fig. 4A). Conversely, the larvae moved in a unidirectional, avoidance manner from high salinity (≈2·85 M NaCl) to lower concentrations (0·05–0·07 M NaCl) (Fig. 4B). Here they described wide looping tracks and changed direction frequently, with the resulting concentration of tracks in this vicinity after 20–30 min (Fig. 4B). They were immobilized within 5 min of exposure (data not shown) when the concentrations of NaCl exceeded 3 M.

Fig. 3. Random migration of Strongyloides stercoralis L3 on a control plate (without NaCl) after 30 min.

Fig. 4. Tracks of groups of 15–20 Strongyloides stercoralis L3 (A) when released ‘down’ gradient (0·01 M NaCl), after 10, 20 and 30 min; and (B) ‘up’ gradient (2·85 M NaCl), after 10, 20 and 30 min.
When 4–6 larvae were released at 0·01 M NaCl they moved ‘up’ the gradient to 1·1 M NaCl where they turned around instead of migrating to higher salinity (Fig. 5A). The larvae eventually migrated to a preferred zone of approximately 0·05 M NaCl (not shown in Fig. 5A). Many larvae (51·1%±26·9) migrated from the 0·01 M-origin to 1·1 M NaCl within 2 min, and most (80%±18·7) did so within 8 min (Fig. 6), covering a distance of 33±11 mm at an average velocity of 4·1±1·4 mm/min. The unidirectional avoidance behaviour of 4–6 S. stercoralis L3 moving from release points at 2·85 M NaCl to lower concentrations including 0·05 M NaCl is shown (Fig. 5B). Repulsion was rapid, and by 2 min 53·6%±21·6 and by 8 min 95%±11·2 had moved away from the release point to 0·05 M (Fig. 6). This was a distance of 49±11 mm, at an average velocity of 6·2±1·1 mm/min. Though the average velocity at which S. stercoralis L3 migrated ‘down’ the gradient (6·2±1·1 mm/min) was faster than migration ‘up’ the gradient (4·1±1·4 mm/min), there was no other apparent difference between migration in the two directions.

Fig. 5. Tracks of 4–6 Strongyloides stercoralis L3 (A) when released ‘down’ gradient (0·01 M NaCl), after 2, 4 and 8 min; and (B) ‘up’ gradient (2·85 M NaCl), after 2, 4 and 6 min.

Fig. 6. Percentage of Strongyloides stercoralis L3 migrating ‘up’ gradient ([bull ]) from 0·01 M to 1·1 M NaCl (n=26); and ‘down’ gradient ([lozf ]) from 2·85 M to 0·05 M NaCl (n=38), in 8 min.
Several authors have investigated the responses of nematodes to salts and other compounds. Ma (1987) found that the infective larvae of Ancylostoma braziliense were attracted by rat and mouse plasma and some salts, especially NaCl with a concentration of 0·15 M. The infective larvae of Neoaplectana carpocapsae, an insect-parasitic nematode, showed an increased rate of accumulation around 0·075 M NaCl (Pye & Burman, 1981). Riddle & Bird (1985) showed that larvae of Rothlenchulus reniformis, a plant-parasitic nematode, were attracted to 0·0005 M NaCl while, according to Ward (1973), C. elegans, a free-living microbiverous species, displayed a threshold concentration of accumulation of 0·002 M Na+ and Cl−.
In this study, the essentially reproducible gradients of NaCl that were established on agarose made it possible to study the chemotactic behaviour of S. stercoralis infective L3 larvae. The migratory behaviour exhibited by the infective larvae indicated that they recognized NaCl concentrations along the gradient, and that they were able to perceive and distinguish between ‘comfortable and uncomfortable’ concentrations. The optimal (‘comfortable’) concentration was in the range of 0·03–0·07 M NaCl, which was confirmed by a quadrant bioassay experiment (Forbes, unpublished observations), using a technique outlined by Hope (1999). Here they wandered ‘aimlessly’, as they did when placed on agarose that contained no salt, changing directions frequently while making loop-like tracks. As a consequence of these movements the larvae remained in this zone primarily. Although occasionally, a few larvae wandered out of this zone but eventually returned. In sharp contrast, when released at concentrations below the optimal region they moved up the gradient to a precise level (≈1·1 M) where they reversed direction and headed back down the gradient, and finally circled and remained in the preferred region. Interestingly, when placed at release points up the gradient, for example at 2·85 M NaCl, the larvae made rapid, unidirectional, avoidance movements that directed them down the gradient to areas of lower salinity (0·03–0·07 M).
The results of this investigation compare favourably with a similar study that examined movement patterns of the infective larvae of a related species, S. ratti, on a sodium chloride gradient (Tobata-Kudo et al. 2000 a). When initially placed at unfavourably high salt concentrations along the gradient, the L3 described unidirectional avoidance movements to lower and more favourable zones where they changed direction frequently while making loop-like patterns of movement (Tobata-Kudo et al. 2000 a). With time, as the larvae moved around in the favourable zone, there was a resulting concentration of larval tracks in this vicinity. Similarly, when placed at concentrations less than 0·02 M NaCl, the larvae initially tended to migrate up the gradient to higher concentrations, but did not linger in areas that exceeded favourable salinity (Tobata-Kudo et al. 2000 a). Whereas this favourable NaCl concentration (≈0·08 M) for S. ratti (Tobata-Kudo et al. 2000 a) is similar to that of S. stercoralis, which had a range of 0·03–0·07 M, the latter species appears more capable of withstanding higher salt concentrations. S. ratti larvae moved up the gradient to no further than a level of 0·15 M NaCl (Tobata-Kudo et al. 2000 a), whereas S. stercoralis infective L3 migrated to as far as the 1·1 M NaCl level. The reason for this disparity in tolerance of salinity between these closely related species is not known. However, it may be attributed in part, to physiological differences between the salt regulatory systems of the hosts they parasitize.
S. stercoralis infects canines and primates including man, whereas S. ratti, as its name suggests, infects rodents (Grove, 1989). In a study on variations in regional sweat compositions in human males (Patterson et al. 2000), it was shown that sweating occurs all over the body due to wide distribution of sweat glands and can result in considerable water loss. McPherson (1960) showed that sweat loss from normal skin might be as high as 3 liters in 4 h. As found by Patterson et al. (2000), the mean concentrations of sodium and chloride ions in sweat collected from 11 different parts of the body, were 36·4 and 31·3 mmol/l (0·0364 and 0·0313 M), respectively. A previous study showed that mean sodium content of sweat (60 mmol/l or 0·06 M) varied in accordance to sweat rate, hormonal control and salt diet (Dobson & Sato, 1972). These findings attested to the high levels of salt in human sweat. In rodents, however, sweat glands are generally most abundant on the paws (Kennedy, Sakuta & Quick, 1984). Loss of water from the body is minimized by the production of highly concentrated urine, dry faeces and low-humidity acclimatization due to a reduced rate of evaporative water loss (Grenot, 2001). By comparison, sweating in rodents is not only reduced, but is expected to have lower concentrations of salts than that of human sweat. Hence, the higher salt concentration on the skin of other mammalian hosts, man in particular, in comparison with that of rodents, could explain the attraction of S. stercoralis L3 to such higher levels of salinity than those attractive to S. ratti.
Infective larvae did not advance beyond 1·1 M NaCl up the gradient, and were immobilized at concentrations exceeding 3 M NaCl, levels of salinity markedly exceeding those occurring naturally on the surface of human skin (0·031–0·06 M). In fact, based on the findings of this study, that is, that the larvae are attracted to 0·03–0·07 M NaCl, skin salinity may, along with other parameters, naturally attract the larvae. For example, as indicated earlier, many nematodes particularly those that are skin-penetrating parasites of homeothermic hosts are positively thermotactic. Li et al. (2000) have confirmed the well-known positive thermotactic response of S. stercoralis L3 on a thermal gradient. Consequently, with the L3 attracted to heat and also to a narrow range of concentrations of NaCl, as demonstrated by this study, it is probable that in the natural environment both attractants play an important role in host finding, host recognition, and parasite development.
Once contact has been established, host penetration may then be stimulated by salt occurring on the skin. Ma (1987), in a chemoattraction study of A. braziliense to salts, suggested that because diffusible NaCl is present at an effective concentration in mammalian plasma, and may form a gradient between the blood and skin surface, salt from the blood may direct the penetrating larvae through the host's skin. Other chemoattractants for example, hydrophilic components that cover not only the skin but also the hairs may also play a role in host penetration. Work by Granzer & Haas (1991) on A. caninum lends support to this view, that host finding and recognition is comprised of a series of behavioural phases which are stimulated by specific environmental and host signals that work in conjunction with each other.
Ward (1973), in a chemotactic behavioural study using C. elegans, showed that the nematode's response to gradients of the following groups of attractants, cyclic nucleotides (cAMP, cGMP), anions (Cl−, Br−, I−), cations (Na+, Li+, K+, Mg2+) and alkaline pH values, involved orientation, movement up the gradient, accumulation and finally habituation. Ward (1973) described 2 assays, which indicated respectively, that sensory receptors on the head alone mediated the orientation response, a chemotaxis, and that the direction of orientation was determined by lateral motion of the head.
Similarly, the ability of S. stercoralis infective larvae to orient themselves on a NaCl gradient and find the region of optimal concentration, implies a capacity for chemotactic behaviour mediated by sensory receptors located at the parasite's anterior end. Ward et al. (1975) reconstructed the complete anterior sensory nervous system of C. elegans from serial electron micrographs, while Bargmann & Mori (1997) indicated that 2 pairs of amphidial neurons, named ASE and ASH in this free-living nematode, function in chemical attraction and avoidance, respectively. Based on the reconstruction of the anterior sensory neuroanatomy of S. stercoralis (Ashton et al. 1995; Ashton, Li & Schad, 1999) using C. elegans as a model, and on the specific response of S. stercoralis L3 to salt concentration on a NaCl gradient, the homologues of these pairs of amphidial neurons may be investigated. This could be done using the NaCl gradient assay technique and laser microbeam ablation technology to elucidate their role in chemotaxis, and ultimately in the infective process of S. stercoralis, and presumably in other parasitic nematodes as well.
This work was supported in part by NIH grant R01 A1 22662 to G. A. Schad and RR02512 to M. Haskins, by the USDA grant 96-35204-3668, and by a grant from the Research Foundation of the University of Pennsylvania.

Table 1. Sodium chloride concentration (M) along a gradient at 20 mm intervals for 80 mm, with time (min)

Fig. 1. (A) Polystyrene box showing NaCl crystals (0·21 g) deposited in a line across the midpoint of the long axis of an agarose substratum to establish opposed, linear NaCl gradients. (B) NaCl concentrations (M) along opposed, linear gradients developed on agarose after 20 h.
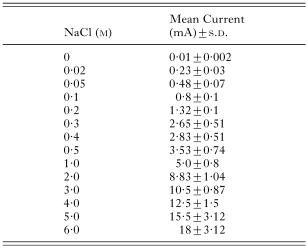
Table 2. Mean current (mA) with standard deviations (S.D.) measured on agarose plates with uniform concentrations of NaCl (M)

Fig. 2. Calibration of sodium chloride gradient using fractional polynomial regression where (A) 10·6 mA=3·01 M NaCl; (B) 0·56 mA=0·05 M NaCl.

Fig. 3. Random migration of Strongyloides stercoralis L3 on a control plate (without NaCl) after 30 min.

Fig. 4. Tracks of groups of 15–20 Strongyloides stercoralis L3 (A) when released ‘down’ gradient (0·01 M NaCl), after 10, 20 and 30 min; and (B) ‘up’ gradient (2·85 M NaCl), after 10, 20 and 30 min.

Fig. 5. Tracks of 4–6 Strongyloides stercoralis L3 (A) when released ‘down’ gradient (0·01 M NaCl), after 2, 4 and 8 min; and (B) ‘up’ gradient (2·85 M NaCl), after 2, 4 and 6 min.

Fig. 6. Percentage of Strongyloides stercoralis L3 migrating ‘up’ gradient ([bull ]) from 0·01 M to 1·1 M NaCl (n=26); and ‘down’ gradient ([lozf ]) from 2·85 M to 0·05 M NaCl (n=38), in 8 min.