Published online by Cambridge University Press: 09 October 2003
A laboratory population of a Danish Gyrodactylus salaris variant founded by 1 single specimen was established and infection studies performed. Rainbow trout as well as Atlantic salmon of 3 different stocks were infected both in cohabitation systems and as single-parasite infections on isolated hosts. Both infection systems revealed that this particular morphotype exhibits low virulence towards Atlantic salmon. Thus, in isolated hosts, the parasites could either not establish or only reproduce to a limited degree on salmon. Rainbow trout, in contrast, proved to be rather susceptible to infection with this G. salaris variant and abundances on this host species always attained significantly higher values in cohabitation systems compared to salmon. Detailed morphological examination confirmed the very high resemblance to G. salaris (sensu stricto), as the range of variation in sclerite characters of the morphotype is almost fully covered by the total range of variation reported for reference G. salaris. Morphological similarities to the closely related congeneric species G. bohemicus were noted. Molecular studies, however, showed that the morphotype most likely represents a G. salaris variant, as it differed only slightly from G. salaris sensu Malmberg, 1957, which is also known to inhabit Danish watercourses. The genomic target region investigated does not allow us to rule out the possibility that it represents a variant form of G. thymalli. Sequences of the ribosomal RNA internal transcribed spacer (ITS) revealed that single individuals contained 2 different ITS sequences, one identical to reference sequence of G. salaris while the other differed at 3 positions. This can be interpreted as an example of a hybrid or, more likely, as intra-individual variation of ITS within single individuals. As one of the nucleotide changes in the variant ITS affects an Hae III restriction site, the current G. salaris variant can be distinguished from G. salaris sensu Malmberg by RFLP diagnosis.
Gyrodactylus salaris Malmberg, 1957, which is recognized as a virulent pathogen of Atlantic salmon (Bakke, Jansen & Hansen, 1990), has caused a marked decimation of infected salmon populations in Norway (Johnsen & Jensen, 1991). Various studies have demonstrated that the high virulence towards East Atlantic salmon is probably a ubiquitous trait of at least Norwegian ‘strains’ of G. salaris, as Scottish stocks of Salmo salar L. have approximately the same degree of susceptibility to infection (Bakke et al. 1990; Bakke & MacKenzie, 1993). Therefore, G. salaris is listed as a List III pathogen under the Fish Health Directive 91/67/EEC (Hudson & Hill, 1993). Proper identification is consequently of outmost importance. New tools for species determination of gyrodactylids complementing traditional morphological techniques have been applied within recent years. These include chaetotaxy (Shinn, Gibson & Sommerville, 1997), probe hybridization to the V4 region of the small subunit ribosomal RNA (rRNA) gene (Cunningham et al. 1995), RFLP of amplified ribosomal RNA internal transcribed spacer (ITS) regions or sequencing of these (Cunningham, 1997), and finally automated image analysis using statistical classifiers (Kay, Shinn & Sommerville, 1999). Such techniques have strongly assisted in the discrimination of cryptic species within the G. salaris-species group such as G. teuchis Lautraite, Blanc, Thiery, Daniel & Vigneulle, 1999 and G. thymalli Žitňan, 1960 (McHugh, Shinn & Kay, 2000; Cunningham et al. 2001; Sterud et al. 2002). However, our knowledge of the infectivity and host range of many of the species and morphotypes within the G. salaris-species complex under laboratory conditions are limited, except for several Norwegian populations of G. salaris. That ‘strains’ or isolated populations of G. salaris with restricted genetic diversity could differ in the context of infectivity and virulence has largely been neglected, with the focus primarily on differences in host susceptibilities (reviewed by Bakke et al. 1992 a; Bakke, Harris & Cable, 2002). Thus, controlled infection experiments with G. salaris have only been carried out with populations/‘strains’ from Norwegian watercourses – primarily from River Drammenselva (e.g. Bakke et al. 1990; Bakke, Jansen & Kennedy, 1991 a; Bakke, Harris & Jansen, 1992 b; Harris, Jansen & Bakke, 1994; Jansen & Bakke, 1991), River Figga (Bakke & MacKenzie, 1993) or River Lierelva (Bakke, Soleng & Harris, 1999; Soleng & Bakke, 2001; Soleng, Bakke & Hansen, 1998; Soleng, Jansen & Bakke, 1999).
Four different species of gyrodactylids have been recorded in wild and farmed salmonids in Denmark (Buchmann & Bresciani, 1997; Lindenstrøm, Nielsen & Buchmann, 1999; Buchmann et al. 2000; Nielsen & Buchmann, 2001): G. derjavini Mikailov, 1975, G. truttae Gläser, 1974, G. teuchis Lautraite, Blanc, Thiery, Daniel & Vigneulle, 1999 and G. salaris. The ITS regions of these Danish populations have been shown to be identical to reference sequences (unpublished) (reference sequences under GenBank accession numbers: G. derjavini: AJ132259; G. truttae: AJ132260; G. teuchis: AJ249350; G. salaris: Z72477). For the current experiments, a single-species laboratory population was established of a gyrodactylid, which was indistinguishable from G. salaris sensu Malmberg by morphology. This population was founded by a single specimen recovered from infected rainbow trout. The progeny was subsequently described by means of biology, morphology and molecular genetics. Thus, specimens were characterized on a morphological as well as a molecular level and infection studies carried out on rainbow trout and 3 different stocks of Atlantic salmon.
Infected rainbow trout, purchased from the fish farm ‘Refsgaard II’ (situated in the River Vejle å, county of Vejle, SE Jutland), carried a mixed infection of Gyrodactylus derjavini and a morphotype tentatively identified as G. salaris based on examination of opisthaptoral structures. Approximately 40% of the gyrodactylid population on these hosts were of the G. salaris type. In order to elucidate the host susceptibility pattern of this morphotype, a pure single-species laboratory population was established on isolated small naïve rainbow trout (n>20). Under anaesthesia, these recipients were infected with a single parasite individually transferred with a fine needle from several infected donor fish. Where infections did establish, additional naïve fish were added to each tank for infection by cohabitation. After propagation of parasites, a sample of Gyrodactylus from each established ‘infection-line’ was collected for morphological identification. One of the parasite metapopulations with G. salaris morphology was chosen and propagated by successively adding naïve hosts. Through this procedure, a laboratory population of a morph resembling G. salaris (assigned Gx) isolated from farmed Danish rainbow trout and founded by 1 single specimen, was established. The laboratory batch of these parasites was held under the same conditions as described below for the infection experiments.
Four different salmonid species and stocks were used in the susceptibility studies: rainbow trout, and 3 stocks of Salmo salar (Conon (Scottish), Corrib (Irish) and Ätran (West-Swedish)). The rainbow trout fry were delivered by Fisher Fish (Zealand), whereas the 3 salmon stocks were purchased at the hatchery ‘Brusgaard Laksehal’ (NE Jutland). Upon arrival in the laboratory, fish were transferred to 300 l grey plastic tanks and allowed to acclimatize for at least 2 weeks before infection trials were initiated. Samples of each stock of fish were subjected to bacteriological and parasitological examination before being subjected to experimental infections. No infectious agents were recovered. Small rainbow trout fry (range 240–259 mg) were used for the establishment of the laboratory population of parasites, whereas rainbow trout (from the same source) used for the susceptibility trials, weighed 2·5–3·0 g. Salmon of the 3 stocks (4 months post-hatch) weighed 2·0–2·5 g.
Two different sets of experiments were performed: individually isolated hosts infected with a single parasite, and infections in cohabitation systems.
Ten rainbow trout and 10 of each of the 3 salmon stocks (thus 40 fish) were infected to establish single-parasite infections. The infections were carried out by transferring individually isolated parasites to anaesthetized recipient fish which, afterwards, were individually isolated in a 10 l plastic aquarium containing 3 l of tap water (changed twice weekly) (Buchmann & Uldal, 1997). The fish were kept at 12 °C under a 12[ratio ]12 h day/night cycle, and fed a restricted diet of pelleted food. The parasite infrapopulations were examined at weekly intervals as described below with the first examination one week post-infection.
Four experimental groups were established with 30 experimental fish each: Corrib, Ätran and Conon salmon, and rainbow trout (total 120 fish). Infection in each of the 4 groups was achieved by cohabitation with 10 infected donor fish (rainbow trout); infection range per cohabitation system: 339–359. Furthermore, an additional group of 10 uninfected and marked (fin clipped) rainbow trout were added to each ‘cohabitation system’, serving as an internal trout:trout group (thus 50 fish per cohabitation system). The experiments were carried out in 128 l glass aquaria (equipped with internal Eheim biofilters) containing 50 l of local aerated tap water changed twice weekly. However, during the first 24 h of exposure, fish tanks were filled with only approximately 25 l of water to increase the probability of contact between infected and naïve fish. The 4 ‘cohabitation systems’ were monitored at weekly intervals by examination of all fish in each subgroup. First parasite enumeration took place 1 week post-infection.
Infection levels were assessed on anaesthetized fish (MS222, 50 mg/l) using a dissecting microscope with subillumination (7–50×magnification). Parasite enumeration was carried out in a thermostat-regulated room (12 °C). The site specificity of infection was noted as outlined by Buchmann & Uldal (1997).
Infection levels were expressed in terms of prevalence, intensity, mean intensity and mean abundance (Bush et al. 1997). Within each cohabitation system, differences in mean abundance between the experimental group and the internal control of rainbow trout was tested with a Mann–Whitney U-test. A Kruskall–Wallis test was applied to test for differences in mean abundance between the experimental groups (rainbow trout and the 3 salmon stocks) in the 4 cohabitation systems. Dunn's pairwise comparison procedure was subsequently used in order to isolate groups with significant differences.
Parasites (live or ethanol fixed) were mounted in ammonium-picrate-glycerine (Malmberg, 1970) and examined under high-power light microscopy (Leitz Dialux 22 equipped with phase-contrast). Fourteen specimens (mounted live) were subjected to more extended examination (Table 1) using the methods described by Mo (1993). Scanning electron microscopy (JEOL) of opisthaptoral hard parts was conducted based on digested single specimens and a slightly modified version of the methods described by Harris et al. (1999). In contrast to Harris et al. (1999), whole parasites were used. After 20 min of rehydration, the water was replaced by lysis solution (proteinase K 60 μg/ml, Tween 20 (0·45%) in TE-buffer) and the slide incubated at 50 °C for up to 20 min or until the parasite was fully dissolved. The digestion procedure was continuously monitored microscopically. Finally, the completely dried preparations were sputter-coated with gold using standard procedures.
Table 1. Dimensions of the opisthaptoral hard parts of the Gx morphotype (NS=Number of specimens; NM=Number of measurements. S.D.=Standard deviation. All measurements carried out on live specimens mounted in ammonium-picrate-glycerine (Malmberg, 1970). Designation of characters as in Mo (1991, 1992).)

With slight modifications, the amplification of the ribosomal RNA internal transcribed spacer (ITS) was accomplished as described by Cunningham (1997). Single ethanol-fixed specimens were allowed to air dry to remove ethanol and then incubated in 7·5 μl of lysis buffer (see above) at 65 °C for 20 min to allow complete digestion followed by 10 min at 95 °C in order to inactivate the proteinase activity. The entire ITS region of the ribosomal DNA array (spanning ITS1-5·8S-ITS2) was amplified using forward 5′-TTTCCGTAGGTGAACCT-3′ and reverse 5′-TCCTCCGCTTAGTGATA-3′ primers (Cunningham, 1997). Primers were used at a concentration of 1 μM with Ready-To-Go-PCR Beads (Amersham Pharmacia Biotech) using 4 μl of the parasite suspension as template (final concentration in reaction: 1·5 U Taq DNA polymerase, 10 mM Tris–HCl, pH 9·0, 50 mM KCl, 1·5 mM MgCl2, 200 μM dNTP; reaction volume 25 μl). Samples were initially denatured for 5 min at 95 °C and then subjected to 28 cycles of 1 min at 94 °C, 1 min at 50 °C and 50 sec at 72 °C. The reaction was finally terminated with an extension period of 5 min at 72 °C.
Aliquots of 8 μl of the PCR products were digested with the restriction enzyme Hae III. Fragment patterns were analysed following electrophoresis through an ethidium bromide-stained agarose gel (2%) alongside digested ITS amplicons from Danish Gyrodactylus derjavini (Paelebro Fish Farm population) as well as Norwegian G. salaris (Driva population) serving as references. RFLP analysis was performed on PCR products from 8 individual Gx parasites.
The internal transcribed spacer (ITS) region of the ribosomal DNA was sequenced from 4 individuals of Gx morphotype (Gx 1, Gx 2, Gx 2a and Gx 41). Two approaches were adopted: ITS PCR products were obtained from individuals Gx 1 and Gx 2 and purified from agarose gel using the Geneclean III Kit (Bio 101). Approximately 90 ng of the purified PCR product was used as template in DNA sequencing reactions using the dRhodamine Terminator Cycle Sequencing Ready Reaction kit (Perkin Elmer) and an ABI Prism 377 automatic sequencer. Both forward and reverse sequencing reactions were carried out using specific primers ITS1 (5′-TTTCCGTAGGTGAACCT-3′), and ITSR3A (5′-GAGCCGAGTGATCCACC-3′) to sequence ITS1, and ITS4·5 (5′-CATCGGTCTCTCGAACG-3′) and ITS 2 (5′-TCCTCCGCTTAGTGATA-3′) to sequence ITS2. The ITS PCR products from 2 further individuals, Gx 2a and Gx 41, were purified from agarose gel and cloned using the pGEM-T Easy Vector System (Promega). Recombinant plasmids were purified using resin column purification (Wizard Plus Miniprep system, Promega) and insert sizes verified following EcoR1 digestion. Sequencing was carried out as above using approximately 300 ng of purified plasmid, and M13/pUC forward and reverse primers.
Figure 1 demonstrates that rainbow trout were significantly more susceptible to infection with Gx than the 3 different salmon stocks when hosts were kept in isolation. Thus, Gx could either not establish on the salmon (e.g. Conon stock; Fig. 1A), or only reproduce to a very limited extent. The infrapopulations on salmon dropped towards zero within 3–4 weeks (Fig. 1). In contrast, prevalences on isolated rainbow trout were consistently high (80–90%) throughout the experimental period (Fig. 1A). Mean intensity of the Gx on isolated rainbow trout increased and peaked at around 40 parasites per fish between weeks 4 and 5 in marked contrast to the salmon infections (Fig. 1B). Thus, the maximum intensity seen on isolated salmon initially infected with 1 single individual of the morphotype was 3.

Fig. 1. Prevalence (A) and mean intensity (B) of Gx on individually isolated hosts initially infected with a single parasite specimen. Ten hosts of either rainbow trout Oncorhynchus mykiss or salmon Salmo salar of the Conon, Ätran and Corrib stock were infected.
A similar picture was evident in the cohabitation systems (Figs 2–5>). Infection with Gx rapidly spread from the infected donors to the uninfected recipients, especially rainbow trout, in all 4 cohabitation systems. Thus, prevalences on naïve rainbow trout (both experimental groups (Fig. 2A) as well as the internal trout:trout groups in the salmon cohabitation systems (Figs 3–5)) increased to 100% within 1–2 weeks post-infection. Prevalences on salmon increased to a much lesser extent (Figs 3–5). The mean abundance in all rainbow trout host groups in the cohabitation systems peaked 4–7 weeks post-infection, whereafter infection levels decreased (Figs 2–5>). A slight increase in mean abundance of Gx could also be observed in the 3 salmon stocks as well as a general tendency towards a small decrease at the end of the experimental period (Figs 3–5). Mean abundances on Corrib salmon were significantly higher than seen on Conon salmon at week 2 post-infection and the subsequent 3 evaluation days (P<0·05). Likewise, from the second week of infection until 4 weeks post-infection, Corrib salmon exhibited significantly higher mean abundances than Ätran (P<0·05). Finally, a higher mean abundance could be observed in the Ätran stock at day 45 p.i., when compared to the infection levels in Conon fish (P<0·05).

Fig. 2. Rainbow trout ‘cohabitation system’. Oncorhynchus mykiss (30 recipients) infected with Gx by cohabitation with 10 infected O. mykiss donors. An additional naïve 10 rainbow trout was included in the system serving as an internal trout[ratio ]trout control. (A) Prevalence. (B) Abundance.

Fig. 3. Conon, Salmo salar ‘cohabitation system’. S. salar (30 recipients, Conon stock) infected with Gx by cohabitation with 10 infected Oncorhynchus mykiss donors. An additional 10 rainbow trout was included in the system serving as an internal trout[ratio ]trout control. (A) Prevalence. (B) Abundance.

Fig. 4. Ätran, Salmo salar ‘cohabitation system’. S. salar (30 recipients, Ätran stock) infected with Gx by cohabitation with 10 infected Oncorhynchus mykiss donors. An additional 10 naïve rainbow trout was included in the system serving as an internal trout[ratio ]trout control. (A) Prevalence. (B) Abundance.

Fig. 5. Corrib, Salmo salar ‘cohabitation system’. S. salar (30 recipients, Corrib stock) infected with Gx by cohabitation with 10 infected Oncorhynchus mykiss donors. An additional 10 naïve rainbow trout was included in the system serving as an internal trout[ratio ]trout control. (A) Prevalence. (B) Abundance.
Mean abundances and peak infections of Gx always proved to be higher on rainbow trout than seen in the 3 different salmon stocks in the cohabitation systems. These differences were always highly significant (P<0·01). This picture of rainbow trout being more susceptible than salmon could also be seen within the level of each cohabitation system, as mean abundances of Gx on S. salar hosts were generally significantly lower than on the internal trout[ratio ]trout group (0·001>P<0·04). However, no significant differences could be observed between the 2 groups of naïve rainbow trout in the rainbow trout cohabitation system (range of P: 0·482–1·00). Although a similar infection pressure was applied to each cohabitation system (differences between donors at the initiation of the experiments not significant, P=0·931), the observed differences in host susceptibility between the 2 species were consistent both within and between cohabitation systems. The response to infection was more significant in rainbow trout than salmon in these cohabitation systems, and occurred approximately 2 weeks earlier than on salmon (Figs 2–5>). Infection levels on salmon of all 3 stocks attained higher values in cohabitation systems than was seen if hosts were isolated (Figs 1–5). The present results demonstrate that the 3 salmon stocks exhibit a significantly lower susceptibility to this Danish Gx morph than rainbow trout.
Caudal and pectoral fins as well as the head and operculum proved to be important microhabitats for the Gx morph infecting rainbow trout during the initial phase of infection (data not shown). However, a change in site of infection was observed with age of infection: the proportion of the parasites on the head region (excluding the cornea) and pectoral fins decreased, whereas the proportion on the caudal fin was constant. Generally, only a small percentage of the parasite population was found on the body. During the final week of the experiment, a translocation of the parasites (from 10% to 20%) to corneal surfaces could be observed, coincident with the period of marked parasite expulsion. The distribution of the parasite population on the salmon hosts differed in some respects compared to rainbow trout, though caudal fins as well as head regions also proved to be important microhabitats on the salmon. However, in contrast to rainbow trout no clear tendency towards a restriction on the head regions (head, operculum and corneal surfaces) after the initial phase of infection could be seen. Thus, corneal surfaces harbour a substantial proportion (13%–42%) of the parasite population on salmon throughout the entire experimental period, whereas this only applies for rainbow trout at the terminal phase of infection.
Measurements of 11 sclerite characters of the Gx morph are presented in Table 1, and depicted in Figs 6 and 7. Anchors appear robust and prominent in relation to size (Table 1, Fig. 6). The ventral bar has a more or less rectangular shape (Fig. 6). As seen in LM, the ventral bar is equipped with relatively small anterolateral processes and the appearance of the ventral bar membrane is tongue-shaped. The marginal hook sickle has a recurved blade forming an open and rounded inner aperture (Fig. 7). The sickle blade is tapering throughout its length from the proximal to the distal part, where the sickle point can be seen extending beyond the toe. However, the recurved appearance of the sickle blade is not equally distributed throughout its length, as the proximal part of the blade is more vertical than curved, thus giving the inner aperture the appearance of an ellipsoid structure. The sickle toe is rather acuminate and constitutes a triangular shape, which drops below the attachment point for the marginal hook handle. The toe tends to slope rather steeply from the base to the point. The ‘shelf’ on the upper edge of the sickle base displays a small recurved thorn at the point, where the toe starts to slope down (Fig. 7). The proximal part of the sickle proper displays a clearly demarcated and weakly pronounced heel.

Fig. 6. Scanning electron micrograph of opisthaptoral hard parts of the Gx morphotype released by proteinase K treatment as described in the Materials and Methods section. Hamuli, ventral bar (ventral bar membrane partly degraded) and marginal hook.
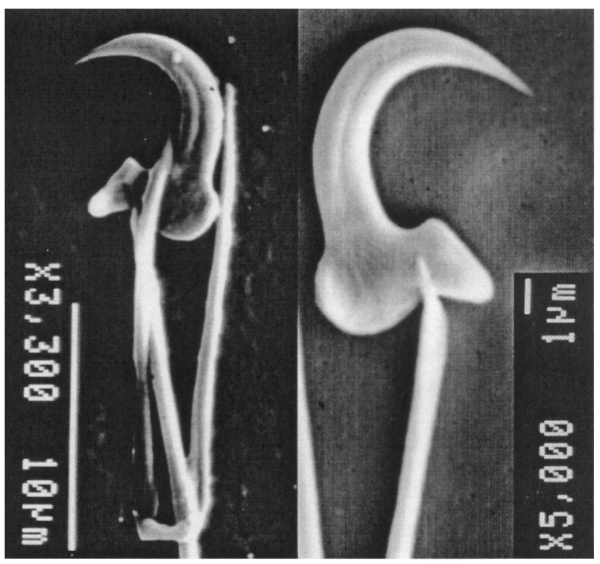
Fig. 7. Scanning electron micrograph of marginal hooks of the Gx morphotype released by proteinase K treatment as described in the Materials and Methods section.
Amplification of the entire ITS region from Gx gave rise to an amplicon of the expected size of approximately 1300 bp (Fig. 8, lane 8). Amplified ITS products were subsequently digested with the restriction enzyme Hae III and run alongside digested G. derjavini (Paelebro population) and G. salaris (Driva population) ITS references. Upon comparison with these, the Danish Gx exhibited a distinct RFLP pattern (Fig. 8). Instead of the 4 fragments seen upon digestion of the ITS PCR products from reference Norwegian G. salaris (511 bp, 399 bp, 234 bp, 154 bp), 5 fragments could be observed on Hae III digested PCR products from the Gx morph (Fig. 8). In addition to the 4 G. salaris fragments, an additional fragment of 554 bp could be observed. The 154 bp fragment appeared very faint in the Danish Gx. Hae III digestion of ITS PCR products from 8 individual specimens of the Gx morph consistently gave the same RFLP pattern.

Fig. 8. Differentiation of the Danish Gx from reference Gyrodactylus salaris (Driva population/‘strain’, Norway) and G. derjavini (Paelebro population/‘strain’, Denmark) by RFLP banding patterns. Agarose gel electrophoresis (2%) of amplified ITS-regions of the rDNA genes subsequently cut by restriction enzyme Hae III. Lanes 1 and 2, Markers (1 kb and 123 bp DNA ladders, respectively); lane 3, Hae III-treated G. derjavini; lane 4, Hae III-treated G. salaris; lane 5, Hae III-treated Gx; lanes 6–8, whole 1300 bp ITS amplicon from G. derjavini, G. salaris and Gx.
A single forward and single reverse sequence was obtained from both ITS1 and ITS2 of purified ITS PCR products from individuals Gx 1 and Gx 2. This sequence has been submitted to the EMBL nucleotide sequence database under accession number AJ515912. On comparison with reference sequences from G. derjavini, G. truttae and G. teuchis from Scotland, and G. salaris from Norway, the sequences were almost identical to G. salaris ITS. Three bases displaying heterogeneity were found; 1 in the ITS 1 region (C/T at position 276), and 2 in the ITS 2 region (C/T at position 911; A/T at position 1090).
Four clones of ITS from Gx 2a, and 3 clones from Gx 41 were sequenced. Clones were either of the reference G. salaris type or of the variant ITS type containing the 3 variations. The variation at position 911 affects a Hae III restriction site. Presence of a C (as found in the reference sequence from Norwegian G. salaris) maintains the proper sequence for enzyme recognition. Presence of T causes loss of the restriction site resulting in a 554 bp fragment where previously 400 and 154 bp fragments were obtained (Fig. 9). A mixture of both types of ITS variants within a PCR product would result in a restriction pattern containing 5 fragments as opposed to the 4 normally obtained on restriction of G. salaris ITS. This was actually observed in the restriction pattern of single Gx individuals (Figs 8 and 9).
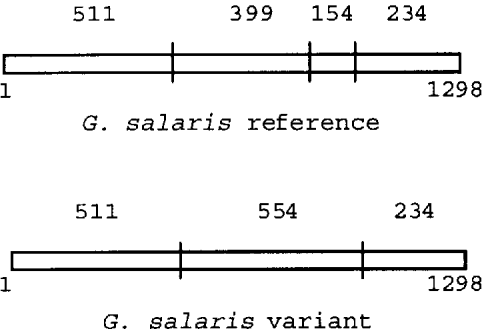
Fig. 9. Diagram showing Hae III restriction sites in the 2 types of ITS variants found within single individuals of Gx as determined by sequencing. Clones from single individuals were either of the reference Gyrodactylus salaris type or of the variant ITS type containing 3 nucleotide substitutions. The variation at position 911 affects a Hae III restriction site. Presence of C (as found in the reference sequence from Norwegian G. salaris) maintains the proper sequence for enzyme recognition. Presence of T causes loss of the restriction site resulting in a 554 bp fragment where previously 400 and 154 bp fragments were obtained. A mixture of both types of ITS variants within a PCR product from single individuals would result in a restriction pattern containing 5 fragments (554, 511, 399, 234, and 154 bp) as opposed to the 4 normally obtained on restriction of G. salaris ITS (511, 399, 234, and 154 bp).
Based on morphological and genetic analysis, the Gx morph examined in this study closely resembles G. salaris. The morphology of the opisthaptoral hard parts of the Gx morph was found to be identical to G. salaris, as the range of variation in the sclerite characters measured is within the total range of variation reported for G. salaris (Mo, 1991). The only morphological character of Gx where the range of variation is not covered by the range reported for G. salaris is the total length of anchors. These appear longer in the Gx morph (80·4–91·2 μm compared to 66·0–85·0 as reported in Mo, 1991), primarily due to the length of the anchor shaft that slightly exceeds the figures reported from G. salaris on rainbow trout (Mo, 1991). The other structures constituting the hamuli, namely length of anchor point and length of anchor root are, by contrast, fully covered by the range reported for G. salaris. However, length of hamuli is not considered to be a reliable character (Mo, 1991). The large anchors could be indicative of G. bohemicus. The steep way, whereby the toe in Gx tends to slope from the base to the point is a trait also seen in G. bohemicus (Ergens, 1992). In G. salaris from salmon, the toe tends to slope more gently (Mo, 1991). However, this is not a general feature for G. salaris, as individuals from rainbow trout in contrast have marginal hooks with a toe displaying a steep slope from its base to its point as seen in the current Gx (compare Fig. 7 with Fig. 7A (S. salar host) and B (O. mykiss host) in Mo, 1991). The small, recurved thorn observed on the base of the hook sickle on Gx is, by contrast, not a feature of G. salaris sensu stricto. G. bohemicus, on the other hand, display such a thorn on its marginal hooks (Ergens, 1992). The validity of this feature as a reliable character has not been investigated. Furthermore, the marginal hook sickle in G. bohemicus is reported to be larger (9–10 μm) (Ergens, 1992) than the current Gx (7·2–9·0 μm; Table 1). The shape of the inner curve of the Gx sickle proper encompasses an oval shape in contrast to the more circular appearance generally seen in G. thymalli. This trait also distinguishes Gx from G. teuchis, which further have a relatively short sickle shaft, a longer and more curved sickle blade with the point tapering well beyond the toe as well as the sickle being wider (approximately 2 μm) in its distal than its proximal part (Cunningham et al. 2001).
No G. bohemicus sequences are available yet, which might shed some light on the G. salaris/G. bohemicus issue. However, the molecular examination supported the similarity with G. salaris, as the genetic differences found were significantly less than between congeners (e.g. G. derjavini, G. truttae and G. teuchis relative to G. salaris), (Cunningham, 1997; Cunningham, Aliesky & Collins, 2000; Cunningham et al. 2001; Sterud et al. 2002). A survey of Gyrodactylus from UK and Denmark has only ever found single nucleotide differences in ITS of G. derjavini (114 sequences) or G. truttae (33 sequences), whereas we have not found any other variants in G. salaris (62 RFLP and 17 sequences) apart from the current Gx. G. derjavini from UK and Czech Republic, two countries with no known exchange of fish in recent years, had identical ITS sequences (Matejusová et al. 2001).
Recently, upon a very large examination of the rDNA ITS including 31 gyrodactylid species from 5 different families of freshwater fish, a similar ITS heterogeneity was only reported in Gyrodactylus carassii (Matejusová et al. 2001). Polymorphism was detected in the ITS2 region, where 6 individuals displayed A/T at position 154 and 1 individual only exhibiting an A. This was explained either by the presence of heterozygosity or the possible existence of polymorphism between individual units of the rDNA repeat, as suggested for the current gyrodactylid. The small differences observed, and the nature of them, do not readily favour and support the erection of Gx as a species distinctly different from G. salaris. Although morphological similarities to G. bohemicus were also noted, the genetic evidence presented here shows that the Gx is a variant of G. salaris sensu Malmberg. However, it must still be remembered that Gyrodactylus thymalli and G. salaris display no difference in their ITS sequences (Cunningham, 1997), yet according to current knowledge they are considered separate species based on i.e. host preference and pathogenicity, even though the issue of conspecificity, which has been raised earlier, is not fully clarified (Soleng & Bakke, 2001; Sterud et al. 2002). The Gx presented here likewise shows differences in pathogenicity in relation to G. salaris. However, it seems obvious that the use of pathogenicity as a species discriminator is problematic and not readily applicable. What then is the relationship of Gx relative to G. salaris (sensu stricto)? From sequences obtained from the Gx individuals, it cannot be determined whether the heterogeneity seen at 3 positions is due to the formation of hybrid individuals between gyrodactylids with ITS sequences differing at the 3 bases in question, or whether the heterogeneity is due to 2 different types of ITS sequences within the ribosomal array of a single individual (van Herwerden, Blair & Agatsuma, 1999). In addition, it might be speculated that the heterogeneity could be related to differences in ITS between the mother and embryon. All 3 base changes occur within 1 repeat unit i.e., not a change in only position 276 in one unit, and a change at position 911 or 1090 in another. This is evident from the sequences obtained from single clones, where each insert showing differences from the reference G. salaris ITS sequence contains all 3 base changes. The sequence variation is not due to variation in techniques or interpretation, as has been found when comparing other Gyrodactylus ITS sequences (Ziętara et al. 2002), as sequencing was carried out in the same laboratory with the same methods as reference G. salaris sequences, and in addition, experimental variation would not be expected to result in such consistent variations or apparent heterozygosity. Evidence of interspecific hybrids has recently been found in the ITS of Fasciola (Agatsuma et al. 2000). However, in the present case of Gx, no individuals displaying only the alternate ITS sequence (with 3 substitutions relative to classical G. salaris sequence and with a predicted Hae III RFLP of 554, 511 and 234 bp) have been recorded despite thorough examination of gyrodactylids from both wild and farmed salmonids in Denmark (Lindenstrøm et al. 1999; Buchmann et al. 2000; Nielsen & Buchmann, 2001). The apparent lack of individuals displaying only the alternate ITS variant does not support the assumption that the present morphotype represents a hybrid between a ‘classical’ G. salaris and a not yet described Gyrodactylus species displaying the 3 ITS substitutions in question. Although the idea of a hybrid cannot be ruled out, it might therefore indicate that the Gx rather represents a unique form of G. salaris displaying intra-specific sequence diversity within the rDNA cluster of single individuals.
No individuals displaying the same RFLP patterns or sequences as the Gx morph have been found outside Denmark despite extensive surveys of Gyrodactylus parasites in Scotland and France as well as Norway. However, morphotypes with the same RFLP pattern have recently been found in 2 other rainbow trout farms in Denmark upon a subsequent survey (Nielsen & Buchmann, 2001). The later records of the Gx are both from fish farms situated in westbound streams (River Holme å – tributary to River Varde å and in River Skjern å) from 2 other counties (Ribe and Ringkøbing) in the Jutland Peninsula (Nielsen & Buchmann, 2001), whereas the fish farm from which the parasite material for this study originated, was located in an eastbound stream (Vejle å). These systems are not interconnected. It would be a remarkable coincidence, if the 3 base changes in the genome of the morph arose at the same positions independently within the 3 different populations. Most plausible, the Gx morph could therefore have been spread by supply of fry or fingerlings from the same sources or as a consequence of trade of fish between farms from the respective streams. Most likely, the Gx represents a geographically isolated morphotype, and the changes within its genome must have only occurred recently. The reproductive isolation of Gyrodactylus populations in fish farms and the extreme fluctuations in population size due to recurrent treatment with anti-parasitic compounds, could favour the fixation of genetic changes, which might appear following genetic bottlenecks and founder effects (Malmberg, 1987). Small qualitative differences in the marginal hooks of Gx relative to G. thymalli were noted, but the genetic examinations could not disprove that Gx could represent a variant of G. thymalli. However, grayling is not a natural host in the stream of Vejle å or any of its tributaries. Despite electrofishing twice a year in Vejle å, the biologists in the county have never caught any grayling in this stream system (county biologists, pers. com.). Furthermore, G. thymalli have not yet been recovered from Danish waters despite thorough examination (Buchmann et al. 2000).
The rainbow trout proved to be relatively susceptible to infection with Gx, compared to the 3 strains of Atlantic salmon tested. This result is in contrast to the current knowledge that East Atlantic stocks of salmon are highly susceptible to infection with G. salaris (Bakke et al. 1990, 1999; Bakke & MacKenzie, 1993). As salmon from the Scottish Conon stock was also used by Bakke & MacKenzie (1993), the observed discrepancy in parasite reproduction and virulence between G. salaris and the present Gx is significant. However, there are differences in experimental design and tanks (Bakke & MacKenzie, 1993), and we cannot exclude that the Conon stock – or the other stocks employed – could be restricted in genetic variability and hence differ from those used in previous studies.
The response to Gx by rainbow trout is in accordance with the study by Bakke et al. (1991 a), where G. salaris-infected rainbow trout, although displaying a marked heterogeneity, were also capable of mounting a response to the infection. The Gx morph exhibits a different microhabitat distribution compared to that reported for G. salaris proper (Jensen & Johnsen, 1992; Mo, 1992; Appleby & Mo, 1997), as caudal fins and head regions represented locations of major importance when infecting S. salar. A marked relocation of parasites to corneal surfaces was evident in the later stages of infection in rainbow trout, coincident with the onset of parasite expulsion. In contrast, a considerable proportion of the Gx parasites infecting salmon were found at the cornea throughout the entire infection period. It could therefore be speculated that salmon, which obviously is not an appropriate host for Gx, possesses substances in the epidermis that the Gx morph, in contrast to G. salaris proper, is unable to cope with and therefore actively seeks shelter in this immunoprivileged site (Buchmann & Bresciani, 1998).
The very limited degree of reproduction on isolated salmon indicates that the disparity in infection course between rainbow trout and salmon in cohabitation systems can not be explained only by differences in transmission efficiencies. The observed differences in abundance in these systems must also rely to a marked extent on the different abilities of the Gx morph to reproduce on the 2 host species. Higher infection levels were consistently seen in the salmon if these hosts were kept in cohabitation rather than isolated. This indicates that infection of the salmon by settlement rather than reproduction is involved and stresses that parasite transfer is taking place in these systems and that the salmon are recurrently infected due to the strong infection pressure being exerted on them. Thus, parasites could be driven from rather susceptible (rainbow trout) to less susceptible (salmon) hosts, especially when the former mounts a response.
Loss of genetic variability as a result of artificial propagation of salmonids, which could lead to an increase in intrastock variability between hatcheries, has previously been reported (Ståhl, 1983; Allendorf, Ryman & Utter, 1987). However, the results may indicate strain variations in parasite virulence rather than differences in host resistance. This suggests that different populations of G. salaris could display different affinities to various salmonid species and strains previously tested (Bakke et al. 1990, 1991 a, 1992 b; Bakke, Jansen & Harris, 1996; Jansen & Bakke, 1995; Soleng & Bakke, 2001). Interestingly, a Loma salmonae (Putz, Hoffman & Dunbar, 1965) variant has recently been described, which exhibits low virulence and preference for species of Oncorhynchus that were generally considered to be the preferred host genus (Sánchez et al. 2001).
Currently, we have no information on the occurrence of the Gx morph on wild fishes. As reproduction of Gx on salmon is almost non-existent, it seriously questions whether the morphotype can establish a self-reproducing parasite population on wild S. salar (Bakke et al. 2002). However, as the Gx morph was observed to survive on individual salmon for more than 20 days (on isolated Corrib and Ätran), this species could be of importance in transmission (Bakke, Jansen & Hansen, 1991 b). The susceptibility of prevailing salmonids in the Jutland peninsula such as Salmo trutta, Coregonus spp. or Thymallus thymallus (the latter 2 with a very scattered distribution in Jutland) towards infection with this particular morphotype is unknown. As Gx and G. salaris share a common host (rainbow trout) which is quite widespread as escapees, it could therefore easily be misidentified due to the very high similarity. However, due to the loss of a Hae III restriction site in the alternate ITS variant of Gx, the RFLP method still remains suitable to differentiate it from G. salaris sensu Malmberg.
This work was financed by EU-project QLRT-2000-01631 (The genetic basis of Gyrodactylus salaris resistance in Atlantic salmon Salmo salar) and in addition by a PhD grant from the Royal Veterinary and Agricultural University, Denmark granted to T. L.

Table 1. Dimensions of the opisthaptoral hard parts of the Gx morphotype

Fig. 1. Prevalence (A) and mean intensity (B) of Gx on individually isolated hosts initially infected with a single parasite specimen. Ten hosts of either rainbow trout Oncorhynchus mykiss or salmon Salmo salar of the Conon, Ätran and Corrib stock were infected.

Fig. 2. Rainbow trout ‘cohabitation system’. Oncorhynchus mykiss (30 recipients) infected with Gx by cohabitation with 10 infected O. mykiss donors. An additional naïve 10 rainbow trout was included in the system serving as an internal trout[ratio ]trout control. (A) Prevalence. (B) Abundance.

Fig. 3. Conon, Salmo salar ‘cohabitation system’. S. salar (30 recipients, Conon stock) infected with Gx by cohabitation with 10 infected Oncorhynchus mykiss donors. An additional 10 rainbow trout was included in the system serving as an internal trout[ratio ]trout control. (A) Prevalence. (B) Abundance.

Fig. 4. Ätran, Salmo salar ‘cohabitation system’. S. salar (30 recipients, Ätran stock) infected with Gx by cohabitation with 10 infected Oncorhynchus mykiss donors. An additional 10 naïve rainbow trout was included in the system serving as an internal trout[ratio ]trout control. (A) Prevalence. (B) Abundance.

Fig. 5. Corrib, Salmo salar ‘cohabitation system’. S. salar (30 recipients, Corrib stock) infected with Gx by cohabitation with 10 infected Oncorhynchus mykiss donors. An additional 10 naïve rainbow trout was included in the system serving as an internal trout[ratio ]trout control. (A) Prevalence. (B) Abundance.
Fig. 6. Scanning electron micrograph of opisthaptoral hard parts of the Gx morphotype released by proteinase K treatment as described in the Materials and Methods section. Hamuli, ventral bar (ventral bar membrane partly degraded) and marginal hook.
Fig. 7. Scanning electron micrograph of marginal hooks of the Gx morphotype released by proteinase K treatment as described in the Materials and Methods section.
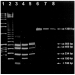
Fig. 8. Differentiation of the Danish Gx from reference Gyrodactylus salaris (Driva population/‘strain’, Norway) and G. derjavini (Paelebro population/‘strain’, Denmark) by RFLP banding patterns. Agarose gel electrophoresis (2%) of amplified ITS-regions of the rDNA genes subsequently cut by restriction enzyme Hae III. Lanes 1 and 2, Markers (1 kb and 123 bp DNA ladders, respectively); lane 3, Hae III-treated G. derjavini; lane 4, Hae III-treated G. salaris; lane 5, Hae III-treated Gx; lanes 6–8, whole 1300 bp ITS amplicon from G. derjavini, G. salaris and Gx.

Fig. 9. Diagram showing Hae III restriction sites in the 2 types of ITS variants found within single individuals of Gx as determined by sequencing. Clones from single individuals were either of the reference Gyrodactylus salaris type or of the variant ITS type containing 3 nucleotide substitutions. The variation at position 911 affects a Hae III restriction site. Presence of C (as found in the reference sequence from Norwegian G. salaris) maintains the proper sequence for enzyme recognition. Presence of T causes loss of the restriction site resulting in a 554 bp fragment where previously 400 and 154 bp fragments were obtained. A mixture of both types of ITS variants within a PCR product from single individuals would result in a restriction pattern containing 5 fragments (554, 511, 399, 234, and 154 bp) as opposed to the 4 normally obtained on restriction of G. salaris ITS (511, 399, 234, and 154 bp).