INTRODUCTION
Schistosoma mekongi infection has been an important public health problem in Laos and Cambodia along the Mekong River and its tributaries since the first case of schistosomiasis was reported in the Mekong River basin (Dupont et al. Reference Dupont, Bernard, Soubrane, Halle and Richter1957). Its clinical manifestations in humans are hepatosplenomegaly and decompensation of portal hypertension (Urbani et al. Reference Urbani, Sinuon, Socheat, Pholsena, Strandgaard, Odermatt and Hatz2002). Although portal fibrosis in human cases has been reported (Wittes et al. Reference Wittes, Maclean, Law and Lough1984; Monchy et al. Reference Monchy, Dumurgier, Heng, Hong, Khun, Hou, Sok and Huerrre2006), infection with hepatitis B, liver flukes, or a history of alcohol consumption must be considered as well. Periportal thickening and portal enlargement were observed on sonography in Cambodia. Annual treatment with praziquantel improved sonographic patterns of liver fibrosis based on the Niamey classification of the World Health Organization in the year 2000, and reduced periportal thickening, but did not improve advanced liver fibrosis and portal hypertension (Urbani et al. Reference Urbani, Sinuon, Socheat, Pholsena, Strandgaard, Odermatt and Hatz2002).
S. mekongi and S. japonicum are closely related phylogenetically, but differ in schistosome egg size (Sornmani, Reference Sornmani1976; Voge et al. Reference Voge, Bruckner and Bruce1978) and in the starting point of egg deposition in mammalian hosts (Voge et al. Reference Voge, Bruckner and Bruce1978). In addition, the echogenic network pattern in the liver, a typical characteristic in S. japonicum infection, has not been noted in S. mekongi infection (Ohmae et al. Reference Ohmae, Sinuon, Kirinoki, Matsumoto, Chigusa, Socheat and Matsuda2004). Sonographic findings in the liver in S. japonicum infection were reported to correspond to histopathological findings (Murakami, Reference Murakami1986; Zilton and Cheever, Reference Zilton and Cheever1993). However, there have been only limited studies of S. mekongi infection (Byram et al. Reference Byram, Imohiosen and von Lichtenberg1978; Byram and Lichtenberg, Reference Byram and von Lichtenberg1980; Owhashi et al. Reference Owhashi, Matsumoto, Imase, Kirinoki, Kitikoon, Chigusa and Matsuda2005).
We therefore conducted this histopathological study of S. mekongi infection to clarify the characteristics of granuloma formation and liver fibrosis in comparison with S. japonicum infection.
MATERIALS AND METHODS
Animals and infection
Female ICR mice, 4 weeks of age, were purchased from Japan SLC. Inc., Hamamatsu, Japan. S. mekongi (Laotian strain) cercariae were obtained from laboratory-infected Neotricula aperta gamma race by shedding. All of these snails were collected annually from the Mekong River located in Cambodia. S. japonicum (Japanese strain) cercariae were shed from laboratory-infected Oncomelania nosophora collected from Yamanashi Prefecture, Japan.
Thirty ICR mice were anaesthetized for S. mekongi infection, and the same number of mice was anaesthetized for S. japonicum infection by subcutaneous injections of a mixed solution of ketamine (Veterinary Ketalar® 50; Sankyo Lifetech Co., Ltd, Tokyo, Japan) and xylazine (Xylazine®; Bayer Health Care, Germany). Each mouse was infected with 20 cercariae of S. mekongi or S. japonicum percutaneously through a shaven abdomen under anaesthesia using the cover-slip method (Maeda et al. Reference Maeda, Irie and Yasuraoka1982). When infected ICR mice died before autopsy, the same number of mice was infected to replace those which had died.
The care and use of all animals complied with the Rules on the Care and Use of Laboratory Animals of Dokkyo Medical University, and the Institutional Review Boards of the author's institution approved the study protocol.
Anatomy
From the S. mekongi- and S. japonicum-infected groups, 6 ICR mice each were chosen at random, euthanized, and dissected at 6, 8, 12, 16, and 20 weeks post-exposure. The adult schistosome worm pairs in the portal vein were picked up by forceps and counted. The chest and abdomen were opened, and the left liver lobe, the lung, and the small intestine were removed from the mice. The left liver lobe was cut into halves, the lung was cut at the section including the apex of the lung, and a few centimeters of the proximal and distal part of the small intestine were cut (Hirose et al. Reference Hirose, Matsumoto, Kirinoki, Shimada, Chigusa, Nakamura, Sinuon, Socheat, Kitikoon and Matsuda2007). The organs were fixed in 20% formalin and embedded in paraffin. Sections of 5 μm thickness were cut and stained with haematoxylin-eosin and Masson's trichrome stain.
Liver granuloma area calculation
The areas (μm2) of granulomas, which contained a single schistosome egg in a section of the liver, were calculated in each mouse. Eight continuous sections of one granuloma were checked to determine whether the granuloma was formed by a single egg and contained an intact or degenerated miracidium. The granulomas were observed with an Olympus AX-80 microscope, photographed with an Olympus DP 71 camera, uploaded by Olympus DP controller with DP manager, and the areas calculated with Win ROOF software package Ver.5.8.1 by tracing the border of granulomas.
Statistical analysis
Two granulomas in each section were chosen at random for the statistical analysis of calculated liver granuloma areas (μm2). The areas of granulomas were analysed by Steel-Dwass's multiple comparison test, and P values of less than 0·05 considered significant.
RESULTS
Schistosome worm burden and mouse survival
Three mice with 4 worm pairs survived until 20 weeks with S. mekongi infection. No mice died during the experimental period even when harbouring 9 worm pairs in the case of S. japonicum infection. Throughout the experimental period, the total number of schistosome worm pairs of S. mekongi was less than that of S. japonicum (Table 1).
Table 1. The number of schistosome worm pairs found in the portal vein

* Mean number of worm pairs±s.d.
** Minimal number–maximal number of worm pairs.
Changes in relative liver weight
The liver weight per mouse body weight was observed for the definition of hepatomegaly during the experimental period. Hepatomegaly began to be seen at 8 weeks in S. mekongi infection, and reached the same level as in S. japonicum infection at 12 weeks. In contrast, hepatomegaly was already seen at 6 weeks in S. japonicum infection (Fig. 1).

Fig. 1. Changes in relative liver weight (%) of ICR mice in Schistosoma mekongi and S. japonicum infections. Filled circles; mean±s.d. of mice with S. mekongi infection. Open circles; mean±s.d. of mice with S. japonicum infection. Filled triangles; mean of normal ICR mice quoted from CLEA JAPAN, INC.
Granuloma area in liver
Liver granulomas varied in size; some appeared very large in the early period and were smaller later in both S. mekongi and S. japonicum infection (Table 2). The granuloma area seemed to be largest at 12 weeks in S. mekongi infection and 8 weeks in S. japonicum infection.
Table 2. Granuloma size in the liver

* Mean area of granulomas±s.d.
Histopathology of granulomas in the liver
In S. mekongi infection, histiocytes and eosinophils were seen around schistosome eggs at 6 weeks. Eosinophil abscess-like granulomas appeared with histiocyte-oriented foam cells in the intralobular area at 8 weeks (Fig. 2A1 and A2), and increased in number at 12 weeks. In the granulomas in the intralobular area, epithelioid cells with eosinophils were seen around the schistosome eggs, which were surrounded by fibrous tissue with eosinophils at 12 weeks (Fig. 2B1 and B2). The fibrosis of granulomas in the intralobular area increased with a mixture of fibrous tissue and eosinophils at 16 weeks. Epithelioid cells around the egg became more like giant cells at 16 weeks (Fig. 2C1 and C2). All stages of granulomas were seen accompanying granulomas with foam cells in the intralobular area at 20 weeks (Fig. 2D1). Inflammatory cells, mainly lymphocytes, infiltrated in the periphery of the granulomas during the experimental period.
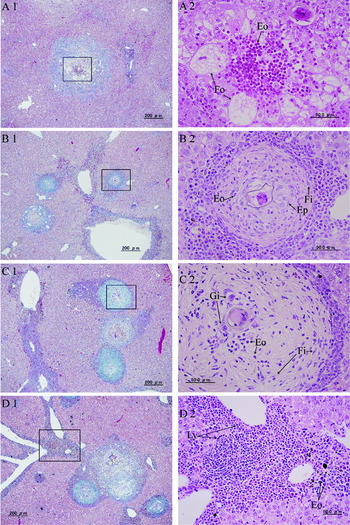
Fig. 2. Histopathology of the liver in Schistosoma mekongi infection. (A1) A granuloma in the intralobular area at 8 weeks (Masson's trichrome stain). (A2) The square shown in A1 contains foam cells (Fo) and eosinophils (Eo) (H&E stain). (B1) Granulomas in the intralobular area at 12 weeks (Masson's trichrome stain). (B2) The square shown in B1 contains epithelioid cells (Ep) with eosinophils (Eo), and surrounding fibrous tissue (Fi) with eosinophils (Eo) (H&E stain). (C1) Granulomas in the intralobular area at 16 weeks (Masson's trichrome stain). (C2) The square shown in C1 contains a mixture of fibrous tissue (Fi) and eosinophils (Eo). Giant cells (Gi) surrounded the schistosome egg (H&E stain). (D1) Periportal inflammatory cell infiltration and granulomas in the intralobular area at 20 weeks (Masson's trichrome stain). (D2) The square shown in D1 contains periportal lymphocyte (Ly) infiltration with fewer eosinophils (Eo) than in the S. japonicum infection (H&E stain).
In S. japonicum infection, eosinophil abscess-like granulomas, which accompanied necrosis of parenchyma cells, were seen in the intralobular area at 6 weeks. In the intralobular area, the granulomas consisted of histiocytes, eosinophils, and gradually increasing fibrous tissue at 8 weeks (Fig. 3A). Granulomas became more fibrous by 16 weeks (Fig. 3B). Lymphocytes and eosinophils infiltrated in the periphery of the granulomas during the experimental period. New granulation was not seen in the intralobular area at 20 weeks, and degenerated eggs were observed to be scattered in normal looking parenchyma (Table 3).
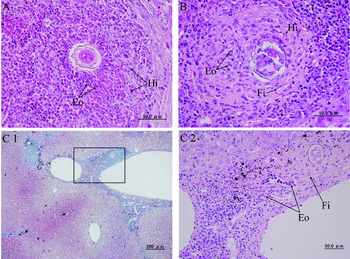
Fig. 3. Histopathology of the liver in Schistosoma japonicum infection. (A) Histiocytes (Hi) and eosinophils (Eo) in a granuloma at 8 weeks (H&E stain). (B) Fibrous tissue (Fi), histiocytes (Hi), and eosinophils (Eo) in a granuloma at 16 weeks (H&E stain). (C1) Portal fibrosis contained a schistosome egg at 20 weeks (Masson's trichrome stain). (C2) The square shown in C1 contains eosinophils (Eo) and fibrous tissue (Fi) around a schistosome egg (H&E stain).
Table 3. Inflammatory cell infiltration of liver granulomas in the intralobular area

* Lymphocytes were seen in the periphery of granulomas in both types of infection.
** At 20 weeks with S. japonicum infection: no newly formed granulomas were observed in the intralobular area.
Periportal lesions
In S. mekongi infection, inflammatory cells, mainly lymphocytes, infiltrated into the periportal area during the experimental period, and no portal fibrosis was seen even at 20 weeks (Fig. 2D2). In S. japonicum infection, inflammatory cells, mainly lymphocytes, infiltrated into the periportal area at 6 weeks. Eosinophil infiltration was increased at 12 weeks. Portal fibrosis was seen at 16 weeks and 20 weeks (Fig. 3C1) along with predominance of eosinophils and fibrous tissue (Fig. 3C2).
Small intestine lesions
Schistosome eggs were deposited in the capillary vessels of the lamina propria mucosa in the early period of S. mekongi and S. japonicum infection with little inflammatory cell infiltration. Schistosome eggs formed granulomas mainly in the submucosa, accompanied by destruction of the muscularis mucosa (Fig. 4A) after 12 weeks in S. mekongi and after 8 weeks in S. japonicum infection. S. mekongi granulomas contained foam cells, neutrophils, and fewer eosinophils than in liver granulomas (Fig. 4B). S. japonicum granulomas were also seen in the submucosa, but did not contain foam cells.

Fig. 4. Histopathology of the small intestine in Schistosoma mekongi infection at 20 weeks. (A) Granulomas in submucosa (H&E stain). (B) The square shown in A contains foam cells (Fo), histiocytes (Hi), neutrophils (Neu), and fewer eosinophils than in liver granulomas (H&E stain).
A large number of schistosome eggs, which contained live miracidia, were located in the lamina propria mucosa with inflammatory cells, mainly neutrophils, and were likely to be released into the lumen with destroyed villi in the later period in both types of infection. The small intestine with S. mekongi infection at 20 weeks is shown in Fig. 4A.
Lung lesions
The number of granulomas, which contained a schistosome egg, was counted in a single section of the lung. In S. mekongi infection, 2 granulomas were seen in one case along with an intact section of single schistosome worm at 16 weeks. One schistosome worm was seen without a granuloma in one case at 16 weeks. Three cases with 1–3 granulomas were seen at 20 weeks. In S. japonicum infection, 2 granulomas were seen in one case at 12 weeks. One case had 3 granulomas and the other had 5 granulomas with male and female schistosome worms at 16 weeks. Three cases had 1–3 granulomas at 20 weeks. Granulomas in both types of infection consisted mainly of histiocytes and lymphocytes. Inflammatory cells infiltrated, but no fibrosis was seen in the alveolar wall (Fig. 5).

Fig. 5. Histopathology of the lung in Schistosoma mekongi infection at 20 weeks. A granuloma with histiocytes (Hi) and lymphocytes (Ly) (H&E stain).
DISCUSSION
Byram and Lichtenberg (Reference Byram and von Lichtenberg1980) reported that mice harbouring 4 or more worm pairs of S. mekongi died prior to 10 weeks, and also reported that hepatomegaly increased towards 25 weeks in ICR mice with exposure to 5–10 cercariae. In this study, 7 mice with 2–6 worm pairs unexpectedly died before 15 weeks post-exposure with S. mekongi infection (data not shown). Throughout the experimental period, the total number of schistosome worm pairs of S. mekongi was less than that of S. japonicum. However S. mekongi worm burden proved fatal in some mice, indicating that infection with S. mekongi was more virulent than that with S. japonicum. Histopathological findings, which included continuous attack of S. mekongi eggs into the intralobular area of the liver, with accumulating damage to the liver parenchyma, may explain the higher virulence of S. mekongi infection compared with S. japonicum infection.
When mice were used as an animal model, hepatomegaly with S. japonicum infection was reported to usually appear by 6 weeks (Warren and Berry, Reference Warren and Berry1972; Warren et al. Reference Warren, Grove and Pelley1978), while that with S. mekongi infection was reported to appear at 8 weeks (Byram and Lichtenberg, Reference Byram and von Lichtenberg1980). These findings were consistent with our results. The delayed hepatomegaly in S. mekongi infection might be related to the delayed egg deposition of schistosome worms when compared to that in S. japonicum infection (Voge et al. Reference Voge, Bruckner and Bruce1978).
In our study, hepatomegaly associated with harbouring of 5 pairs in S. mekongi infection was less than that with harbouring of 1 pair in S. japonicum infection at 8 weeks, showing that schistosome egg deposition and granulation had more effect on hepatomegaly than the number of schistosome worms. In S. mekongi infection, the maximal granuloma was reported to appear at 8 weeks (Byram and Lichtenberg, Reference Byram and von Lichtenberg1980). In this study, 1 granuloma showed a relatively large area at 8 weeks in comparison to the other 6 granulomas, thus resulting in a high standard deviation at 8 weeks in S. mekongi infection. Statistically speaking, the largest granuloma was seen at 12 weeks in S. mekongi infection in this study. Granulomas containing foam cells tended to be larger than the granulomas without foam cells in S. mekongi infection, and large granulomas with foam cells increased in number at 12 weeks, which might explain why the largest granulomas could be seen at 12 weeks. The largest size of the granuloma area in S. japonicum infection was reported to appear at 6 weeks in previous studies (Warren et al. Reference Warren, Grove and Pelley1978; Owhashi et al. Reference Owhashi, Maruyama and Nawa1996), but it was observed at 8 weeks in this study.
Kupffer cells in the liver are reported to play an important role in granuloma formation; they are transformed into epithelioid cells and giant cells in mice by injection of glucan, an insoluble polysaccharide (Naito and Takahashi, Reference Naito and Takahashi1991; Takahashi et al. Reference Takahashi, Naito, Umeda and Shultz1994). Histiocytes seen in S. mekongi infection were also transformed into epithelioid cells and giant cells after presenting as foam cells. The reaction of histiocytes to glucan and S. mekongi eggs was not similar to that of histiocytes to S. japonicum eggs. The foamy appearance of histiocytes, which contain droplets in the cytoplasm through the process of receptor-mediated endocytosis, accounts for foam cells (Brown and Goldstein, Reference Brown and Goldstein1983). The foam cells detected in this study were probably the same cells as the vacuolocytic macrophages previously reported in S. mekongi infection (Byram and Lichtenberg, Reference Byram and von Lichtenberg1980). Although the droplets in foam cells are usually cholesteryl ester stored in the cytoplasm, the droplets appearing in S. mekongi infection were reported to have only scattered lipid droplets (Byram and Lichtenberg, Reference Byram and von Lichtenberg1980). The foam cells appearing in S. mekongi infection might be formed not only by the uptake of lipid released from necrotized hepatocytes but also that of substances related to the S. mekongi egg itself.
The eosinophil reaction to S. mekongi eggs was reported to be intense from the earliest time of egg deposition in mice (Byram et al. Reference Byram, Imohiosen and von Lichtenberg1978; Byram and Lichtenberg, Reference Byram and von Lichtenberg1980), and also reported to be intense to S. japonicum eggs with more fibrous reaction at 8 weeks (Warren et al. Reference Warren, Boros, Hang and Mahmoud1975, Reference Warren, Grove and Pelley1978; Owhashi et al. Reference Owhashi, Maruyama and Nawa1996). In our study, the eosinophil reaction was intense in the liver in both S. mekongi and S. japonicum infection, and the neutrophilic reaction was more predominant in lesions in the small intestine in S. mekongi infection.
At 8 weeks in both types of infection, granulomas were seen in the intralobular area not in the periportal area in mice. It is not the smaller egg volume (Voge et al. Reference Voge, Bruckner and Bruce1978) but the non-fibrous portal reaction to eggs that allowed S. mekongi eggs to continuously infiltrate into the intralobular area and form granulomas. In S. japonicum infection, portal fibrosis containing schistosome eggs could be induced by increasing infiltration of eosinophils into the periportal area. Portal fibrosis may block schistosome eggs from infiltrating into the intralobular area. In S. mekongi infection, on the other hand, lymphocytes were predominant until 20 weeks in the periportal area, and portal fibrosis was not seen even at 20 weeks. Instead of the delayed egg deposition, the low rate of eosinophil infiltration into the periportal area explains the lack of portal fibrosis in S. mekongi infection. In S. mekongi infection, the continuous granuloma formation in the intralobular area and the non-fibrous portal reaction to eggs are strikingly different from S. japonicum infection. These morphological changes in the periportal area appeared to be associated with the induction of portal hypertension by periportal thickening and portal enlargement in S. mekongi infection (Urbani et al. Reference Urbani, Sinuon, Socheat, Pholsena, Strandgaard, Odermatt and Hatz2002) and pipe-stem fibrosis in S. japonicum infection (Warren and Berry, Reference Warren and Berry1972). Portal inflammatory cell infiltration, mainly by lymphocytes, is also seen in human cases of S. mekongi infection (Wittes et al. Reference Wittes, Maclean, Law and Lough1984; Monchy et al. Reference Monchy, Dumurgier, Heng, Hong, Khun, Hou, Sok and Huerrre2006).
In the small intestine, schistosome eggs were seen initially in capillary vessels in the lamina propria mucosa, which were connected to the portal vein, from where the schistosome worms came to deposit eggs. S. mekongi granulomas were seen mostly in the submucosa as previously reported (Byram and Lichtenberg, Reference Byram and von Lichtenberg1980), contained fewer eosinophils than the liver granulomas, and contained more foam cells which might have been directly induced by schistosome eggs. Granulomas hardened the submucosa, which aided release of live eggs into the lumen. Schistosome eggs in the lamina propria mucosa, surrounded mainly by neutrophils, were likely to be released into the lumen with destroyed villi. Release of a large number of live eggs into the lumen is effective in protecting hosts from accumulation of schistosome eggs in the body, and also enabled parasites to survive.
Minimal fibrosis of the lung in S. japonicum infection has been reported in mice (Hirata et al. Reference Hirata, Takushima, Kage and Fukuma1993) and in rabbits (Cheever et al. Reference Cheever, Duvall and Minker1980). In our study, no fibrosis was seen in the alveolar walls either in S. mekongi or S. japonicum infection. Multiple lung granulomas, which contained degenerated eggs, were observed in both types of infection at 16 and 20 weeks. Eggs in the liver located close to the central vein in S. mekongi infection possibly reached the lung, but eggs trapped in the portal wall in S. japonicum infection could not reach the lung. Schistosome eggs laid in the portal vein might be able to reach the lung through collateral vessels when liver fibrosis was advanced, but lung granulomas could be seen without portal fibrosis along with schistosome worms in the lung. Although schistosome worms were unexpectedly seen in the lungs both in S. mekongi and S. japonicum infection, schistosome worms in the lungs were previously reported in human cases of S. haematobium infection (Buchanan and Gelfand, Reference Buchanan and Gelfand1970), in laboratory-infected rabbits with S. japonicum infection (Inokuchi et al. Reference Inokuchi, Maki and Tsutsumi1979), and in field rats in Leyte, Philippines with S. japonicum infection (Oshima et al. Reference Oshima, Yasuraoka, Irie, Blas, Noseñas and Santos1978). Although we also reported the presence of S. mekongi worms in the lungs of laboratory-infected ICR mice, the question remained how and when the schistosome worms came to reside in the lungs. The possibility of direct egg laying in the lungs in schistosome infection should be mentioned, since the lung granuloma formation appeared to be more common than previously thought and could be seen with or without portal fibrosis in both S. mekongi and S. japonicum infection.
In conclusion, schistosome eggs flowing from the portal vein to the hepatic lobule impair liver parenchyma cells by inducing eosinophil abscess-like granulomas and cellular or fibrous granulomas with histiocytes. Generally, granulation gradually decreased when miracidia in the eggs degenerated with regeneration of the liver parenchyma. The granulation in the liver, the intestine, and the lung might be one of the protective measures taken by hosts against schistosomiases, with induction of degeneration of miracidia in the eggs. The advanced portal fibrosis resembled pipe-stem fibrosis which has not been seen in S. mekongi infection, is effective in preventing eggs from infiltrating into the intralobular area before impairment of the liver parenchyma. The absence of portal fibrosis in S. mekongi infection allows schistosome eggs to infiltrate continuously into the intralobular area, and this could be what lies behind the ultrasonographic difference; the echogenic network pattern as was seen in S. japonicum infection, has not been noted in S. mekongi infection.
ACKNOWLEDGEMENTS
The authors thank to Ms Fumie Yokotsuka, Clinical Research Center, Dokkyo Medical University for her valuable technical support and encouragement.
FINANCIAL SUPPORT
This study was supported by the Sasakawa Memorial Health Foundation, Parasitic Diseases Control Program (1997–2006), and the Ohyama Health Foundation (2002, 2008).










