Introduction
Understanding how a parasite interacts with its hosts is like piecing together a puzzle with constantly changing parts. Increases in our knowledge base are iterative, requiring re-evaluation of earlier interpretations and assumptions. This review summarizes the current knowledge of the biology of a myxozoan parasite, Ceratonova shasta, its interactions with its vertebrate and invertebrate hosts and how it is affected by its environment, in the context of the reason we study it: its ability to cause severe enteronecrosis in Pacific salmon, an iconic and economically important group of fish.
Ceratonova shasta was initially described from hatchery rainbow trout Oncorhynchus mykiss in California, USA (Noble, Reference Noble1950; Schafer, Reference Schafer1968) and subsequently recognized to infect other species of Oncorhynchus and other genera within the family Salmonidae. The parasite is established in most major river drainages of the Pacific Northwest of the United States (including California, Washington, Oregon, Idaho and Alaska) and British Columbia, Canada, including some of the largest salmon-producing rivers in the world (reviewed by Hallett and Bartholomew, Reference Hallett, Bartholomew, Woo and Buchmann2012). Among myxozoans, C. shasta is unusual in its restriction to this geographic range despite the natural migration and human introduction of Pacific salmon and trout to nearly all corners of the globe. Even within the endemic range of C. shasta, its distribution is a mosaic, with the parasite present in some rivers and absent in its tributaries and adjacent rivers.
Previous reviews summarized what was known about the resistance and susceptibility spectrum of host species (Bartholomew, Reference Bartholomew1998); the distribution, pathobiology, detection and control of the pathogen (Hallett and Bartholomew, Reference Hallett, Bartholomew, Woo and Buchmann2012; Jones et al., Reference Jones, Bartholomew, Zhang, Okamura, Gruhl and Bartholomew2015); and the effects of climate change on the parasite life cycle and disease severity (Ray et al., Reference Ray, Alexander, De Leenheer, Bartholomew, Okamura, Gruhl and Bartholomew2015). Here, we expand on these topics to incorporate new information and to examine how this information changes our understanding of parasite ecology and disease management. Much of the recent progress comes from studies conducted in the Klamath River Oregon/California, USA, where this parasite is considered a primary factor affecting salmon recovery (Fujiwara et al., Reference Fujiwara, Mohr, Greenberg, Foott and Bartholomew2011), and where management actions have been implemented based on data from long-term monitoring and research (Lehman et al., Reference Lehman, Johnson, Adkison, Burgess, Connon, Fangue, Foott, Hallett, Martinez–López, Miller, Purcell, Som, Valdes–Donoso and Collins2020). Common to many major rivers in the USA, the Klamath has been divided by a series of hydropower dams for over a century (Hamilton et al., Reference Hamilton, Rondorf, Tinniswood, Leary, Mayer, Gavette and Casal2016). Reconnection of the basins through dam removal is imminent (Thompson et al., Reference Thompson, Anderson, Clemento, Campbell, Pearse, Hearsey, Kinziger and Garza2020) and will provide novel opportunities to study a multi-host aquatic parasite under changing environmental conditions.
Ceratonova shasta life cycle and development
Ceratonova shasta (Cnidaria: Myxozoa: Myxosporea) follows what can be considered the typical myxosporean life cycle model with 2 spore stages, the actinospore (Fig. 1A) and myxospore (Fig. 1H), developing in an aquatic annelid (definitive host) and a member of the family Salmonidae (intermediate host), respectively (Bartholomew et al., Reference Bartholomew, Whipple, Stevens and Fryer1997). Among the characteristics that make this parasite unique is its invertebrate host, Manayunkia occidentalis (Atkinson et al., Reference Atkinson, Bartholomew and Rouse2020), which is a member of a small clade of freshwater annelids. The ability to artificially passage infections by injection of ascites from infected fish enabled some of the first research on myxozoan disease progression and host susceptibility (Johnson et al., Reference Johnson, Sanders and Fryer1979; Bower and Margolis, Reference Bower and Margolis1985; Ibarra et al., Reference Ibarra, Gall and Hedrick1991). However, the development of molecular diagnostic tools (Palenzuela et al., Reference Palenzuela, Trobridge and Bartholomew1999; Hallett and Bartholomew, Reference Hallett and Bartholomew2006) and the establishment of the life cycle in the laboratory (Bjork and Bartholomew, Reference Bjork and Bartholomew2009a) have enabled experimentation that has informed our understanding of transmission between and infection in both hosts.

Fig. 1. The complex life cycle of Ceratonova shasta involves 2 spore stages and 2 obligate hosts: a salmonid fish and the annelid Manayunkia occidentalis. Clockwise from left: waterborne actinospores (A) sense the fish and discharge their nematocysts (B). The parasite sporoplasm penetrates the gill epithelium (C) where it begins to proliferate (D), then enters the host bloodstream (E). The parasite travels to the intestine, migrates between tissue layers (F), further proliferates and then sporulates (G). Mature myxospores (H) are released with feces or from the decomposed carcass. Waterborne myxospores encounter the annelid host, discharge their nematocysts (I) and penetrate the annelid intestine. The parasite migrates to the body wall where it proliferates (J, K), then sporulates into actinospores (L, M), which burst from the body wall (N). Scale: black bar = 10 μm, hollow bar = 100 μm.
Infection and development in the salmonid host
After emergence from the annelid host, passively distributed waterborne actinospores (Fig. 1A) attach to fish gills, facilitated by discharging tubules from their nematocysts (polar capsules) (Bjork and Bartholomew, Reference Bjork and Bartholomew2010) (Fig. 1B, C). The binucleate amoeboid sporoplasm released from the attached actinospore migrates between the cells of the gill epithelium to the gill blood vessel and encysts within the vessel epithelium (Fig. 1D). Autogamy and cell division result in presporogonic stages that are released into the bloodstream (Fig. 1E). Parasites invade the intestine about 1 week following infection (Fig. 1F) and continue proliferating, triggering an acute inflammatory response (Bartholomew et al., Reference Bartholomew, Smith, Rohovec and Fryer1989; Bjork and Bartholomew, Reference Bjork and Bartholomew2010; Barrett and Bartholomew, Reference Barrett and Bartholomew2021; Barrett et al., Reference Barrett, Estensoro, Sitjà-Bobadilla and Bartholomew2021). Depending on temperature and possibly some chemosensory trigger, the parasite initiates sporulation, forming disporic pseudoplasmodia that culminate in mature myxospores (Yamamoto and Sanders, Reference Yamamoto and Sanders1979; Fig. 1G). Invasion of other tissues and organs appears to be secondary to intestinal infection, achieved through blood circulation as the host immune system weakens (Bartholomew et al., Reference Bartholomew, Smith, Rohovec and Fryer1989; Bjork and Bartholomew, Reference Bjork and Bartholomew2010). Clinical signs of disease vary with parasite genotype, parasite dose, fish species and fish age, and may include inappetance, anorexia, lethargy, darkening, distention of the abdomen with ascites, exophthalmia and a swollen and haemorrhagic vent (Hallett and Bartholomew, Reference Hallett, Bartholomew, Woo and Buchmann2012). Disease severity is directly related to temperature and may culminate in death, which can occur as early as 15 days post-exposure in fish held at 15°C (Ray et al., Reference Ray, Holt and Bartholomew2012). Myxospores typically mature during terminal stages of infection with the majority released when fish die (although there is a notable exception described below in which spores are shed from apparently healthy fish), possibly triggered by factors produced as tissues become necrotic (Kent et al., Reference Kent, Soderlund, Thomann, Schreck and Sharpton2014).
Myxospores released from the salmon (Fig. 1H) are similar in size (6–8 μm × 14–17 μm) to those of other myxozoan species (Yamamoto and Sanders, Reference Yamamoto and Sanders1979) and sink in the water column (settling rate of 35 cm day−1; authors' and Deas' unpublished data), which facilitates encounter with the benthic invertebrate host (Alexander et al., Reference Alexander, Kerans, Hallett, Stevens, Okamura, Gruhl and Bartholomew2015).
Infection and development in the annelid host
Infection of the definitive host M. occidentalis occurs when the filter-feeding annelid ingests C. shasta myxospores released from infected fish. Discharge of the nematocyst tubules (Fig. 1I) likely serves to anchor the spore in the intestine and allows the binucleate sporoplasm, released when the spore valves separate, to invade the gut epithelium. The parasite migrates between gut epithelial cells and as early as 6 h post-ingestion, sporoplasm aggregates are found intracellularly within the epidermis (Fig. 1J, K), with development occurring along the nerve cord (Meaders and Hendrickson, Reference Meaders and Hendrickson2009). Proliferation (schizogony and gametogony) and sporogonic development in the epidermis is asynchronous and culminates in a pansporocyst of 8 actinospores (Fig. 1L, M; Bartholomew et al., Reference Bartholomew, Whipple, Stevens and Fryer1997; Meaders and Hendrickson, Reference Meaders and Hendrickson2009). Release of actinospores may occur through secretory pores (Bartholomew et al., Reference Bartholomew, Atkinson and Hallett2006; Bjork, Reference Bjork2010) or directly through the epidermal layer (Fig. 1N; Meaders and Hendrickson, Reference Meaders and Hendrickson2009), mechanisms that could allow both asynchronous spore release and survival of the host.
The released actinospore is a small (14 × 11 μm; Fig. 1A), tetrahedral spore with 3 nematocysts and no valve cell inflation (Bartholomew et al., Reference Bartholomew, Whipple, Stevens and Fryer1997). Ceratonova shasta actinospores are small in comparison with actinospores of other freshwater myxozoans. Thus, they have presented challenges for research as they required modification of existing methods for capture, enumeration and detection (Hallett and Bartholomew, Reference Hallett and Bartholomew2006; Bjork and Bartholomew, Reference Bjork and Bartholomew2009a). The lack of caudal processes to provide buoyancy and the presence of only 1 binucleate sporoplasm contrasts with many other myxozoan actinospores (Eszterbauer et al., Reference Eszterbauer, Atkinson, Diamant, Morris, El-Matbouli, Hartikainen, Okamura, Gruhl and Bartholomew2015) and must also affect parasite transmission strategy.
Parasite factors driving disease
Advances in genomic sequencing, transcriptomics and proteomics have changed our understanding of C. shasta, particularly earlier conclusions about its distribution and virulence. This section reviews the major advances in our understanding of parasite factors that affect the outcome of infection in the salmonid host (Fig. 2).

Fig. 2. Multipartite components of enteronecrosis.
Ceratonova shasta genotypes: our evolving understanding of a complex species
Originally described as a species of the predominantly marine myxozoan genus Ceratomyxa (Noble, Reference Noble1950), analysis of C. shasta's distinguishing characters (host, tissue, habitat, rDNA sequences) supported its reclassification into a new genus Ceratonova (Atkinson et al., Reference Atkinson, Foott and Bartholomew2014). This genus comprises 2 formally described freshwater intestinal parasites (Atkinson et al., Reference Atkinson, Bartholomew and Rouse2020), and heretofore undescribed species detected only in eDNA samples (Atkinson and Bartholomew, Reference Atkinson and Bartholomew2010b; Richey et al., Reference Richey, Kenelty, Hopkins, Stevens, Martínez-López, Hallett, Atkinson, Bartholomew and Soto2020). Ceratonova shasta comprises 3 distinct genetic lineages (genotypes; Fig. 3) distinguished by polymorphisms in the parasite's ribosomal internal transcribed spacer region 1 (ITS-1) and differing in fish host specificities – primarily different Oncorhynchus species (Atkinson and Bartholomew, Reference Atkinson and Bartholomew2010a, Reference Atkinson and Bartholomew2010b; Stinson et al., Reference Stinson, Atkinson and Bartholomew2018; Breyta et al., Reference Breyta, Atkinson and Bartholomew2019). Genotype 0 is the most genetically distinct and is specific for steelhead/rainbow trout (O. mykiss) and cutthroat trout (Oncorhynchus clarkii) (Stinson et al., Reference Stinson, Atkinson and Bartholomew2018), which are likely the ancestral hosts (Breyta et al., Reference Breyta, Atkinson and Bartholomew2019). Genotype I is specific to Chinook salmon (Oncorhynchus tshawytscha), in which it can cause dose-dependent mortality (Hallett et al., Reference Hallett, Ray, Hurst, Holt, Buckles, Atkinson and Bartholomew2012). In contrast, genotype II, while primarily infecting coho salmon (Oncorhynchus kisutch), is a generalist in its ability to infect multiple host species. Genotype II is the dominant strain detected from sockeye/kokanee (Oncorhynchus nerka) and chum salmon (Oncorhynchus keta), from non-native salmonids, and from native salmonids with origins in watersheds where the parasite is not present (allopatric strains) (Stinson et al., Reference Stinson, Atkinson and Bartholomew2018). Genotype II is highly virulent in allopatric rainbow trout (Hurst and Bartholomew, Reference Hurst and Bartholomew2012). A third genotype ‘III’ was originally ascribed to an additional ITS sequence variant, however, subsequent genetic work identified it as the same as type II (Atkinson et al., Reference Atkinson, Hallett and Bartholomew2018). While mixed genotype infections have been observed, particularly in allopatric fish stocks, typically only the host-specific genotype persists and sporulates (Stinson and Bartholomew, Reference Stinson and Bartholomew2012; Hurst et al., Reference Hurst, Wong, Hallett, Ray and Bartholomew2014).
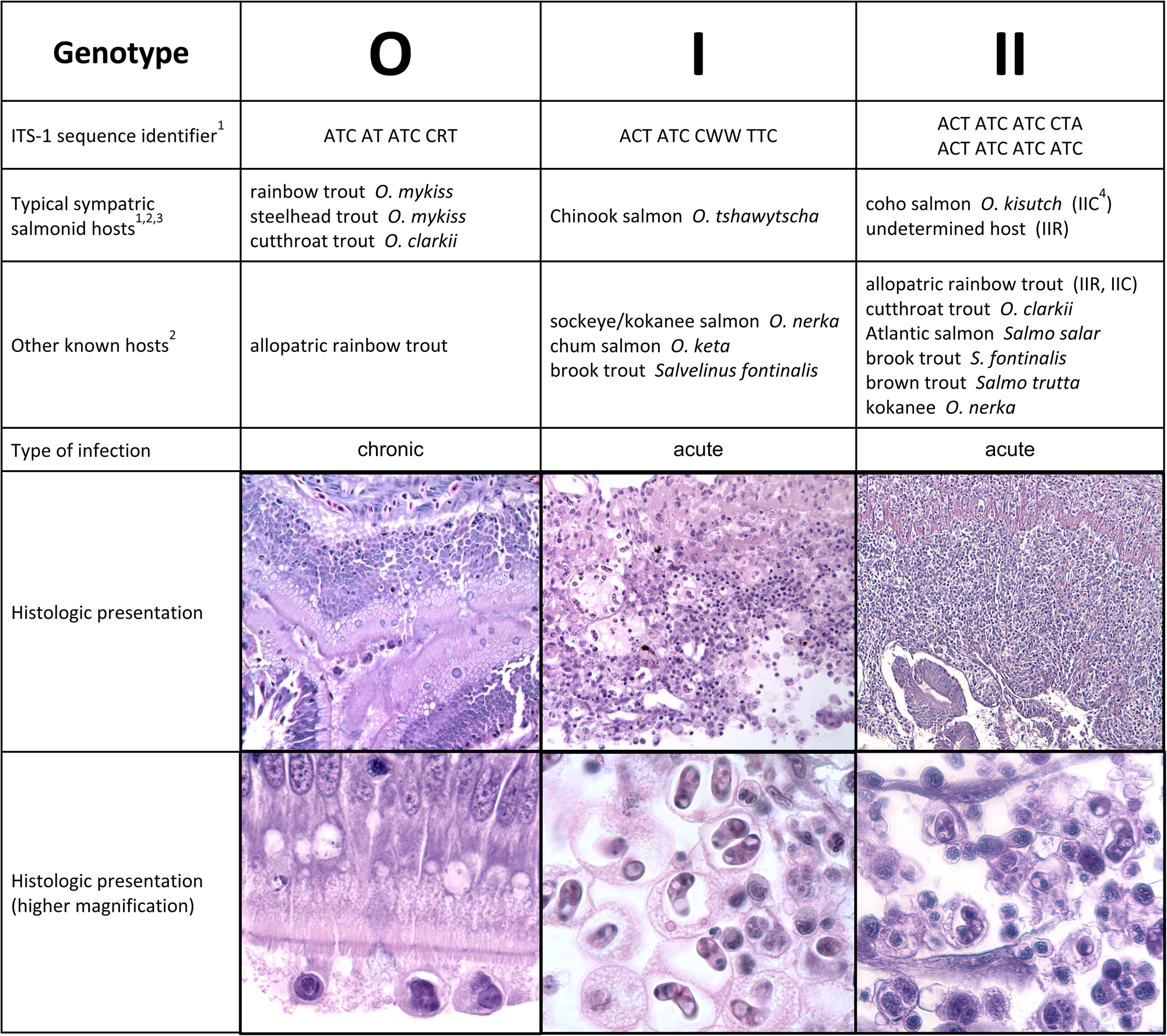
Fig. 3. Ceratonova shasta genotypes and their distinguishing characters. Sympatric/allopatric designators are used to characterize stocks with origins from waters with or without C. shasta, respectively. Histological sections of the intestine stained with H&E; O and II = allopatric rainbow trout, I = Chinook salmon. [1] Atkinson and Bartholomew (Reference Atkinson and Bartholomew2010b); [2] Stinson et al. (Reference Stinson, Atkinson and Bartholomew2018); [3] Stinson and Bartholomew (Reference Stinson and Bartholomew2012); [4] Hurst et al. (Reference Hurst, Holt and Bartholomew2012).
Data from fish infection studies support further characterization of genotype II: IIR, typically detected in allopatric rainbow trout, and IIC, found in coho salmon. This distinction, initially demonstrated in field-exposed fish (Atkinson and Bartholomew, Reference Atkinson and Bartholomew2010b) then in laboratory challenges (Hurst et al., Reference Hurst, Holt and Bartholomew2012), was supported by phylogenomic analysis (Alama-Bermejo et al., Reference Alama-Bermejo, Meyer, Atkinson, Holzer, Wiśniewska, Kolísko and Bartholomew2020) that showed genotype IIC was more closely related to genotype I than to genotype IIR. This also correlates with the evolutionary distance between their fish hosts, as Chinook (I) and coho (IIC) salmon are more closely related to each other than to rainbow trout (IIR) (Crête-Lafrenière et al., Reference Crête-Lafrenière, Weir and Bernatchez2012). It was hypothesized that the high virulence of IIR in allopatric rainbow trout is a consequence of a relatively recent host shift from a common ancestor of genotypes I and IIC, and suggests that evolution of genotype II is being guided by adaptation to new hosts (Alama-Bermejo et al., Reference Alama-Bermejo, Meyer, Atkinson, Holzer, Wiśniewska, Kolísko and Bartholomew2020).
The multiple C. shasta genotypes exist in sympatry in rivers from California to British Columbia, and have the same host associations across this range (Stinson et al., Reference Stinson, Atkinson and Bartholomew2018). Genotypes 0 and I appear to be so tightly adapted to their hosts that they are not present in waters where those species are not found, despite the presence of other salmonids. This pattern is particularly evident above barriers to migration like dams or impassable waterfalls, and suggests that as connectivity is restored for salmon in these rivers following dam removal, parasite redistribution will also occur where the obligate invertebrate host is also present (Hurst et al., Reference Hurst, Holt and Bartholomew2012; Stinson and Bartholomew, Reference Stinson and Bartholomew2012).
Behaviour of the different genotypes in their fish hosts also reflects their close host–parasite adaptation, and suggests different transmission strategies to the invertebrate host. Chinook and coho salmon are semelparous, dying after they return to freshwater to spawn. Infections in adult salmon by both genotypes I and II are characterized by release of spores only upon the death of the fish, and the process of sporulation continues post-mortem in adult Chinook salmon (Kent et al., Reference Kent, Soderlund, Thomann, Schreck and Sharpton2014; Foott et al., Reference Foott, Stone, Fogerty, True, Bolick, Bartholomew, Hallett, Buckles and Alexander2016). This provides explanation for earlier studies on the effects of C. shasta on pre-spawn mortality where fish that were killed for egg taking had a much lower apparent infection prevalence (18% myxospore positive) compared to fish that died prior to spawning (65% myxospore positive) (Chapman, Reference Chapman1986). The input of myxospores on spawning grounds, mainly in the fall, brings the parasite to the top of the river system. Dispersal from carcasses likely relies on high winter flows to disturb the sediments and mobilize myxospores. This is particularly important in tributary spawning grounds where the annelid host is typically absent. Juvenile fish encounter and become infected with C. shasta in the spring during their migration to the ocean, thus spreading the parasite downriver to infect lower mainstem annelid host populations.
In contrast, Pacific trout (steelhead/rainbow, cutthroat) are iteroparous and live to spawn more than once, and the interaction between genotype 0 and its fish host (both sympatric and allopatric strains) is less virulent. Thus, the adaptation of genotype 0 to Pacific trout allows sporulation and release over a long period of time. In contrast to the severe damage to the mucosal epithelium characteristic in genotype I and II infections, genotype 0 parasites migrate through the layers of the intestine causing inflammation but minimal damage to the intestinal epithelia (Fig. 3; Taggart-Murphy et al., Reference Taggart-Murphy, Alama-Bermejo, Dolan, Takizawa and Bartholomew2021). Genotype 0 appears to have adapted a coelozoic lifestyle, developing and maturing within the lumen of the intestine. In these chronic infections spores are observed in fecal casts as long as 2 years after they became infected (Richard Holt, OSU, pers. comm.).
Virulence factors: molecular tools of the trade
The first high-throughput sequencing datasets for C. shasta paved the way towards understanding parasite virulence at the molecular level (Alama-Bermejo et al., Reference Alama-Bermejo, Meyer, Atkinson, Holzer, Wiśniewska, Kolísko and Bartholomew2020). Studies on virulence factors have focused on candidate genes related to parasite motility and adhesion, proteases and their inhibitors, venom-like compounds (VLCs) and signalling molecules. The first transcriptome-wide comparisons of proteases and motility genes revealed their association with different C. shasta genotypes, suggesting that these genes are likely under positive selection pressure and linked to virulence (Alama-Bermejo et al., Reference Alama-Bermejo, Meyer, Atkinson, Holzer, Wiśniewska, Kolísko and Bartholomew2020). To decipher mechanisms of C. shasta virulence, genotypes 0 and IIR were studied due to their different infection outcomes in allopatric rainbow trout. Genotype 0 produces a chronic low-virulence infection, with limited proliferation, delayed spore production and no host mortality over >12 months (Alama-Bermejo et al., Reference Alama-Bermejo, Holzer and Bartholomew2019; authors’ unpublished data). In contrast, genotype IIR results in a highly virulent infection, with rapid and massive parasite proliferation and 100% host mortality in <1 month (Alama-Bermejo et al., Reference Alama-Bermejo, Holzer and Bartholomew2019). Using this model, C. shasta virulence was characterized, identifying key molecular effectors and differences in parasite proliferation and migration.
Parasite motility, proliferation and adhesion as virulence factors
In the fish host, C. shasta developmental stages are motile and have high phenotypic plasticity, suggesting a high capacity to respond to their microenvironment. In vivo observations of parasite motility from genotype IIR in rainbow trout showed that proliferative stages are capable of producing different cell protrusions (filopodia, lamellipodia and blebs) and of switching between motility modes, with specific functions like anchoring/adhesion, crawling and blebbing (Fig. 4; Alama-Bermejo et al., Reference Alama-Bermejo, Holzer and Bartholomew2019). Observation of in vivo motility was not possible for genotype 0 because of its low proliferation, but histological observations of luminal parasite stages showed filopodia attached to epithelial cells (Taggart-Murphy et al., Reference Taggart-Murphy, Alama-Bermejo, Dolan, Takizawa and Bartholomew2021). In vivo observation of genotype I stages (in Chinook salmon) showed the same types of protrusions, but the parasite displayed an exploratory behaviour with simultaneous projection of all 3 cell protrusions and a unique long and extensible posterior filament that acted as a root or uropod (Fig. 4F; Alama-Bermejo et al., Reference Alama-Bermejo, Holzer and Bartholomew2019). Histological and transcriptomic comparisons of genotypes 0 and IIR in rainbow trout revealed major differences in migration, gene expression patterns and proliferation rate in the host. Virulent genotype IIR had initial rapid proliferation with bleb-based migration to the gut, and showed higher small subunit (SSU) rDNA parasite copy numbers in the intestine (21–152-fold) than in genotype 0 infected fish. As a result of its more rapid proliferation, there was higher expression of cytoskeleton-related genes β-actin and ras homolog family member A (RhoA) and genotype IIR produced myxospores earlier than genotype 0 (Alama-Bermejo et al., Reference Alama-Bermejo, Holzer and Bartholomew2019; Taggart-Murphy et al., Reference Taggart-Murphy, Alama-Bermejo, Dolan, Takizawa and Bartholomew2021). Proteomic evidence suggests that cytoskeleton related genes are important during sporogenesis in genotype IIR, with changes from a dynamic to a stabilized actin network when transitioning from early stages to myxospores (Brekhman et al., Reference Brekhman, Ofek-Lalzar, Atkinson, Alama-Bermejo, Maor-Landaw, Malik, Bartholomew and Lotan2021). In the gut, parasite adhesion increased, mediated by adhesion factors integrin-β and talin, and induced massive interaction and disruption of the host intestinal extracellular matrix (ECM). This correlated with the extraordinary affinity of genotype IIR stages to fibronectin, a glycoprotein component of the ECM (Alama-Bermejo et al., Reference Alama-Bermejo, Holzer and Bartholomew2019). In response to this parasite-induced pathology, the host responds with upregulation of host genes related to ECM and cell adhesion (Barrett and Bartholomew, Reference Barrett and Bartholomew2021). Destruction of the intestinal tissue resulted in systemic infection with spread of the parasite to other organs, combined with a massive inflammatory response and rapid host mortality (<1month). In contrast, genotype 0 had low proliferation rates and slow/early direction-driven mesenchymal migration, evidenced by only early-stage expression of adhesive factors, low parasite copy numbers in the gut and continual/chronic maturation and release of myxospores (Alama-Bermejo et al., Reference Alama-Bermejo, Holzer and Bartholomew2019). In summary, motility studies showed that parasite adhesion shapes C. shasta virulence, and thus adhesion effectors such as integrins are promising candidate targets for chemotherapeutic interventions in myxozoans.

Fig. 4. Ceratonova shasta cell protrusions. (A) Developmental stages, 2 of them showing abundant static filopodia; (B) Parasite stages with lamellipodia and small filopodia projecting in the external margin; (C) 3D radiating pattern of filopodia; (D) active blebbing parasite stages with 5 overlapping blebs (white arrowheads); (E) external appearance of 2 blebs; (F) stage with profuse blebbing at the anterior end and a posterior end with extensible filaments that anchored the stage to other cells. A–E: genotype IIR, rainbow trout ascites; F: genotype I, Chinook salmon ascites; A, D, F: light microscopy; B–C, E: scanning electron microscopy.
Proteases and inhibitors
Proteases and inhibitors have roles in pathogen invasion, migration, feeding and immunomodulation of host responses. Large protease repertoires have been found in myxozoans: e.g. at least 6% of the C. shasta nematocyst proteome are proteases (Piriatinskiy et al., Reference Piriatinskiy, Atkinson, Park, Morgenstern, Brekhman, Yossifon, Bartholomew and Lotan2017), and all main catalytic types of protease were found in C. shasta developmental stages (Alama-Bermejo et al., Reference Alama-Bermejo, Meyer, Atkinson, Holzer, Wiśniewska, Kolísko and Bartholomew2020; Ahmad et al., Reference Ahmad, Debes, Pukk, Kahar, Hartikainen, Gross and Vasemägi2021). Differential expression, in silico characterization and annotation of 4 proteases and an inhibitor provided the first insights on the involvement of these enzymes in parasite virulence (Alama-Bermejo et al., Reference Alama-Bermejo, Bartošová-Sojková, Atkinson, Holzer and Bartholomew2022). Virulent genotype IIR upregulates an aspartic protease (cathepsin D) in concert with the occurrence of increased parasite proliferation at 15 days post exposure (dpe), coincident with upregulation of the pro-inflammatory cytokines interleukin (IL)-6 and IL-8 (Taggart-Murphy et al., Reference Taggart-Murphy, Alama-Bermejo, Dolan, Takizawa and Bartholomew2021). This suggested that C. shasta cathepsin D may induce an inflammatory response in the host, as has been shown for other parasite aspartic proteases (Cassone et al., Reference Cassone, Vecchiarelli and Hube2016). A cysteine protease inhibitor (stefin) in C. shasta was upregulated in IIR infections, with peaks of expression at 15 dpe and to a lesser extent at 29 dpe (Alama-Bermejo et al., Reference Alama-Bermejo, Bartošová-Sojková, Atkinson, Holzer and Bartholomew2022). Stefins are usually involved in regulation of endopeptidases, especially during metabolically active proliferative stages in parasites (Lee et al., Reference Lee, Song, Moon, Lee, Jha, Danne, Cha, Yu, Kong, Chung and Hong2013). In genotype IIR, the stefin was present in proteomic data from ascites but absent from myxospores (Brekhman et al., Reference Brekhman, Ofek-Lalzar, Atkinson, Alama-Bermejo, Maor-Landaw, Malik, Bartholomew and Lotan2021), indicating some cell regulatory function in developmental stages. A contrasting expression profile between genotypes and the cathepsin D and the stefin 3D protein structures suggests these molecules are key for the rapid proliferation and metabolism of genotype IIR (Bartošová-Sojková et al., Reference Bartošová-Sojková, Kyslík, Alama-Bermejo, Hartigan, Atkinson, Bartholomew, Picard-Sánchez, Palenzuela, Faber, Holland and Holzer2021; Alama-Bermejo et al., Reference Alama-Bermejo, Bartošová-Sojková, Atkinson, Holzer and Bartholomew2022).
The cysteine proteases cathepsin L and Z were of greater importance for the low virulence genotype 0, with upregulation at all time points and especially when the parasite proliferated in the intestine. These proteases could contribute to the lack of acute inflammatory response in genotype 0, as it has been shown that parasite-derived cysteine proteases can neutralize immune pathways by degrading host immune factors (Bird et al., Reference Bird, Trapani and Villadangos2009). A metalloprotease or aminopeptidase-N was found to be expressed in both genotypes mostly at later time points and coincident with the onset of sporogenesis, which agreed with previous proteomic data where this protease was observed to be a significant component of IIR nematocysts (Piriatinskiy et al., Reference Piriatinskiy, Atkinson, Park, Morgenstern, Brekhman, Yossifon, Bartholomew and Lotan2017; Brekhman et al., Reference Brekhman, Ofek-Lalzar, Atkinson, Alama-Bermejo, Maor-Landaw, Malik, Bartholomew and Lotan2021). These results aligned with other parasite aminopeptidases involved in sporulation (Gras et al., Reference Gras, Byzia, Gilbert, McGowan, Drag, Silvestre, Niepceron, Lecaille, Lalmanach and Brossier2014). These first studies on C. shasta proteases suggest that these molecules are key players in host–parasite interactions in myxozoans and important to understand parasite virulence.
Venom-like compounds
Cnidarians are the oldest extant venomous animals. Myxozoans have retained a few venom-like compounds (VLCs) similar to those present in free-living species. In C. shasta genotype IIR, 8 VLCs were identified in the nematocyst proteome and a time-series infection transcriptome (Americus et al., Reference Americus, Hams, Klompen, Alama-Bermejo, Lotan, Bartholomew and Atkinson2021). Those from the nematocyst proteome included a lactadherin-like protein, a peptidase inhibitor-16-like protein and a C-type lectin-like protein. The infection transcriptome contained 2 metallopeptidase-like transcripts (astacin and reprolysin-like), 2 Kunitz-type protease inhibitor-like transcripts, a hyaluronidase-like transcript and the same peptidase-inhibitor 16-like transcript and C-type lectin-like transcript found in the nematocyst proteome. Functional annotation indicated possible roles of the VLCs as anticoagulants, spreading factors, protease inhibitors and in protein lysis. These compounds had closely related sequences in omics data from other Myxozoa, which suggested there is a conserved venom profile across these parasites and an overall reduction in venom diversity relative to free-living cnidarians (Americus et al., Reference Americus, Hams, Klompen, Alama-Bermejo, Lotan, Bartholomew and Atkinson2021).
Ceratonova shasta (genotype IIR) expressed the VLCs differently over a 3-week infection in rainbow trout. Three were most highly expressed at 1 dpe in the fish's gills and expression of 4 VLCs peaked at 21 dpe in the intestines, coinciding with formation of myxospores. The reprolysin-like metallopeptidase had similar expression at all timepoints. The early-expressed VLC genes (C-type-lectin, a Kunitz-type protease inhibitor, hyaluronidase-like transcript) were upregulated prior to the development of nematocysts, suggesting these VLCs in C. shasta have been repurposed to facilitate parasite invasion and initial proliferation within the host. The later-expressed genes (a Kunitz-type protease inhibitor, astacin-like metallopeptidase, Peptidase inhibitor 16, lactadherin-like compound) probably represent compounds being synthesized for packaging in the myxospore nematocysts for infection of the annelid host. Two origins of the VLCs were suggested from molecular phylogenetics analyses: the lactadherin-like protein, the C-type lectin-like protein and hyaluronidase-like transcript were inherited from a cnidarian ancestor, whereas the other VLCs were more closely related to sequences from venomous non-cnidarian organisms (spiders, scorpions) and thus may have gained qualities of venom components via convergent evolution (Americus et al., Reference Americus, Hams, Klompen, Alama-Bermejo, Lotan, Bartholomew and Atkinson2021). Myxozoan parasite venomics is only in its infancy, and presents both the challenge and opportunity of identifying ‘venom’ compounds that do not fit the ‘predation and defence’ narrative of venom function in free-living cnidarians, and instead show that venoms may have a diverse utility in parasitic organisms.
Salmonid host factors affecting disease severity
One of the unique aspects of disease induced by C. shasta is the clear-cut differences in disease severity that occur within and among its different host salmon species (reviewed in Bartholomew, Reference Bartholomew1998). Therein, C. shasta is one of the best examples of heritable disease resistance in fishes (Ibarra et al., Reference Ibarra, Hedrick and Gall1992; Bartholomew, Reference Bartholomew1998; Bartholomew et al., Reference Bartholomew, Whipple and Campton2001; Nichols et al., Reference Nichols, Bartholomew and Thorgaard2003b) and a good model system for studying disease resistance against myxozoans. Advances in annotation of the host genome and transcriptome, and the establishment of laboratory cultures of pure genotypes have allowed investigation of sympatric and allopatric host responses to known genotypes at both the genomic and phenotypic levels.
Host life history
Salmonids exhibit a wide range of rearing, migratory and spawning strategies, and these affect when and how long they will be exposed to C. shasta. For example, Chinook salmon are semelparous and are classified into spring, summer or fall types, based on their timing of entry into freshwater. Spring Chinook typically enter rivers in spring and spawn in late summer to early fall (Quinn, Reference Quinn2018) and likely encounter the parasite during the peak infectious period and when temperatures are highest, potentially making them the most vulnerable, but also likely to have evolved increased resistance to disease. Returning adult fall Chinook may avoid the highest peak of infection in the spring, but their migration often coincides with a second, smaller parasite peak in the fall. These differences may explain why in rivers like the Klamath River, which is now dominated by fall Chinook salmon (Thompson et al., Reference Thompson, Bellinger, O'Rourke, Prince, Stevenson, Rodrigues, Sloat, Speller, Yang, Butler, Banks and Miller2019), prespawn mortality of adult salmon is not commonly observed. Nevertheless, observed infection prevalence is generally high (62–91%) for returning Klamath Basin adult Chinook salmon (Foott et al., Reference Foott, Stone, Fogerty, True, Bolick, Bartholomew, Hallett, Buckles and Alexander2016). Iteroparous species like steelhead/rainbow trout have resident freshwater forms and a broader period of spawning (Quinn, Reference Quinn2018), both of which translate to a greater period of overlap with the parasite. As discussed earlier (section on C. shasta genotypes), this has resulted in a different adaptive strategy for genotype 0.
Juvenile migration strategies also vary, with coho and spring Chinook salmon spending their first year of life in freshwater, while fall Chinook migrate to the ocean in their first year (Quinn, Reference Quinn2018). The shorter time in freshwater may explain the more rapid maturation of the parasite in Chinook salmon, an adaptation to enable it to encounter its freshwater annelid host. Developmental degree days (DDD, total number of days × temperature above specific threshold) required from infection (actinospore encounter) to myxospore formation and release appear to vary, with genotype I developing more rapidly (283 DDD in Chinook) than genotype II (324 DDD in coho) (authors' unpublished data). Infection prevalence in out-migrating juvenile salmon varies considerably inter-annually (e.g. 17–91% in the Klamath River) and does not necessarily correlate with severity of infection (Voss et al., Reference Voss, Benson and Freund2022). These juvenile fish could avoid parasite exposure by utilizing tributaries and cold water refugia (Chiaramonte et al., Reference Chiaramonte, Ray, Corum, Soto, Hallett and Bartholomew2016) or by migrating at times that avoid peak parasite release (Margolis et al., Reference Margolis, McDonald and Whitaker1992). While there are other reasons for these behaviours (e.g. thermal regulation, physiological factors), fish with these behavioural traits may have an adaptive advantage when parasite abundance is high.
Parasite dose
As with most infectious pathogens, C. shasta parasite dose relates directly to disease severity. However, temperature, parasite genotype and fish host species and strain also play determining roles in the outcome of parasite exposure. Development of laboratory culture systems and quantitative molecular assays has enabled controlled dose infection studies and enumeration of exposure dose in the field. A controlled dose challenge (Bjork and Bartholomew, Reference Bjork and Bartholomew2009a) confirmed an earlier estimate (Ratliff, Reference Ratliff1983) that susceptible rainbow trout are fatally infected by as few as 1 actinospore. Because of the high parasite numbers required for these studies, determining a lethal parasite dose for resistant strains of fish in the laboratory has been more difficult and has been expressed as a mortality threshold, or the dose at which mortalities begin to occur. For one resistant strain, Iron Gate Klamath River Chinook salmon, the threshold at 18°C was approximately 7.7 × 104 actinospores (Ray et al., Reference Ray, Rossignol and Bartholomew2010). However, this threshold will vary with fish strain depending on their evolutionary association (sympatric/allopatric) with the parasite. For example, for Trinity River Chinook salmon, which have a more restricted exposure to the parasite, 22% mortality occurred at a dose approximately 5-fold lower (1.4 × 104; Foott et al., Reference Foott, Stone and True2007). The concept of a mortality threshold has been useful in defining a level of parasite abundance in the river that is likely to result in mortality, particularly as an easy-to-understand metric for assessing immediate risk to particular fish species. For example, a lethality threshold of 40% was reached with 10 spores L−1 for Klamath River Chinook salmon, and 5 spores L−1 for coho salmon (Hallett et al., Reference Hallett, Ray, Hurst, Holt, Buckles, Atkinson and Bartholomew2012).
Genetic differences in susceptibility
Intraspecies differences in host resistance to disease by C. shasta demonstrate the role of the parasite as a strong selective pressure on fish population genetics in rivers where the parasite is endemic. Disease resistance is a spectrum, with allopatric fish strains generally being susceptible to disease while fish that are sympatric with the parasite may become infected but have a high level of resistance to disease development. Based on what we now know, it is likely that the first recognition of C. shasta as a pathogen by Noble (Reference Noble1950) was a result of genotype II infections in an allopatric host. Similarly, the inability to establish coastal strains of steelhead in the Willamette River OR, USA (Buchanan et al., Reference Buchanan, Sanders, Zinn and Fryer1983) was likely that they were susceptible to genotype II. In contrast, steelhead and cutthroat trout that recovered from C. shasta infections in a hatchery (WA, USA) were likely infected with genotype 0 (Bartholomew et al., Reference Bartholomew, Ray, Torell, Whipple and Heidel2004).
A series of inheritance studies confirmed that resistance to C. shasta is genetically controlled and conferred by multiple loci (Ibarra et al., Reference Ibarra, Hedrick and Gall1992, Reference Ibarra, Hedrick and Gall1994; Bartholomew et al., Reference Bartholomew, Whipple and Campton2001). As the O. mykiss genome became available (Nichols et al., Reference Nichols, Young, Danzmann, Robison, Rexroad, Noakes, Phillips, Bentzen, Spies, Knudsen, Allendorf, Cunningham, Brunelli, Zhang, Ristow, Drew, Brown, Wheeler and Thorgaard2003a; Palti et al., Reference Palti, Genet, Gao, Hu, You, Boussaha, Rexroad and Luo2011), this allowed for estimating the number and location of gene regions associated with C. shasta resistance. An initial study that used clonal lines of rainbow trout for genetic analysis to test for the co-segregation of molecular markers revealed at least 3 genomic loci associated with resistance to C. shasta (Nichols et al., Reference Nichols, Bartholomew and Thorgaard2003b), confirming polygenic control of resistance and identifying potential linkage groups and markers. More recently, a genome-wide association study identified a chromosomal region of OMY9 with a major effect on resistance to the parasite and another region on OMY11 with a lesser contribution to survival (Barrett, Reference Barrett2020). The identification of genetic markers has implications for selective breeding programmes and development of therapeutics, and potentially providing a non-lethal means of assessing a stock's resistance.
Immune response to the parasite
The degree of fish host reaction to the myxozoan invader (reviewed by Holzer et al., Reference Holzer, Piazzon, Barrett, Bartholomew and Sitjà-Bobadilla2021) is central to understanding differences in disease severity among allopatric/susceptible and sympatric/resistant fish hosts. Disease pathology in C. shasta infections is typically associated with a dysregulated, and often excessive T-cell response that fails to control parasite proliferation (Barrett and Bartholomew, Reference Barrett and Bartholomew2021; Barrett et al., Reference Barrett, Estensoro, Sitjà-Bobadilla and Bartholomew2021). Resulting infections are characterized by severe intestinal inflammation, with lymphocytic infiltration of the infected tissues. In an RNA-seq time-series study of allopatric and sympatric steelhead trout response to C. shasta genotype II infection, an initial downregulation of immune genes in both phenotypes, particularly those associated with the ifnγ signalling pathway, suggests that parasite-induced immunosuppression may play a role in invasion (Barrett and Bartholomew, Reference Barrett and Bartholomew2021). However, the response to the parasite diverged after this point, with resistant fish responding rapidly with upregulation of genes for innate immune receptors, including nlrc5, a pathogen recognition receptor. This, combined with the induction of Th1 markers in the intestine of resistant fish, suggests that early specific recognition of C. shasta is a critical factor in resistance. The upregulation of ifnγ and nlrc5 is also suggestive of an intracellular phase early in the infection, which is supported by the presence of parasite in the endothelium of blood vessels starting at 3 days post-infection (Fig. 1D; Bjork and Bartholomew, Reference Bjork and Bartholomew2010). In addition to the rapid upregulation of key immune factors in resistant fish, there was also a massive upregulation of caspase-14, a protein involved in keratinocyte differentiation that might reflect epithelial repair mechanisms that limit the spread of the parasite (Barrett et al., Reference Barrett, Estensoro, Sitjà-Bobadilla and Bartholomew2021). In contrast, susceptible fish did not respond until the parasite was proliferating and causing pathology in the intestine. At this time they showed both a vigorous Th1 (ifnγ, il12, Th1 transcription factors) and Th2 (il4, il13, gata3) response (Barrett and Bartholomew, Reference Barrett and Bartholomew2021). This response appeared ineffective and likely contributed to host pathology, as parasite numbers continued to increase exponentially and the intestinal structure broke down rapidly.
Upregulation of pro-inflammatory cytokines in the intestine, particularly of interferon (IFNγ), was also noted in response to genotype I infections in allopatric Chinook salmon, and in genotype II infections in rainbow trout (Bjork et al., Reference Bjork, Zhang, Hurst, Alonso-Naveiro, Alexander, Sunyer and Bartholomew2014; Hurst et al., Reference Hurst, Alexander, Dolan, Jia and Bartholomew2019; Taggart-Murphy et al., Reference Taggart-Murphy, Alama-Bermejo, Dolan, Takizawa and Bartholomew2021). Although genotype 0 infections in allopatric rainbow trout were characterized histologically by a proliferation of immune cells, upregulation of inflammatory cytokines (IL-6, IL-8, IL-10, IFNγ) was reduced and delayed compared with genotype II infections, and tissues underwent reparative processes (fibrosis) (Taggart-Murphy et al., Reference Taggart-Murphy, Alama-Bermejo, Dolan, Takizawa and Bartholomew2021), consistent with the ability of fish infected with this genotype to survive infection. The presence of atypical stefins in myxozoans may explain the increased expression of the anti-inflammatory IL-10 (Bartošová-Sojková et al., Reference Bartošová-Sojková, Kyslík, Alama-Bermejo, Hartigan, Atkinson, Bartholomew, Picard-Sánchez, Palenzuela, Faber, Holland and Holzer2021). These secretory proteins have immunomodulatory roles that may enable the parasite to interfere with specific fish immune responses and facilitate parasite migration within host tissues.
The role of a specific antibody response in resolving C. shasta infections is not clear. In chronic or prolonged infections, there is generally increasing expression of IgT and IgM transcripts and production of protective antibodies may be effective in delaying disease progression and reducing disease severity (e.g. non-lethal strains of C. shasta or C. shasta infections in resistant fish strains). For example, the role of IgT as a mucosal antibody was first described in rainbow trout (Zhang et al., Reference Zhang, Salinas, Li, Parra, Bjork, Xu, LaPatra, Bartholomew and Sunyer2010) infected with the chronic, non-lethal genotype 0, which may have allowed the fish time to respond to the infection. However, when infections are acute (e.g. virulent strains of C. shasta or infections in susceptible fish strains), there is little evidence for a role for B cells. In resistant and susceptible steelhead infected with genotype II, at 21 days post-infection (when the parasite was proliferating in the intestine) resistant fish had higher expression of IgM (14 vs 2-fold) and IgT (337 vs 65-fold) (Barrett et al., Reference Barrett, Estensoro, Sitjà-Bobadilla and Bartholomew2021). This difference in expression is likely a result of the delayed recognition of the parasite by susceptible fish. Thus, in susceptible fish the antibody response occurs too late to be effective, while resistant fish that are better able to maintain their intestinal structure and mount an earlier adaptive response are successful in resolving infection.
The ability to evade or modulate these host immune responses plays a role in persistence of myxozoan infections (reviewed in Holzer et al., Reference Holzer, Piazzon, Barrett, Bartholomew and Sitjà-Bobadilla2021). Ceratonova shasta likely takes advantage of embedding in the endothelial cells of gill blood vessels to evade the immune response early in the infection. Myxozoans also alter their antigenic epitopes during their development, and monoclonal antibodies produced against C. shasta that reacted with host leucocytes suggest that the parasite may utilize cross-reacting carbohydrate epitopes to evade host recognition (Bartholomew et al., Reference Bartholomew, Smith, Rohovec and Fryer1989). As discussed earlier, another evasion mechanism is motility, and C. shasta utilizes integrin-based motility to promote adhesion to the ECM of host cells, facilitating rapid directional movement and exploitation of host cells (Alama-Bermejo et al., Reference Alama-Bermejo, Holzer and Bartholomew2019). Similarly, proteolytic enzymes encoded by pathogens are also weapons of defence by degrading host defences (Alama-Bermejo et al., Reference Alama-Bermejo, Meyer, Atkinson, Holzer, Wiśniewska, Kolísko and Bartholomew2020).
Annelid host factors affecting salmonid disease severity
The annelid host of C. shasta was originally reported as Manayunkia speciosa (Bartholomew et al., Reference Bartholomew, Whipple, Stevens and Fryer1997). However, it was observed that although this host was present in the Eastern US, C. shasta had not become established there, despite the introduction of Pacific salmon and trout (Hallett and Bartholomew, Reference Hallett, Bartholomew, Woo and Buchmann2012). This apparent paradox was informed by the discovery that infected West Coast annelids were a distinct species, M. occidentalis, and presumably the only permissive annelid host for C. shasta (Atkinson et al., Reference Atkinson, Bartholomew and Rouse2020). This annelid also serves as host to Parvicapsula minibicornis, another important myxozoan parasite of salmonids that is also restricted to the Pacific Northwest of North America with its annelid host (Atkinson et al., Reference Atkinson, Bartholomew and Rouse2020; Bartholomew et al., Reference Bartholomew, Atkinson and Hallett2006).
Manayunkia occidentalis is a cryptic, tube-dwelling suspension feeder that can exhibit rapid population expansion and high plasticity of habitat use (Stocking and Bartholomew, Reference Stocking and Bartholomew2007; Alexander et al., Reference Alexander, Hallett, Stocking, Xue and Bartholomew2014, Reference Alexander, Wright, Som, Hetrick and Bartholomew2016; Fig. 5). Annelid hosts encounter and consume myxospores while foraging (n.b., suspension and deposit feeding have been suggested; Stocking and Bartholomew, Reference Stocking and Bartholomew2007; e.g. Taghon et al., Reference Taghon, Nowell and Jumars1980), and once infected, likely remain infected for the duration of their lives.

Fig. 5. Annelid host of Ceratonova shasta. (A) Collection by SCUBA; (B) annelid tubes at low density anchored in encrusting periphyton; (C) annelid tubes at high density; (D) annelids feeding from their tubes; (E) mature annelid, bar 1 mm (authors' images).
Infection prevalence in natural populations
The prevalence of myxosporean infections in natural populations of invertebrate hosts is typically considerably lower than in their vertebrate counterparts (Yokoyama et al., Reference Yokoyama, Ogawa and Wakabayashi1993; Xiao and Desser, Reference Xiao and Desser1998; Hallett et al., Reference Hallett, Erséus, O'Donoghue and Lester2001; DuBey and Caldwell, Reference DuBey and Caldwell2004; Holzer et al., Reference Holzer, Sommerville and Wootten2004; Krueger et al., Reference Krueger, Kerans, Vincent and Rasmussen2006; Alexander et al., Reference Alexander, Kerans, Koel and Rasmussen2011; Lodh et al., Reference Lodh, Stevens and Kerans2011), and C. shasta is no exception. The prevalence of C. shasta in M. occidentalis varies considerably among and within systems and seasons but <1–5% has been reported (e.g. Stocking and Bartholomew, Reference Stocking and Bartholomew2007; Alexander et al., Reference Alexander, Hallett, Stocking, Xue and Bartholomew2014).
Low levels of infection prevalence in the annelid hosts are explained by parasite (myxospore) encounter rates, which are influenced by annelid host densities and hydraulic conditions (Bjork and Bartholomew, Reference Bjork and Bartholomew2009b; Alexander et al., Reference Alexander, Kerans, Hallett, Stevens, Okamura, Gruhl and Bartholomew2015). Annelid host distribution and density are driven by hydraulic conditions, with higher densities associated with stable environments that have not been disturbed. Annelids can form large assemblages (>1 million m−2; Fig. 5C) when conditions permit, and C. shasta-infected annelids are more frequently detected in high-density assemblages.
Parasite dose
The relationship between myxospore dose and infection in annelids has not been well characterized. In most experiments, myxospores have been added unquantified to annelid cultures (e.g. Meaders and Hendrickson, Reference Meaders and Hendrickson2009; but see Bjork and Bartholomew, Reference Bjork and Bartholomew2009a). A more quantified approach involved exposure of annelids to C. shasta myxospores in 6-well cell culture plates. Ten annelids were placed in each of 5 replicate wells per dose, in which each annelid was exposed to 0, 1, 10, 500 or 1000 myxospores, held at 18°C for 6 weeks, then examined with a combination of microscopy and polymerase chain reaction. No infections were detected in the annelids exposed to 1 myxospore, but infections were detected in 18–46% of the annelids exposed to 10 or more myxospores, suggesting more than a single myxospore is required to initiate infection in this host (authors' unpublished data).
Factors affecting development and release in Manayunkia occidentalis
Actinospore development occurs within 49 days at 17–21°C but can continue for over 12 weeks following exposure (Meaders and Hendrickson, Reference Meaders and Hendrickson2009). Timing of actinospore release is related to DDD, myxospore dose, parasite genotype and environmental conditions. As in fish, DDD required from infection (myxospore encounter) to actinospore release appear to vary among parasite genotypes, but data are limited and those that have been reported can be difficult to interpret. For example, Meaders and Hendrickson (Reference Meaders and Hendrickson2009) reported actinospore release at 49 days (35 days at 17.5°C + 14 days at 21.5°C; from which we calculated 917 DD) but they used myxospores derived from both Chinook (genotype I) and rainbow trout (genotype II). Differences in DDD have been observed among genotypes in annelids held in laboratory mesocosms (J. D. Alexander, unpublished data) suggesting ~650 DDD are required for genotype I, ~800 for genotype II and ~1000 for genotype 0. Estimates of actinospore release over 1 day by an infected annelid host were comparable in 2 different studies: ~375 day−1 (Bartholomew et al., Reference Bartholomew, Atkinson and Hallett2006), 340 ± 155 day−1 (Meaders and Hendrickson, Reference Meaders and Hendrickson2009), and are in agreement with the estimated release over a 2-week period of 4759 ± 2173 (Bjork and Bartholomew, Reference Bjork and Bartholomew2009a). However, the initial myxospore dose also plays a role in actinospore release. Release of >20 000 actinospores in 24 h following exposure to doses of 500 myxospores host−1 (genotype II) has been observed from a single infected worm (J. D. Alexander, unpublished data). Although this factor is likely unimportant under natural conditions where the likelihood of myxospore encounter is low, it shows a similar dose-dependent response as in fish hosts.
Finally, annelid host environment likely plays an important role in actinospore release. Meaders and Hendrickson (Reference Meaders and Hendrickson2009) suggest that rather than actinospore release occurring asynchronously, spores may be ‘stored’ until there is a cue for release (e.g. thermal threshold, mechanical disturbance). Evidence of a thermal threshold response in laboratory experiments and field data has been observed (authors' unpublished data). For example, when annelids were exposed to myxospores and then held at 8 or 16°C, actinospores were not observed in any of the 8°C treatment except during periods of accidental temperature increases when experimental chambers warmed to above 10°C, supporting the possibility of a thermal threshold. This 10°C threshold typically co-occurs with the descending limb of the river hydrograph (disturbance) and emergence of salmon fry in the spring. The mechanism driving actinospore release from the annelid hosts is probably explained by a combination of DDD and environmental cues, and should be further investigated.
Environmental drivers of disease
Previous reviews have characterized relationships between abiotic factors and disease caused by C. shasta (see Bartholomew, Reference Bartholomew1998; Hallett and Bartholomew, Reference Hallett, Bartholomew, Woo and Buchmann2012; Jones et al., Reference Jones, Bartholomew, Zhang, Okamura, Gruhl and Bartholomew2015; Ray et al., Reference Ray, Alexander, De Leenheer, Bartholomew, Okamura, Gruhl and Bartholomew2015). River water temperature and flow conditions are the primary environmental drivers of disease. These factors play important roles throughout the complex life cycle of C. shasta, driving spore dispersal and viability and infection and disease in both hosts. Below we provide a brief review of these topics in the context of parasite ecology.
Water temperature influences all phases of the C. shasta life cycle, from interactions within both hosts to spore viability in the environment. In fish hosts, water temperature is correlated with disease progression, severity and mortality, due to the increased rate of parasite replication at higher temperatures, although temperature effects on immune function of the fish host are also important (Alcorn et al., Reference Alcorn, Murra and Pascho2002; Scharsack and Franke, Reference Scharsack and Franke2022). Water temperature is correlated with annelid host population expansion resulting in increased host availability and foraging rates (e.g. Vaughn et al., Reference Vaughn, Spooner and Galbraith2007), both of which increase likelihood of myxospore encounter and infection. In addition, water temperature is also correlated with increased parasite proliferation and duration of actinospore release in annelid hosts, and release appears to be regulated by a combination of accumulation of degree days (DDD, see above) and a temperature threshold (8°C) (authors' unpublished data).
Water temperature is inversely correlated with the longevity of myxozoan stages in the environment; however, myxospores and actinospores exhibit differential viabilities and persistence. Actinospores are more affected by temperature than myxospores, which are comparatively stable perhaps due to the hardened valves that surround the sporoplasm. Spore viability is inversely related to temperature. Actinospores remain viable for ~7 days at 4°C, but only for ~4 days at 20°C and myxospores persisted for >150 days at 4°C and 50 days at 20°C (Foott et al., Reference Foott, Stone and True2007; Bjork, Reference Bjork2010; authors' unpublished data). Field studies suggest similar results, with infectivity for fish demonstrated for 13 days at 11°C (Ratliff, Reference Ratliff1981) and 3–7 days at 18°C. The variability in viability and persistence of the different spore stages presumably correlates with differences in host–parasite encounter between the 2 spore stages; the parasite increases its odds of host encounter through persistence in the environment (myxospores) and through relatively long temporal periods of release (actinospores).
The effects of river discharge and velocity on phases of the C. shasta life cycle are just as important as those of temperature. Studies on the effects of water velocity on C. shasta–host interactions (Bjork and Bartholomew, Reference Bjork and Bartholomew2009b; Ray and Bartholomew, Reference Ray and Bartholomew2013) showed that water velocity and infection prevalence were negatively correlated in both vertebrate and invertebrate hosts. River discharge influencing parasite spore encounter is 1 mechanism to explain why velocity and infection prevalence are negatively correlated. Myxospores are released over relatively short periods and are likely dispersed to annelid hosts in ‘bursts’ associated with flow events, and annelid host distributions (patchy) are driven by hydraulic conditions during peak flow events (Alexander et al., Reference Alexander, Wright, Som, Hetrick and Bartholomew2016). Actinospores are released from annelids over relatively longer periods of time sometimes co-occurring with flow events that ‘dilute’ actinospore stages and in turn temporarily reduce host–parasite encounter.
Monitoring disease effects
Acquiring data collected under different water years at set index sites is imperative for understanding disease dynamics in a complex riverine ecosystem. These long-term datasets are necessary for model development, predicting effects and informing management decisions, both short and long term. One of the best known on-going monitoring programmes for a myxozoan parasite is that for C. shasta in the Klamath River. That programme was initiated in response to the chronic, often high prevalence of infection in out-migrating juveniles and the recognition that this parasite was a key factor limiting salmon recovery in that system (Fujiwara et al., Reference Fujiwara, Mohr, Greenberg, Foott and Bartholomew2011). The monitoring approach for C. shasta is guided by the parasite's life cycle, and consists of fish studies (sentinel exposures and wild fish sampling), annelid sampling and waterborne parasite sampling.
This continuing multi-year monitoring effort (beginning in 2002) has enabled the identification of metrics that matter for informing population-level impacts and directing adaptive management decisions (Ray et al., Reference Ray, Rossignol and Bartholomew2010, Reference Ray, Perry, Som and Bartholomew2014; Ray and Bartholomew, Reference Ray and Bartholomew2013). For example, juvenile Chinook salmon can clear subclinical infections within 60 days post infection (authors' observations) and prevalence of infection and severity of infection are not always correlated, with unrecoverable disease observed at times when fewer than 30% of fish are infected yet not when 80% are infected (Voss et al., Reference Voss, Benson and Freund2022). Thus, severity of infection, sometimes referred to as prevalence of mortality, is the metric that matters and requires a management action, rather than prevalence of infection. For waterborne stages, it is the density that corresponds to a 40% lethality threshold, which is 10 spores L−1 of type I for Chinook salmon and 5 spores L−1 for coho (Hallett et al., Reference Hallett, Ray, Hurst, Holt, Buckles, Atkinson and Bartholomew2012); these 2 genotypes occur independently of each other and therefore must be managed separately for their respective salmon host. For annelids, both density of hosts and infection prevalence in those hosts are measured, but it is the combined metric of density of infected annelids (infected individuals per m2) that corresponds to waterborne parasite stages and infection dose for salmon. Recognition of these meaningful metrics has enabled the identification of thresholds for management actions (e.g. in places like the Klamath River).
Management and control
Although C. shasta was first described as a disease of hatchery fish, its impacts on wild fish populations can present a challenge to the recovery of threatened and endangered wild salmon populations in rivers where the parasite is endemic (Fujiwara et al., Reference Fujiwara, Mohr, Greenberg, Foott and Bartholomew2011). Two strategies for decreasing disease that occur naturally in wild salmon are avoidance of the parasite through migration timing (Margolis et al., Reference Margolis, McDonald and Whitaker1992) and evolution of disease resistance, discussed earlier. The most widely used hatchery management strategies, stocking resistant salmonids and timing release of hatchery fish to occur during periods when parasite abundance is low (Buchanan et al., Reference Buchanan, Sanders, Zinn and Fryer1983; Hallett and Bartholomew, Reference Hallett, Bartholomew, Woo and Buchmann2012; Jones et al., Reference Jones, Bartholomew, Zhang, Okamura, Gruhl and Bartholomew2015), mirror these natural strategies. Prevention and control strategies applicable to hatchery and wild scenarios have been reviewed (Hallett and Bartholomew, Reference Hallett, Bartholomew, Woo and Buchmann2012; Jones et al., Reference Jones, Bartholomew, Zhang, Okamura, Gruhl and Bartholomew2015), and here we will focus on mitigating disease effects on wild salmon populations (Fig. 6).

Fig. 6. Possible management actions and goals to affect each phase of the Ceratonova shasta life cycle, targeting both hosts and the parasite directly. *Intestines removed from carcasses before out-planting.
While options for controlling disease in the wild are limited, some general strategies have been tested in field and laboratory studies. An epidemiological model developed to inform management approaches (Ray et al., Reference Ray, Alexander, De Leenheer, Bartholomew, Okamura, Gruhl and Bartholomew2015) predicted that reducing transmission of myxospores from adult salmon to winter invertebrate populations could be effective in disrupting the parasite life cycle. However, a study that removed adult carcasses from spawning grounds did not support that this approach would be successful; for although most fish were infected (up to 86%), only a few (<10%) contributed high numbers of myxospores (Foott et al., Reference Foott, Stone, Fogerty, True, Bolick, Bartholomew, Hallett, Buckles and Alexander2016).
Reducing transmission of actinospores to juvenile salmon in spring through increased flows has shown more promise. Laboratory studies demonstrated reduced parasite transmission and disease severity at higher water velocities (Bjork and Bartholomew, Reference Bjork and Bartholomew2009b; Ray and Bartholomew, Reference Ray and Bartholomew2013). Similarly, disease risk was low following a 10-year flood event in the Klamath River, due in part to effects on the invertebrate host populations (Alexander et al., Reference Alexander, Hallett, Stocking, Xue and Bartholomew2014). Thus, in rivers with dams, the ability to manage flows offers potential for reducing parasite density and fish exposure duration, and this has been used to some effect in the Klamath River. In this river system, 3 types of flow are utilized to mitigate C. shasta (Hillemeier et al., Reference Hillemeier, Belchik, Soto, Tucker and Ledwin2017) and their influence on disease dynamics depends upon their timing (season), magnitude and duration. For example, a deep flushing flow of high discharge for short duration (approximating a 5–10 year magnitude flood, 24 h) in spring every other year is prescribed to move armoured bed layer sediments and reduce annelid populations (Curtis et al., Reference Curtis, Poitras, Bond and Byrd2021). A surface flushing flow of moderate discharge (equivalent to ~2–5 year interval magnitude flood) for 72 h every winter moves surface (fine) sediments, again to reduce annelid populations. Whereas, in the spring, when disease thresholds (spore density and mortality prevalence) are met, an enhanced flow (also referred to as a dilution flow or emergency disease flow) can be prescribed to dilute waterborne infectious stages and encourage fish outmigration. While the third action has an immediate and short-term impact (via dilution) on parasite density and dose, the effect of the first 2 actions can be more extensive through actual removal of the annelid host. Because water management is so contentious, predictive models have been developed for annelid hosts based on outputs from 2-dimensional hydraulic models to simulate conditions during peak discharge. The resultant tool describes annelid distribution under various discharge scenarios (e.g. dams out, drought, flow manipulations; Alexander et al., Reference Alexander, Wright, Som, Hetrick and Bartholomew2016) and is being used to predict the distribution and density of infected annelids and evaluate the effects of managed flow events. A mechanistic model describing factors driving actinospore abundance during the period of juvenile salmon outmigration was also developed to evaluate the effects of flow manipulation (Robinson et al., Reference Robinson, Alexander, Bartholomew, Hallett, Hetrick, Perry and Som2022). The model describes onset of spore release and inter-annual variation and has potential application as a management tool to assess the impact of proposed flow regimes on disease-induced mortality of juvenile salmonids, a priori. Changes to discharge regimes also encourage anadromous fish migration, which can reduce the overlap of the 2 organisms, and once fish reach the cooler water of the Pacific Ocean disease progression is slowed and fish may recover.
One development that has informed river management is the application of molecular water sampling techniques that both quantify the parasite and distinguish between parasite genotypes (Hallett and Bartholomew, Reference Hallett and Bartholomew2006; Hallett et al., Reference Hallett, Ray, Hurst, Holt, Buckles, Atkinson and Bartholomew2012; Atkinson et al., Reference Atkinson, Hallett and Bartholomew2018). This allows prediction of fish species most likely to become infected, identifies focal points of infection and, when combined with data on temperature and flow, can be used to predict mortality (Ray et al., Reference Ray, Perry, Som and Bartholomew2014). Because the data can be available within days, these methods allow decisions to be made about water allocation and fish release from hatcheries when they would be most effective. The following are 2 examples of how these data were applied. In spring 2020, a surface flushing flow was implemented in response to the measurement of high densities of C. shasta (>100 spores L−1) in the reaches of the Klamath River accessible to anadromous fishes (below the dams). Monitoring occurred at index sites to measure the immediate impact of this action: daily water sampling, pre- and post-flow annelid sampling and sentinel fish exposures. During the flow event the density of waterborne spores decreased by an order of magnitude, then remained reduced after discharge returned to baseline. Fish mortality, annelid density and the density of C. shasta-infected annelids were lower after the flow event. All considered, the prescribed flow event reduced disease risk for native salmonids in the Klamath River by lowering spore abundance. The following year, in 2021, high parasite densities were measured in early spring and infection was determined to be unrecoverable in over half the wild fish sampled (Voss et al., Reference Voss, Benson and Freund2022). However, because of continued drought in the region there was no water available to implement a managed flow. Instead, hatchery managers deviated from usual practices and did not release millions of salmon that spring, but rather held them through the summer until river conditions (primarily parasite density and water temperature) became more favourable in the fall. This action likely not only circumvented the death of millions of hatchery juveniles but also the amplification of the parasite by these fish and seeding the system, which will have implications for both wild and hatchery stocks.
Future directions and research questions and opportunities
Studies conducted prior to 2010 were interpreted without the knowledge of C. shasta genotypes, and their conclusions need to be re-evaluated in the context of parasite genotype and host specificity differences. These include the reported doses for infection of resistant strains from laboratory studies. Additional questions that arose as the current knowledge about C. shasta was considered for this review include:
• Are there genetic differences among the annelid host populations and how do these differences map across different river basins and relate to C. shasta genotype abundances?
• What is the source of genotype II where there are no coho salmon present?
• Do juvenile coho salmon clear infection and survive to adulthood?
• What are the fish species-specific dose thresholds that result in clearing an infection vs succumbing to infection?
• Can C. shasta persist in adult fish through their ocean phase, and remain viable to develop when the fish reenter freshwater?
• How will C. shasta genotypes redistribute following changes in connectivity (Hurst et al., Reference Hurst, Holt and Bartholomew2012; Stinson and Bartholomew, Reference Stinson and Bartholomew2012)?
Addressing these data gaps will enable the development of more robust models and position us to better inform short- and long-term management decisions that impact critical salmonid species with broad importance. Data gaps have been highlighted by modelling approaches. For example, modelling by Robinson et al. (Reference Robinson, Alexander, Hallett and Som2020) suggested that prevalence of C. shasta infection in out-migrating hatchery fish was associated with waterborne C. shasta densities in subsequent seasons, which highlighted data gaps surrounding effects of hatchery operations and management of flow regimes. Modelling effects of future climate scenarios (e.g. Ray et al., Reference Ray, Alexander, De Leenheer, Bartholomew, Okamura, Gruhl and Bartholomew2015) on C. shasta in the Klamath River demonstrated that high winter discharge would have greater effects than temperature on future disease dynamics, highlighting the importance and context of discharge regimes. The models still require ground-truthing and will be strengthened by the addition of parasite genotype-specific parameters, and testing hypotheses developed in the Klamath River in other rivers where the parasite is endemic. Further, as riverine ecosystems are restored, opportunities for study of C. shasta epidemiology in fish and annelid host populations under changing scenarios such as in response to reconnections, including dam removal, will provide additional data for model validation and refinement.
Data availability
The majority of data is from published studies and is referenced. Unpublished data (pers. com.) are available on request from the authors.
Conflict of interest
All authors contributed to the writing and editing of this review article. J. B. provided historical background, reviewed infection and development in the fish host and detailed host responses. J. A. reviewed infection and development in the annelid host, and annelid host and ecological factors affecting disease. S. H. summarized monitoring, management and control. G. A. B. reviewed parasite virulence factors and provided Fig. 4 and S. A. reviewed parasite genotypes, venom-like compounds and provided all other figures.
Financial support
This publication was funded in part by the Bureau of Reclamation (Reclamation), U.S. Department of Interior. Funding was provided by Reclamation as part of its mission to manage, develop and protect water and related resources in an environmentally and economically sound manner in the interest of the American public. Funding was provided through Interagency Agreement #R15PG00065 and #R19PG00027. The views in this report are the authors' and do not necessarily represent the views of Reclamation. Original author research was funded in-part by: United-States-Israel Binational Agricultural Research and Development (BARD) grant IS-5001-17C and United-States-Israel Binational Science Foundation (BSF), Jerusalem, Israel grant 2019063.
Conflict of interest
None.
Ethical standards
Not applicable.










